Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Dec 15, 2024; 16(12): 4565-4578
Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4565
Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4565
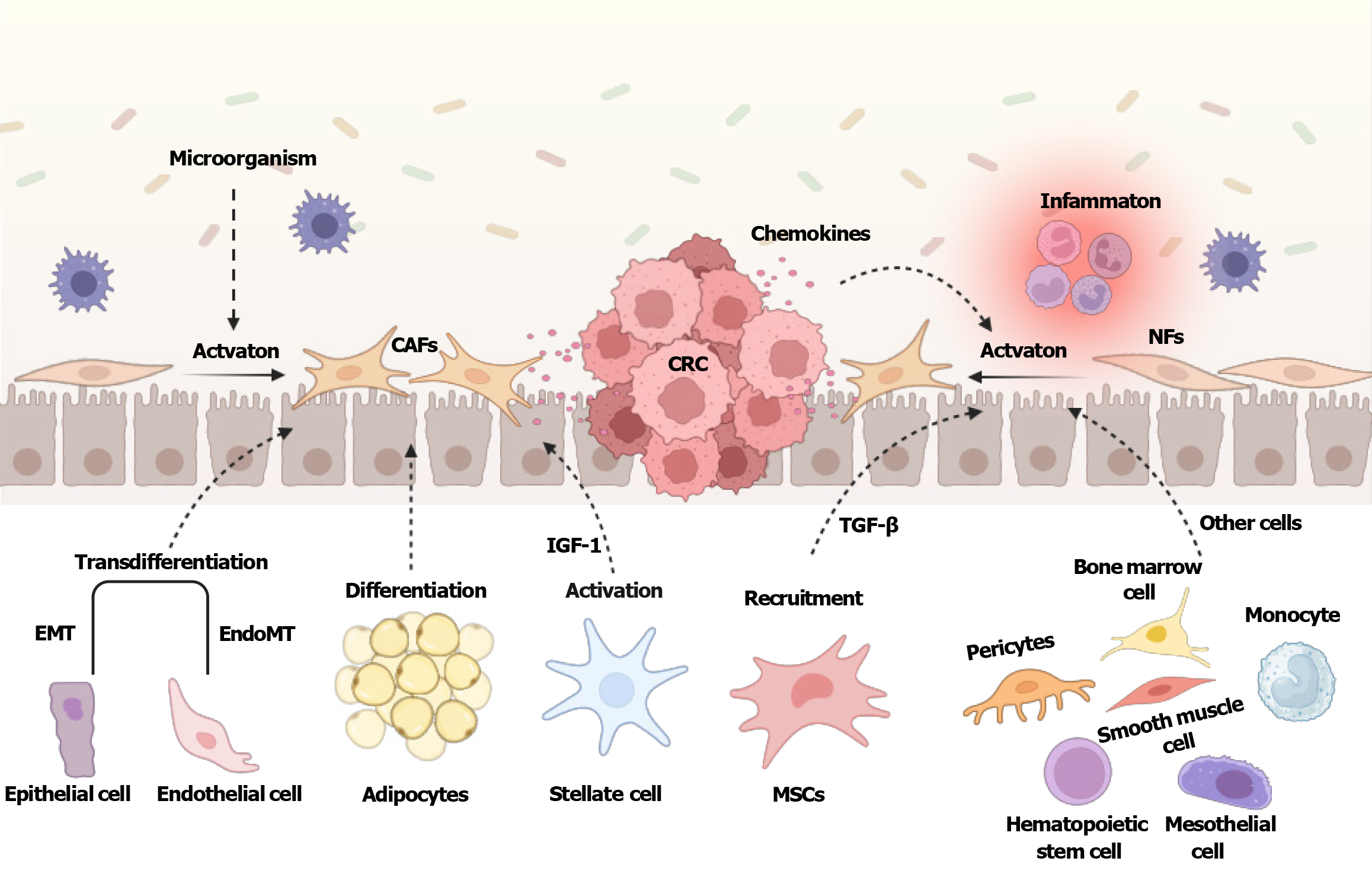
Figure 1 Origins of cancer-associated fibroblasts in colorectal cancer.
