Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. May 15, 2024; 16(5): 1705-1724
Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1705
Published online May 15, 2024. doi: 10.4251/wjgo.v16.i5.1705
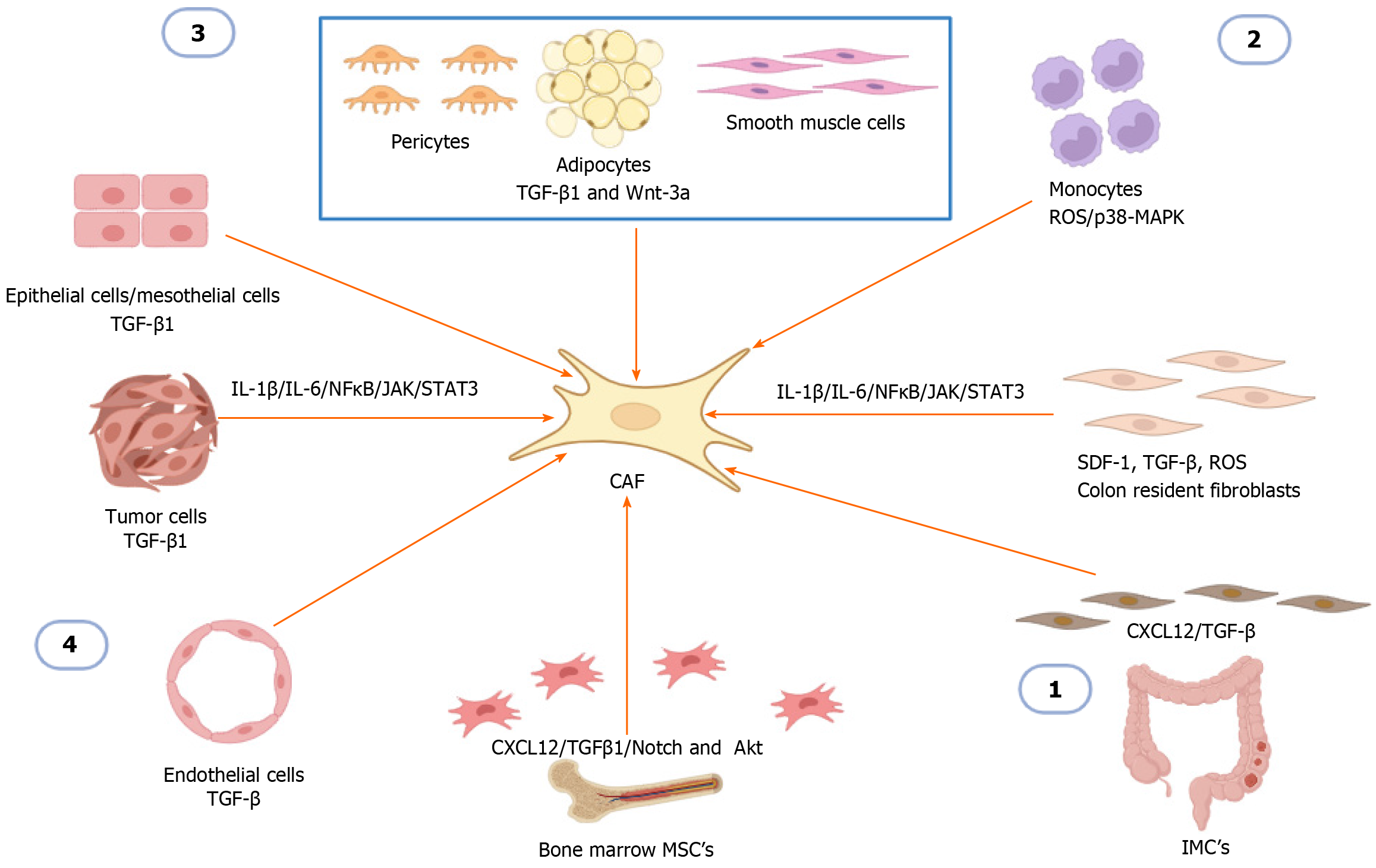
Figure 1 The cellular origins of cancer-associated fibroblasts in solid tumors and colorectal cancer.
