Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Jan 15, 2024; 16(1): 234-243
Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.234
Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.234
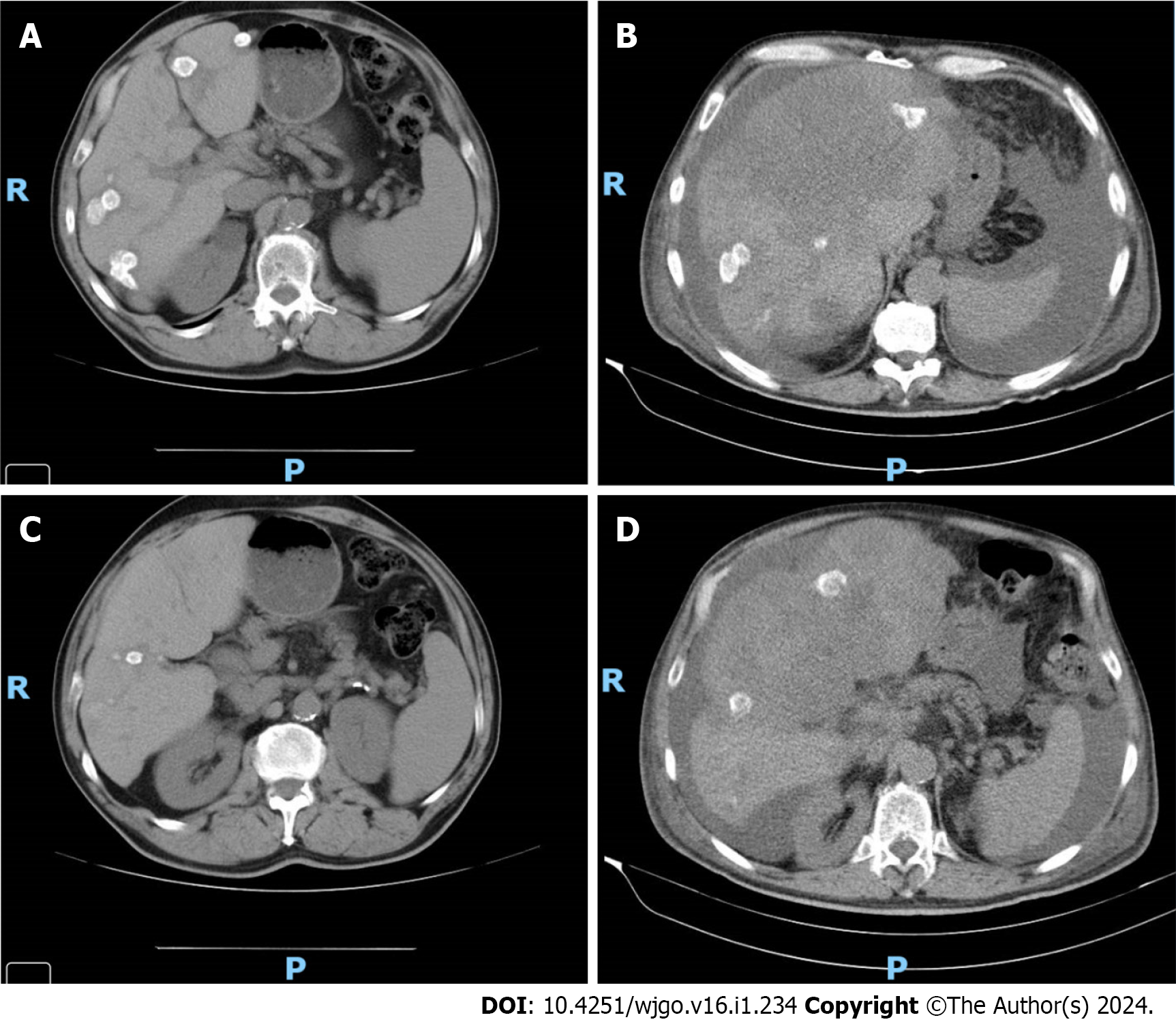
Figure 1 Imaging evaluation of Case 11.
A: Imaging evaluation at baseline (July 2021), upper axial plane, [flat chemotherapy (CT) scan with IV contrast]; B: Imaging evaluation at 3 mo (October 2021, on the right) upper axial plane, (flat CT scan with IV contrast); C: Imaging evaluation at baseline (July 2021), lower axial plane, (flat CT scan with IV contrast); D: Imaging evaluation at 3 mo (October 2021), lower axial plane, (flat CT scan with IV contrast).