Normal fibroblasts can be activated by various factors including inflammation, microorganisms, and chemokines, and transform into cancer-associated fibroblasts. In addition, epithelial cells, endothelial cells, hepatic stellate cells, adipocytes, mesenchymal stem cells, pericytes, monocytes, mesothelial cells, hematopoietic stem cells, bone marrow cells, and smooth muscle cells, are all potential precursors of cancer-associated fibroblasts. Created with BioRender.com (Supplementary material). CAFs: Cancer-associated fibroblasts; CRC: Colorectal cancer; TGF-β: Transforming growth factor-β; NFs: Normal fibroblasts; EMT: Epithelial-to-mesenchymal transition; IGF-1: Insulin-like growth factor-1; MSCs: Mesenchymal stem cells.
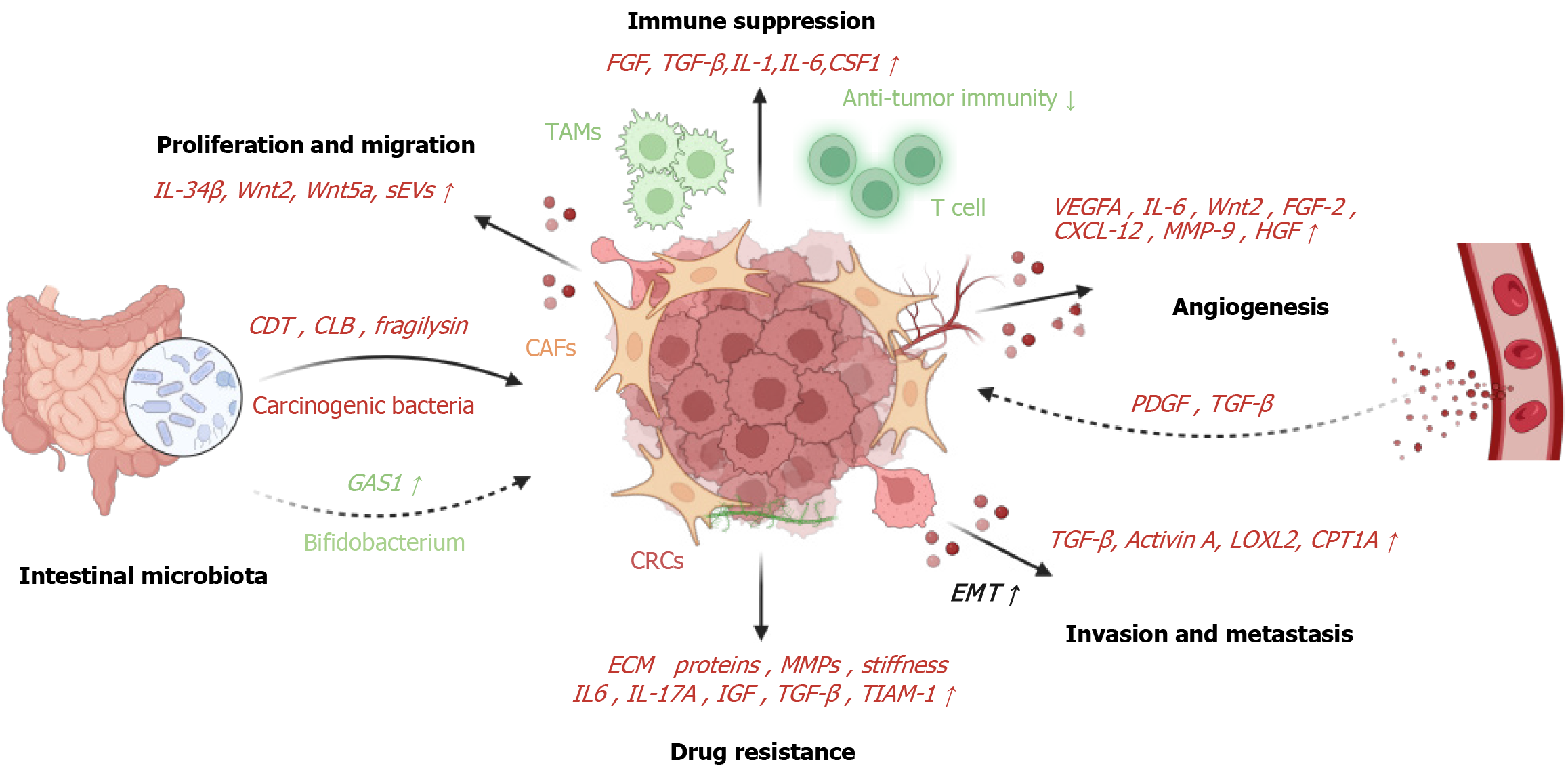
Figure 2 Roles of cancer-associated fibroblasts.