(1) Colon resident fibroblasts, intestinal mesenchymal cells and mesenchymal stem cells are converted to cancer-associated fibroblasts (CAFs) by stimulation of different activators including stroma-derived factor 1, transforming growth factor beta (TGF-β), TGF-β1, C-X-C chemokine ligand 12, reactive oxygen species, and the Notch and Akt signaling pathways; (2) Monocytes are transformed to CAF through monocyte-myofibroblast transdifferentiation; (3) Pericytes, adipocytes and smooth muscle cells are transformed to CAF by stimulation of the activators TGF-β1 and Wnt-3a; and (4) Epithelial cells, mesothelial cells, tumor cells are transformed into CAF through the process of epithelial mesenchymal transition by stimulation of the activator TGF-β1, endothelial cells are transformed into CAF by the process of endothelial-mesenchymal transition by stimulation of the TGF-β activator. In addition to all these factors involved in myofibroblast activation, interleukin 1 β (IL-1β), IL-6 through nuclear factor kappa B, and Janus kinase have been reported to function as transducers and activators of the signal transducer and activator of transcription 3 to promote CAF activation. Created with BioRender.com. TGF-β: Transforming growth factor beta; IMC: Intestinal mesenchymal cells; MSCs: Mesenchymal stem cells; CAFs: Cancer-associated fibroblasts; CXCL12: C-X-C chemokine ligand 12; IL: Interleukin; NF-κB: Nuclear factor kappa B; SDF-1: Stroma-derived factor 1; ROS: Reactive oxygen species.
