Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Apr 15, 2024; 16(4): 1453-1464
Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1453
Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1453
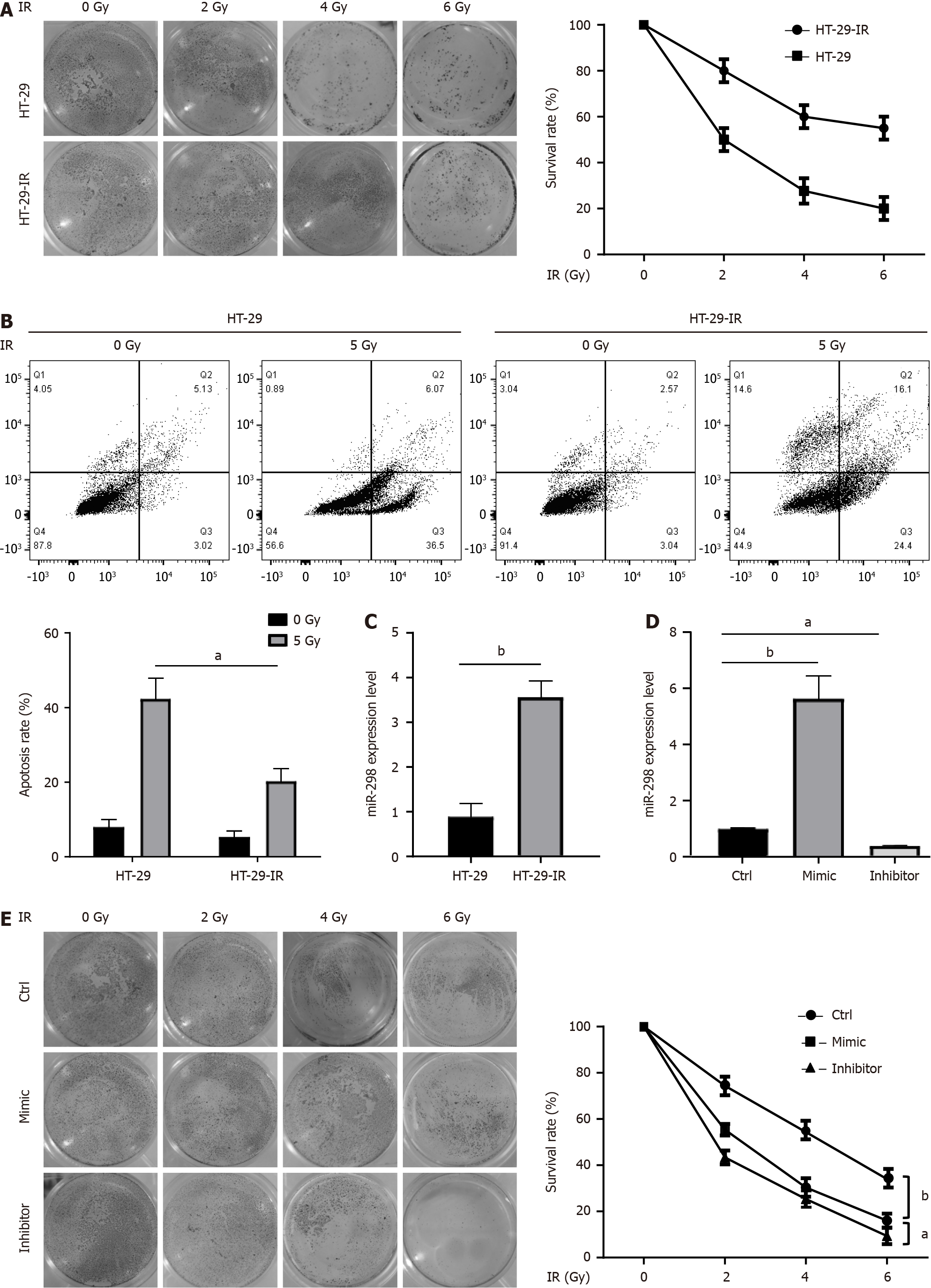
Figure 1 MicroRNA-298 overexpression promoted the radiation resistance of colorectal cancer cell strains.
