Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Oct 15, 2023; 15(10): 1771-1783
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1771
Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1771
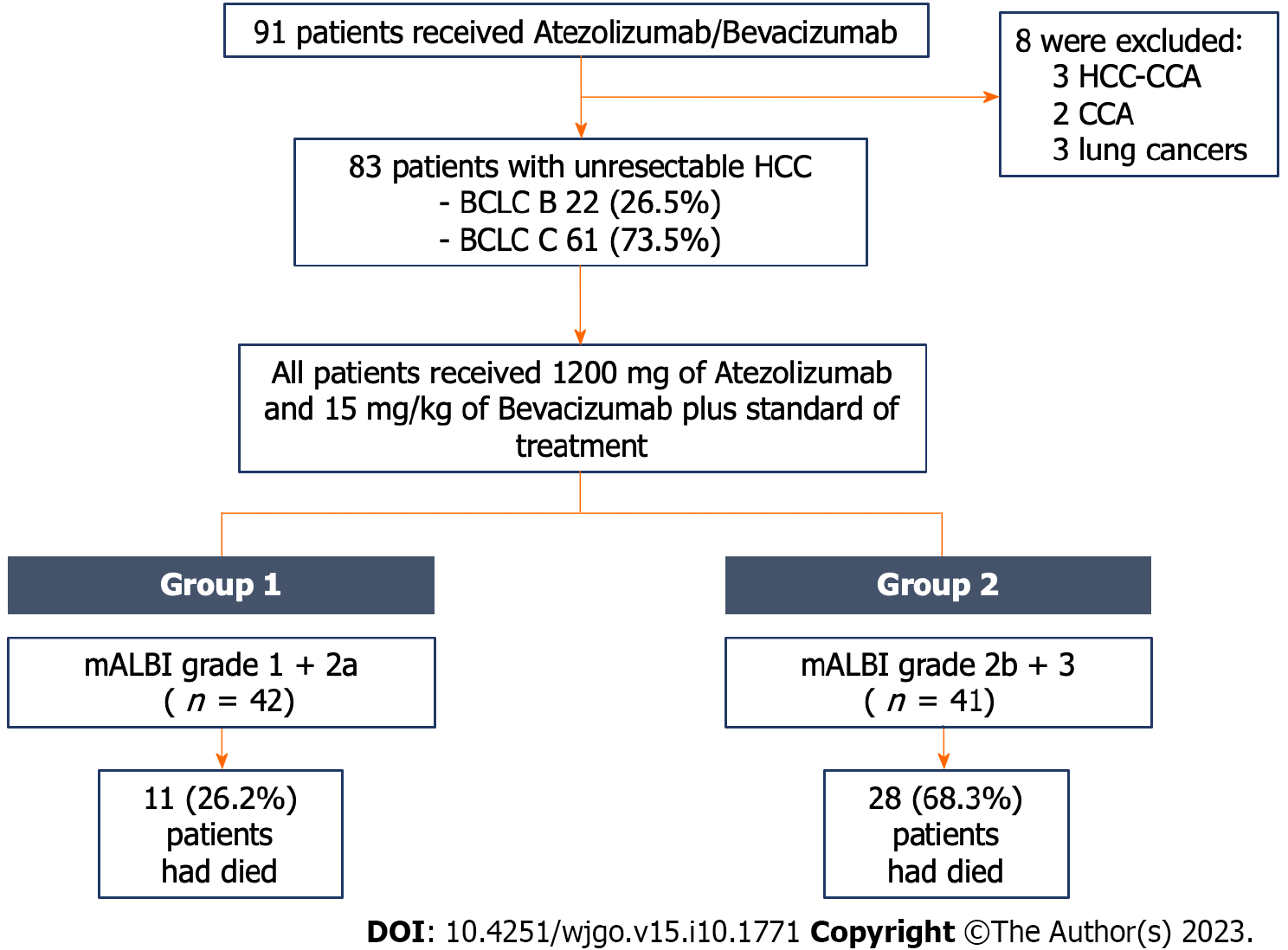
Figure 1 Flow diagram of patient enrollment.
BCLC: Barcelona Clinic Liver Cancer; CCA: Cholangiocarcinoma; HCC: Hepatocellular carcinoma; mALBI: Modified albumin-bilirubin.