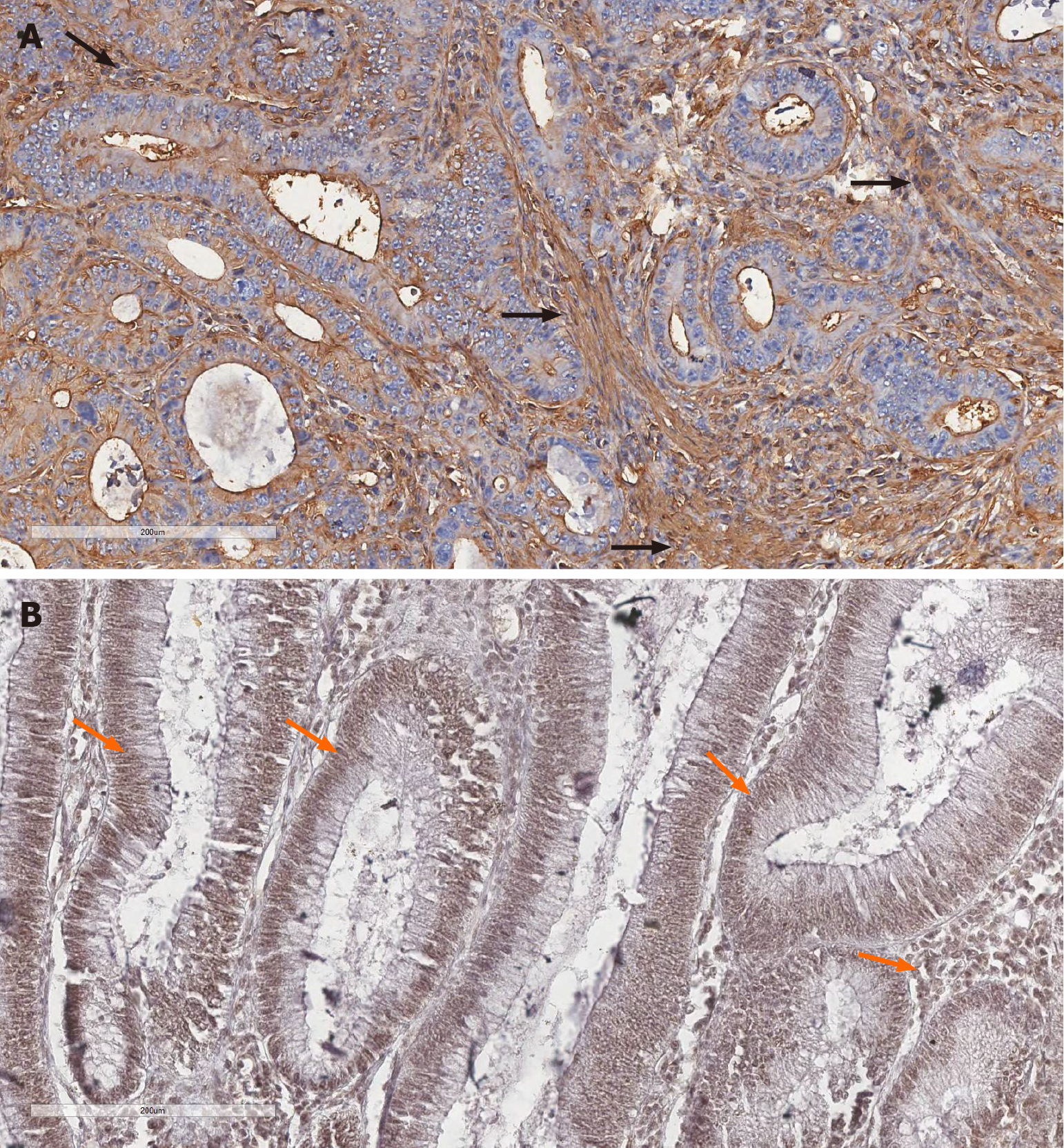
Figure 2 Immunohistochemistry detection of alpha-smooth muscle actin and the signal transducer and activator of transcription 3 in representative images of colorectal cancer.
A: Cytoplasmic expression of alpha-smooth muscle actin (α-SMA) in stromal cells; B: Diffuse expression of activated smooth muscle actin and the transcription signaling pathway 3 (STAT3) in the nuclei of both glandular and stromal neoplastic cells. Black arrows (α-SMA-positive stroma), orange arrows (STAT3-positive tumor and stroma). Magnification 20 ×. Scale bars: 200 μm.
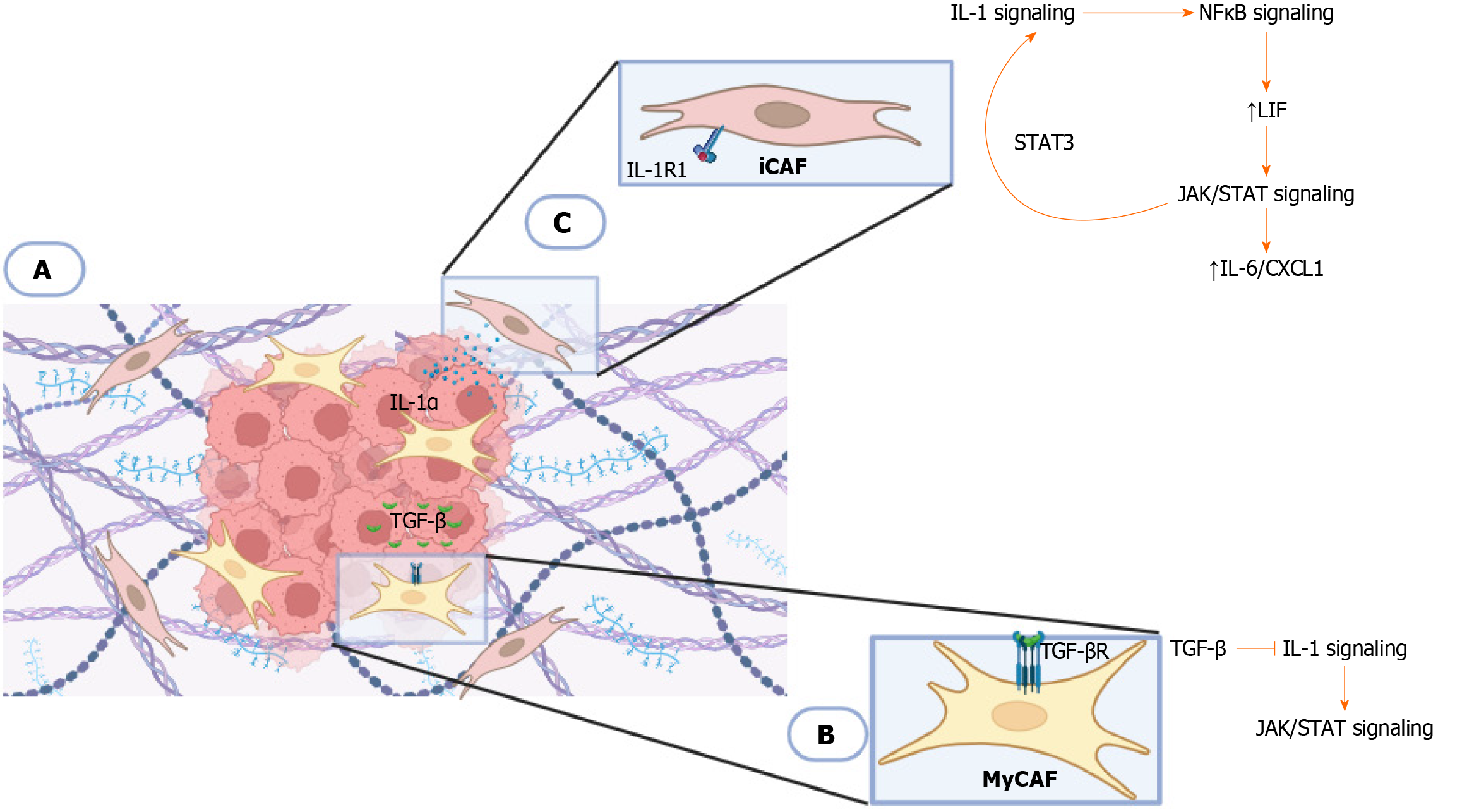
Figure 3 Plasticity between cancer-associated fibroblasts[117].
A: This model demonstrates the antagonistic signaling pathway that determines the ability of cancer-associated fibroblasts (CAFs) to transform into tumor or inflammatory promoter phenotypes. Two subtypes of CAF have been reported in the different zones within the tumor microenvironment in pancreatic ductal adenocarcinoma; inflammatory tumor-associated fibroblasts located in the tumor periphery, and tumor-promoting myofibrolasts (myCAFs) close to the tumor; B: Tumor cells secrete transforming growth factor β ligand (TGF-βL), which allows activation of TGF-β receptor (TGF-βR) in adjacent myCAFs, inhibiting interleukin 1 receptor type 1 (IL-1R1) expression; C: Tumor cells secrete IL-1 alpha (IL-1α) which