A: As demonstrated by clonogenic assay, radiation-resistant cell lines (HT-29-IR) exhibited a notable increase in survival rates compared to the radio-sensitive parental cell lines (HT-29) upon exposure to radiation treatment; B: Annexin V/PI staining was performed on HT-29 and HT-29-IR exposed to 5 Gy irradiation. The results indicated that HT-29-IR cells were highly resistant to radiation-induced apoptotic cell death than HT-29; C: Real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) was performed to determine microRNA-298 (miR-298) expression in HT-29 and HT-29-IR; D: RT-qPCR was conducted to assess miR-298 levels in HT-29 transfection with either the miR-298 mimic or miR-298 inhibitor; E: HT-29 cells were transfected with miR-298 mimic or miR-298 inhibitor and then exposed to radiation, followed by cell survival assessment by clonogenic assay. aP < 0.05, bP < 0.01. miR-298: MicroRNA-298; HT-29-IR: Radiation-resistant cell lines; IR: Ionizing radiation.
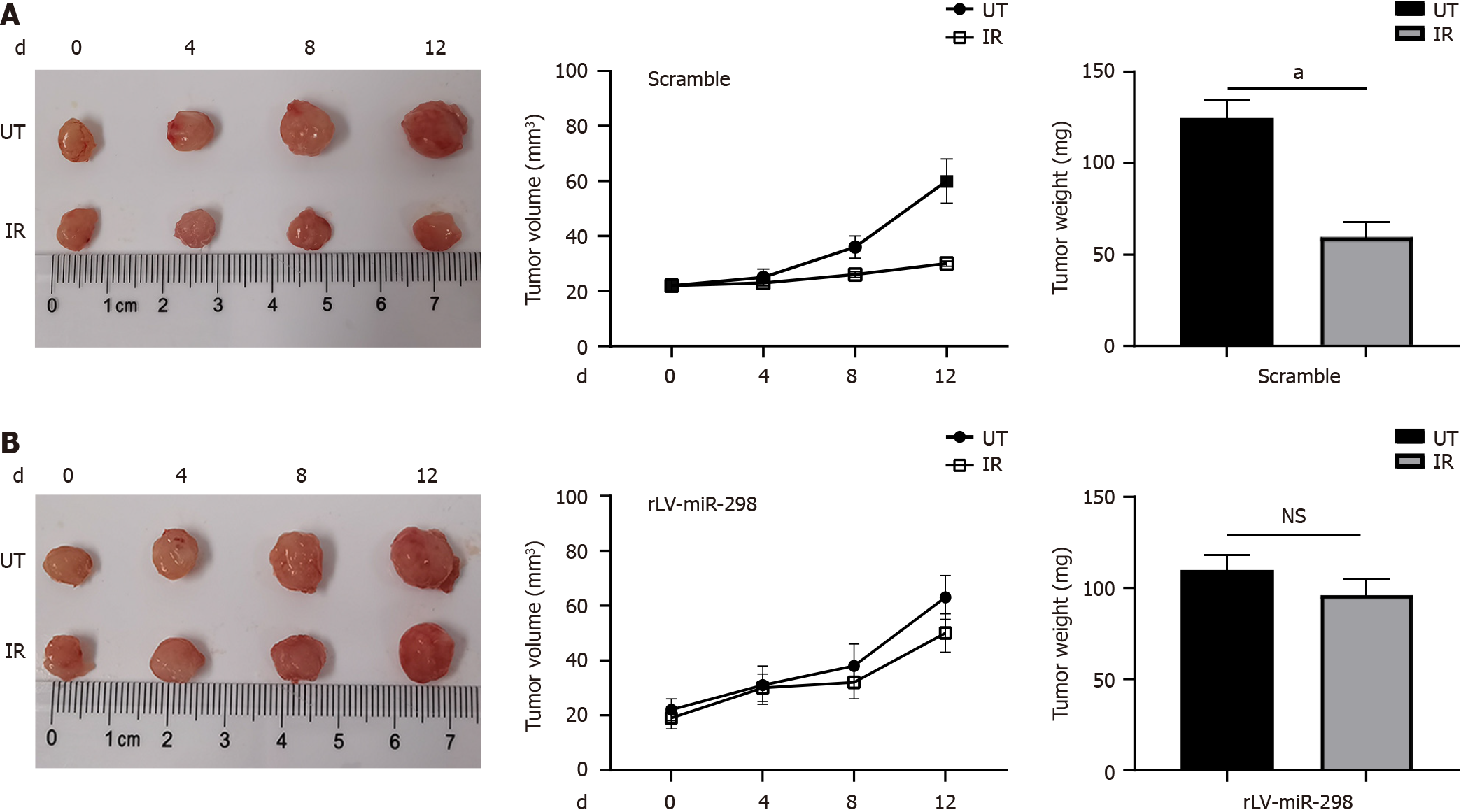
Figure 2 MicroRNA-298 overexpression decreases radio-sensitivity in the tumor of ectopic colorectal cancer model.