Cancer-associated fibroblasts (CAFs) secrete a variety of cytokines, including interleukin (IL)-34, Wnt2, Wnt5a, and small extracellular vesicles, which actively promote colorectal cancer proliferation and migration. Additionally, CAFs intricately manage tumor immune evasion through a range of complex mechanisms, including the secretion of diverse cytokines and chemokines such as fibroblast growth factor, transforming growth factor-β (TGF-β), IL-1, IL-6, and colony-stimulating factor 1. And CAFs contribute to tumor initiation by releasing pro-tumor factors that stimulate angiogenesis, including vascular endothelial growth factor A, IL-6, Wnt2, fibroblast growth factor 2, CXC motif chemokine ligand 12, matrix metalloproteinase-9, and hepatocyte growth factor. In turn, the leaky vasculature within tumors releases pro-angiogenic factors such as platelet-derived growth factor and TGF-β through degranulation, which further activates fibroblasts. The secretion of TGF-β, activin A, and lysyl oxidase-like 2 by CAFs, along with the upregulation of carnitine palmitoyl-transferase 1A expression, promotes epithelial-to-mesenchymal transition in colorectal cancer, thereby enhancing the invasion and metastasis of colorectal cancer. Moreover, CAFs produce ECM proteins and remodel the matrix via matrix metalloproteinases, creating a physical barrier and increasing matrix stiffness and interstitial pressure. And CAFs secrete key soluble factors, including IL-6, IL-17A, and insulin-like growth factor-1. “Carcinogenic bacteria” secrete bacterial toxins such as cytolethal distending toxin, colibactin, and fragilysin, influencing the tumor microenvironment to foster tumor progression and immune evasion. Meanwhile, Bifidobacterium exhibits anticancer effects by activating CD143+ CAFs. Created with BioRender.com (Supplementary material). IL: Interleukin; sEVs: Small extracellular vesicles; FGF: Fibroblast growth factor; TGF-β: Transforming growth factor-β; CSF1: Colony-stimulating factor 1; TAMs: Tumor-associated macrophages; VEGFA: vascular endothelial growth factor A; CXCL-12: CXC motif chemokine ligand 12; MMP: Matrix metalloproteinase; HGF: Hepatocyte growth factor; CDT: Cytolethal distending toxin; CLB: Colibactin; CAFs: Cancer-associated fibroblasts; PDGF: Platelet-derived growth factor; GAS1: Growth arrest specific 1; CRC: Colorectal cancer; IGF: Insulin-like growth factor; TIAM-1: T-lymphoma invasion and metastasis-inducing protein-1; EMT: Epithelial-to-mesenchymal transition; LOLX2: Lysyl oxidase-like 2; CPT1A: Carnitine palmitoyl-transferase 1A.
- Citation: Cui JY, Ma J, Gao XX, Sheng ZM, Pan ZX, Shi LH, Zhang BG. Unraveling the role of cancer-associated fibroblasts in colorectal cancer. World J Gastrointest Oncol 2024; 16(12): 4565-4578
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4565.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4565