activates the IL-1 signaling pathway in CAFs. IL-1 signaling induces a cytokine cascade that includes IL-6 and C-X-C chemokine ligand 1, allowing activation of Janus kinase/transcription signaling pathway (JAK/STAT) signaling through nuclear factor kappa B and autocrine leukemia inhibitory factor signaling. Activated JAK/STAT signaling establishes a positive feedback loop involving transcription signaling pathway 3 (STAT3) by upregulation of IL-1R1 expression. Created with BioRender.com. iCAF: Inflammatory tumor-associated fibroblasts; IL: Interleukin; JAK/STAT: Janus kinase/transcription signaling pathway; myCAFs: Tumor-promoting myofibrolasts; LIF: Leukemia inhibitory factor; CXCL: C-X-C chemokine ligand; TGF-β: Transforming growth factor β; NF-κB: Nuclear factor kappa B. Citation: Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discovery 2019; 9 (2): 282-301, with permission from American Association for Cancer Research (Supplementary material).
- Citation: Sánchez-Ramírez D, Mendoza-Rodríguez MG, Alemán OR, Candanedo-González FA, Rodríguez-Sosa M, Montesinos-Montesinos JJ, Salcedo M, Brito-Toledo I, Vaca-Paniagua F, Terrazas LI. Impact of STAT-signaling pathway on cancer-associated fibroblasts in colorectal cancer and its role in immunosuppression. World J Gastrointest Oncol 2024; 16(5): 1705-1724
- URL: https://www.wjgnet.com/1948-5204/full/v16/i5/1705.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i5.1705