A and B: Mice were treated with ionizing radiation when tumor volume reached 20 mm3, and then tumor growth was monitored for 12 d. Data are mean ± SD values from 5 individual mice in each group. Tumor weights were measured after mice were sacrificed on day 20, representative tumors from corresponding treatments groups. The control group (A) was more sensitive to radiation than the microRNA-298 overexpression group (B). aP < 0.05; NS: Not significant. miR-298: MicroRNA-298; UT: Untreated; IR: Ionizing radiation.
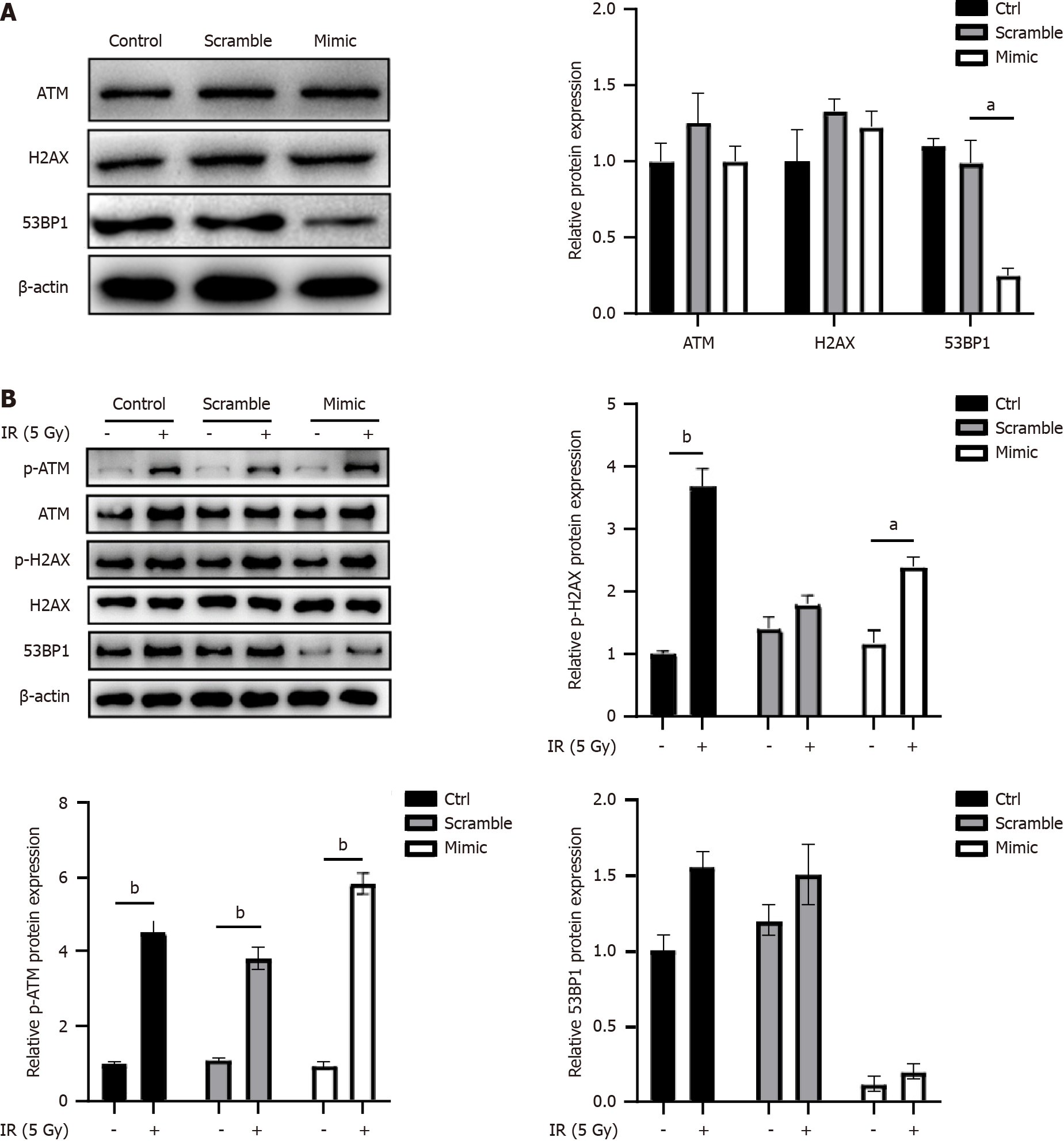
Figure 3 MicroRNA-298 overexpression decreases p53 binding protein 1 expression.