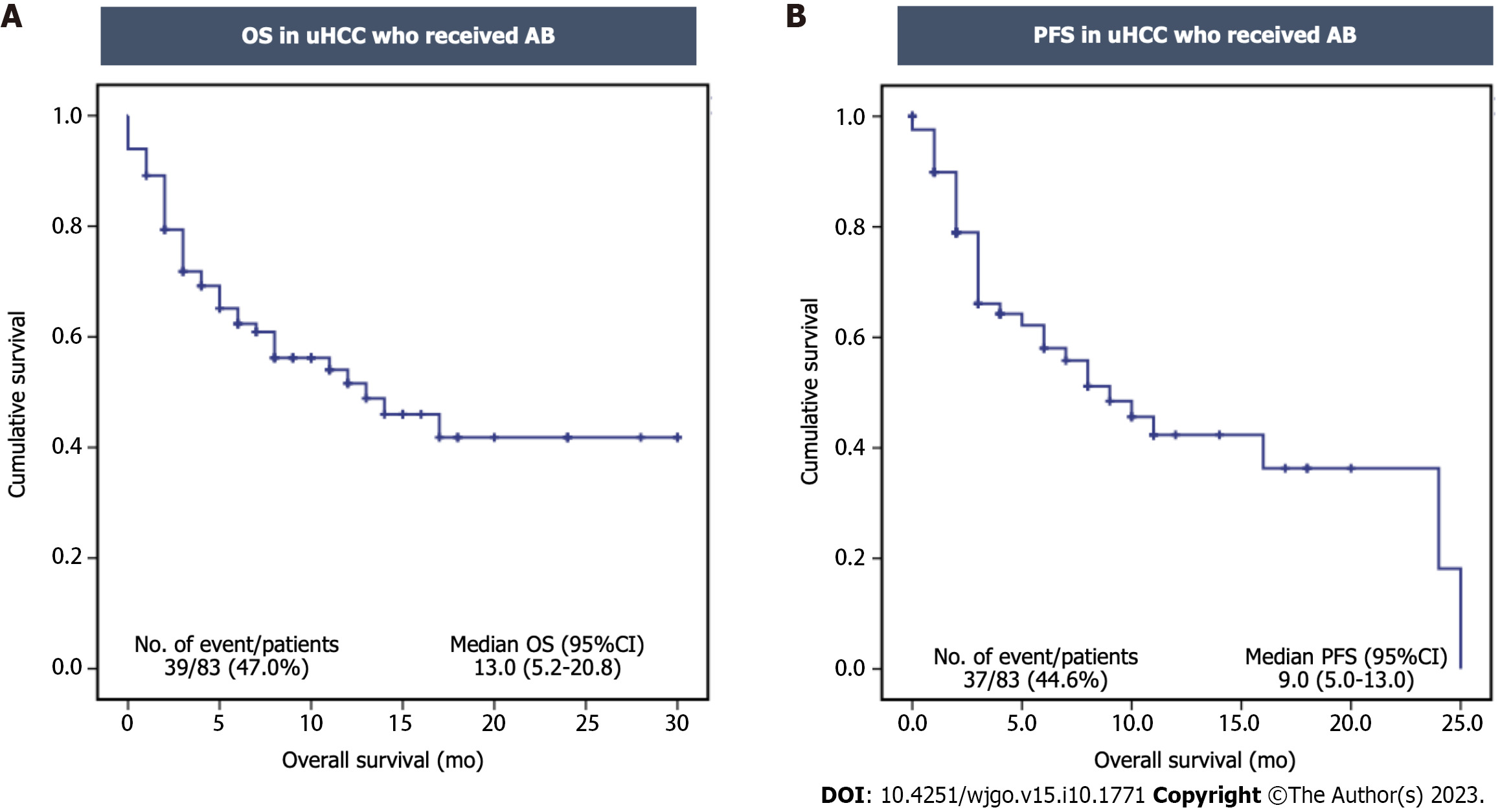
Figure 2 Kaplan–Meier analysis of overall survival and progression free survival of the unresectable hepatocellular carcinoma patients who received atezolizumab plus bevacizumab therapy.
A: Overall survival (OS) in unresectable hepatocellular carcinoma (uHCC) patients who received atezolizumab plus bevacizumab (AB); B: Progression-free survival (PFS) in uHCC patients who received AB. CI: Confidence interval.