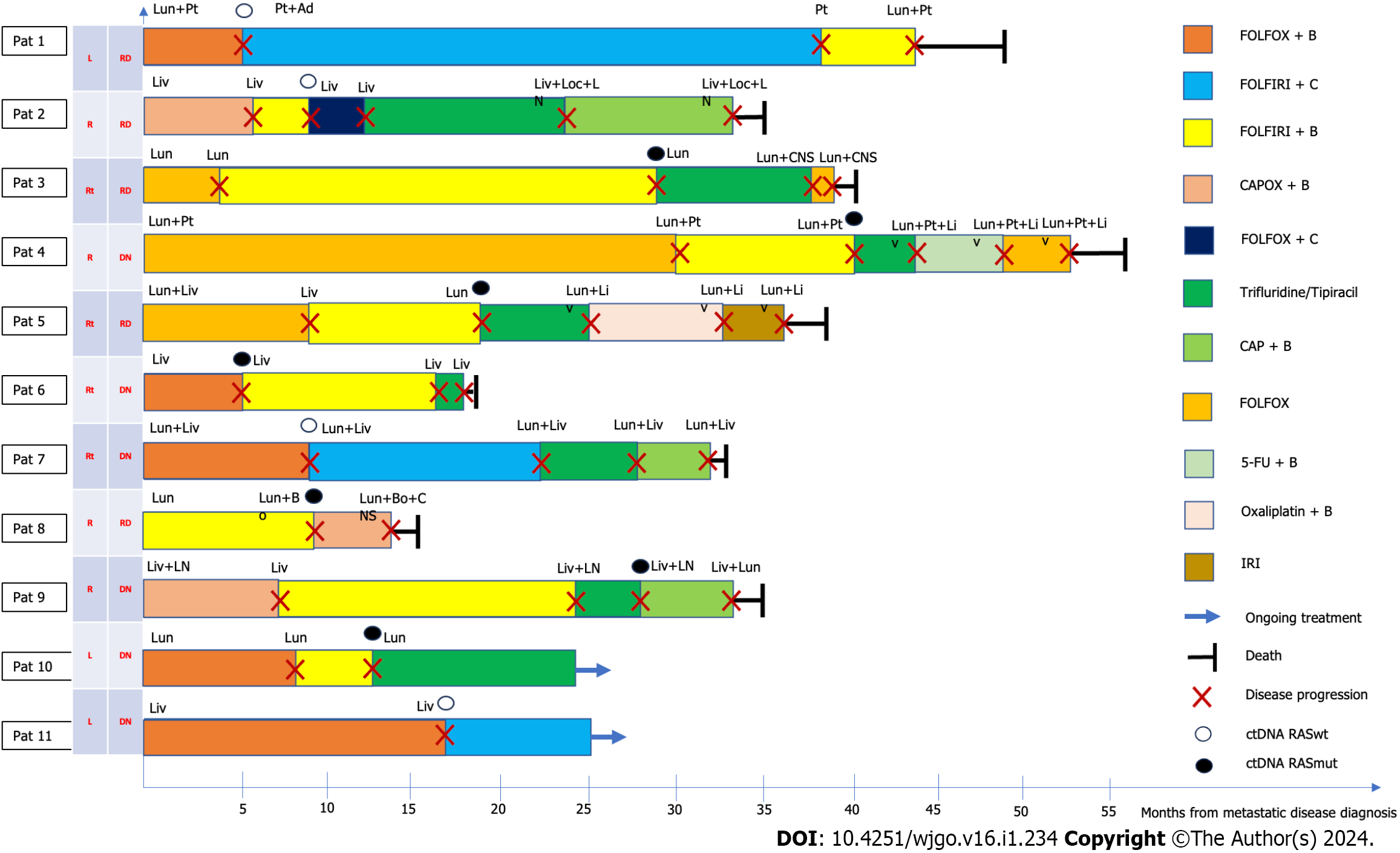
Figure 2 Disease progression after second-line treatment.
5-FU: 5-fluorouracil; Ad: Adrenal; B: Bevacizumab; Bo: Bone; C: Cetuximab; CAP: Capecitabine; CAPOX: Capecitabine + oxaliplatin; CNS: Central nervous system; ctDNA: Circulating DNA; DN: De novo disease; FOLFIRI: Folinic acid + fluorouracil + irinotecan; FOLFOX: Folinic acid + fluorouracil, and oxaliplatin; IRI: Irinotecan; Liv: Liver; Loc: Local; LN: Lymph node; Lun: Lung; Oxali: Oxaliplatin; Pat: Patient; Pt: Peritoneal; RASmut: RAS mutated; RASwt: RAS wild-type; RD: Relapsing disease; Rt: Rectum.
- Citation: Gramaça J, Fernandes IG, Trabulo C, Gonçalves J, dos Santos RG, Baptista A, Pina I. Emerging role of liquid biopsy in rat sarcoma virus mutated metastatic colorectal cancer: A case report. World J Gastrointest Oncol 2024; 16(1): 234-243
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/234.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.234