A: After scramble or microRNA-298 (miR-298) mimic transfection, HT-29 cells were detected by Western blotting for ATM, H2AX, and p53 binding protein 1 (53BP1) protein levels; B: HT-29 cells were subjected to radiation exposure after scamble or miR-298 mimic transfection. Western blotting was performed to detect the total and phosphorylated ATM, H2AX, and 53BP1 expression. aP < 0.05, bP < 0.01. miR-298: MicroRNA-298; IR: Ionizing radiation; ATM: Ataxia telangiectasia mutated; H2AX: H2A.X variant histone; 53BP1: p53 binding protein 1.
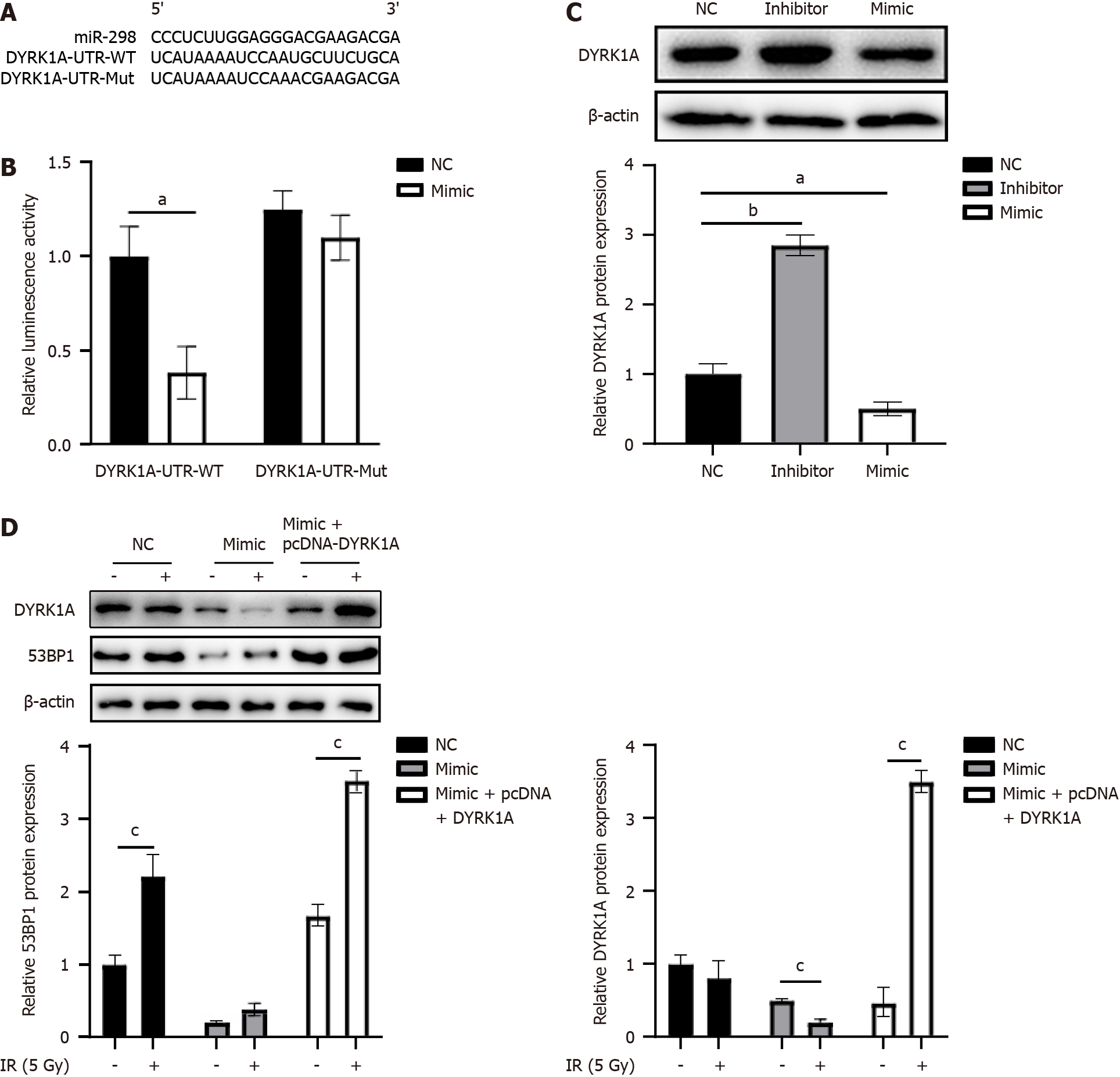
Figure 4 Human dual-specificity tyrosine(Y)-regulated kinase 1A is microRNA-298’s target.
A: A schematic diagram depicts the putative binding loci of microRNA-298 (miR-298) within the 3’ untranslated region (3’UTR) of the human dual-specificity tyrosine(Y)-regulated kinase 1A (DYRK1A) gene; B: A luciferase reporter assay was carried out to assess the direct association of miR-298 with DYRK1A in HT-29. These cells were co-transfected with the miR-298 mimic plus a luciferase reporter construct containing either the wild-type (WT) DYRK1A 3’UTR or a mutant (Mut) version that disrupted the putative miR-298 binding sites; C: Following negative control (NC), miR-298 inhibitor, or miR-298 mimic transfection, and Western blotting was carried out to determine DYRK1A protein expression in HT-29 cells; D: HT-29 cells were subjected to NC, or miR-298 mimic transfection, or miR-298 mimic plus pcDNA-DYRK1A vector co-transfection, followed by western blotting determination of DYRK1A and p53 binding protein 1 expressions. aP < 0.05, bP < 0.01. miR-298: MicroRNA-298; IR: Ionizing radiation; DYRK1A: Human dual-specificity tyrosine(Y)-regulated kinase 1A; NC: Negative control.
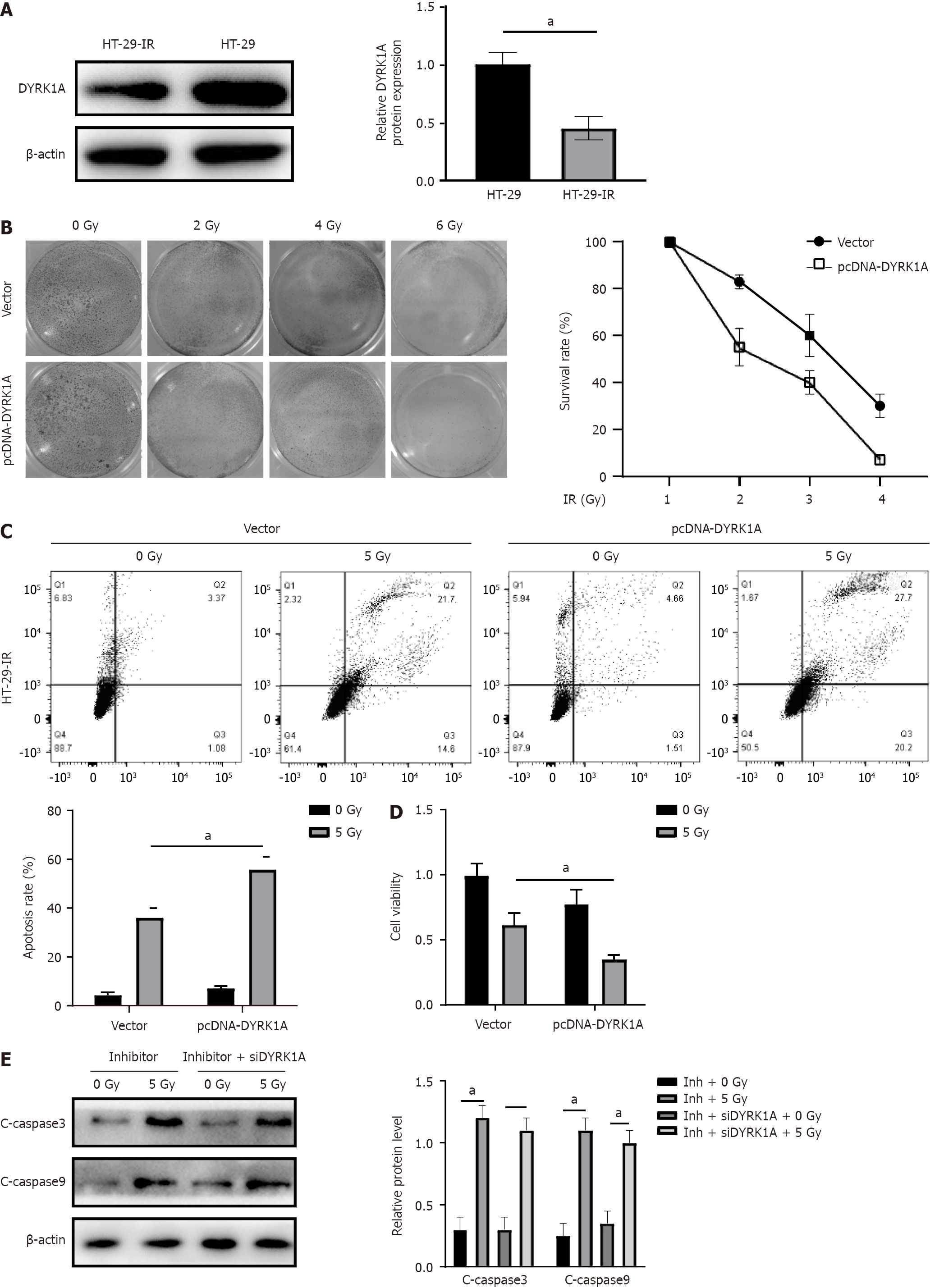
Figure 5 MicroRNA-298 knockdown regulates radio-induced apoptosis depended on human dual-specificity tyrosine(Y)-regulated kinase 1A expression.
A: Western blotting was carried out to examine human dual-specificity tyrosine(Y)-regulated kinase 1A (DYRK1A) levels in HT-29 and HT-29-IR cell lines; B: HT-29 cells were exposed to radiation following empty vector or pcDNA-DYRK1A vector transfection. Clonogenic assay was performed to assess cell survival; C: HT-29 cells were treated with radiation exposure after empty vector or pcDNA-DYRK1A vector transfection, followed by cell apoptosis examination by Annexin V/PI staining; D: HT-29 cells were treated with radiation exposure after empty vector or pcDNA-DYRK1A vector transfection, and cell viability was then determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; E: HT-29 cells were transfected with inhibitor, or co-transfected with inhibitor and si-DYRK1A, and then exposed to radiation. Western blotting was carried out to measure cleaved-caspase 3/9 protein levels. aP < 0.05.
- Citation: Shen MZ, Zhang Y, Wu F, Shen MZ, Liang JL, Zhang XL, Liu XJ, Li XS, Wang RS. MicroRNA-298 determines the radio-resistance of colorectal cancer cells by directly targeting human dual-specificity tyrosine(Y)-regulated kinase 1A. World J Gastrointest Oncol 2024; 16(4): 1453-1464
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1453.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1453