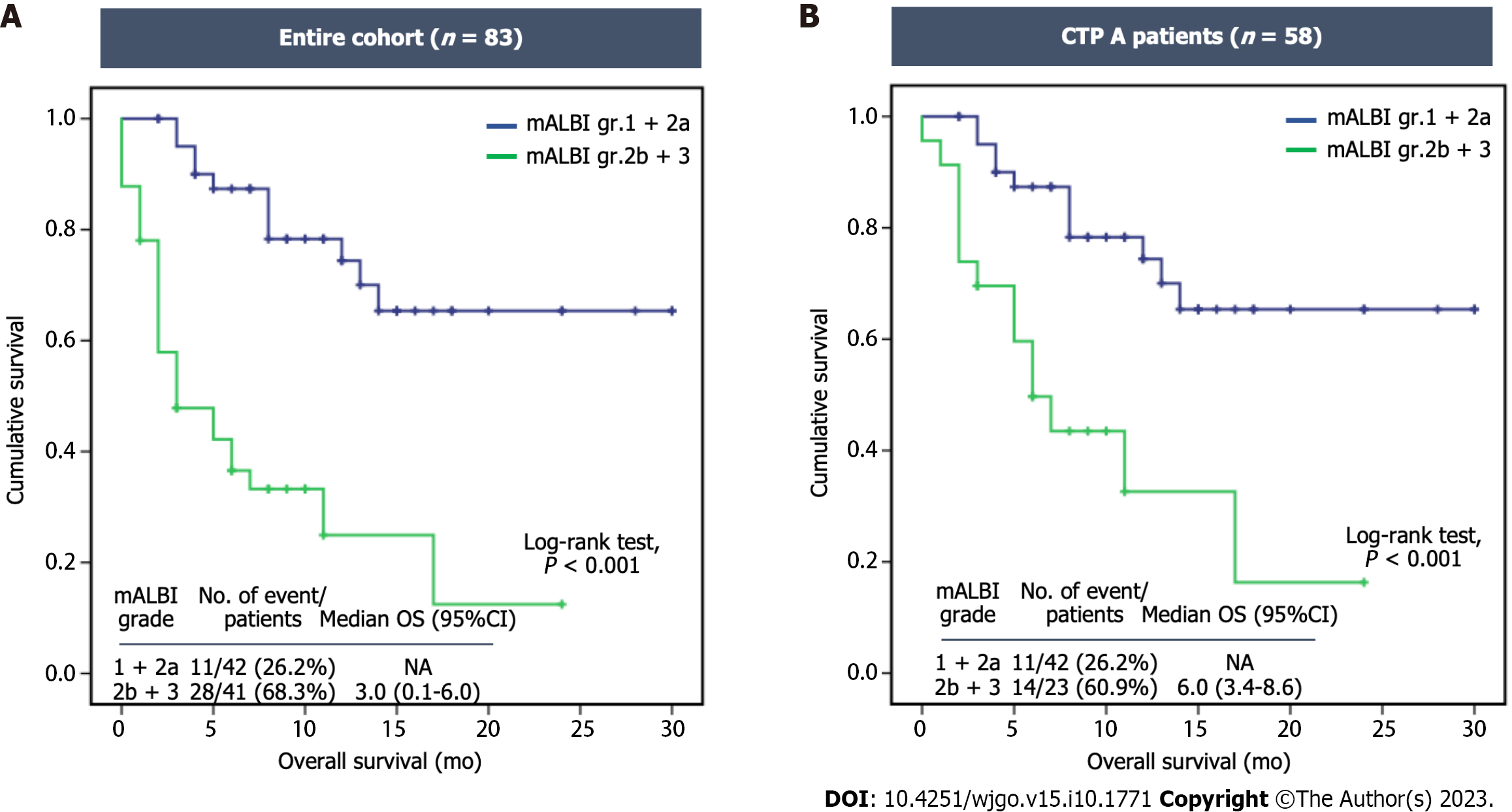
Figure 3 Kaplan-Meier curves for overall survival in patients undergoing atezolizumab plus bevacizumab therapy stratified by modified albumin-bilirubin grade.
A: Entire cohort; B: Child-Turcotte-Pugh (CTP) class A. CI: Confidence interval; gr: Group; mALBI: Modified albumin-bilirubin; NA: Not available; OS: Overall survival.
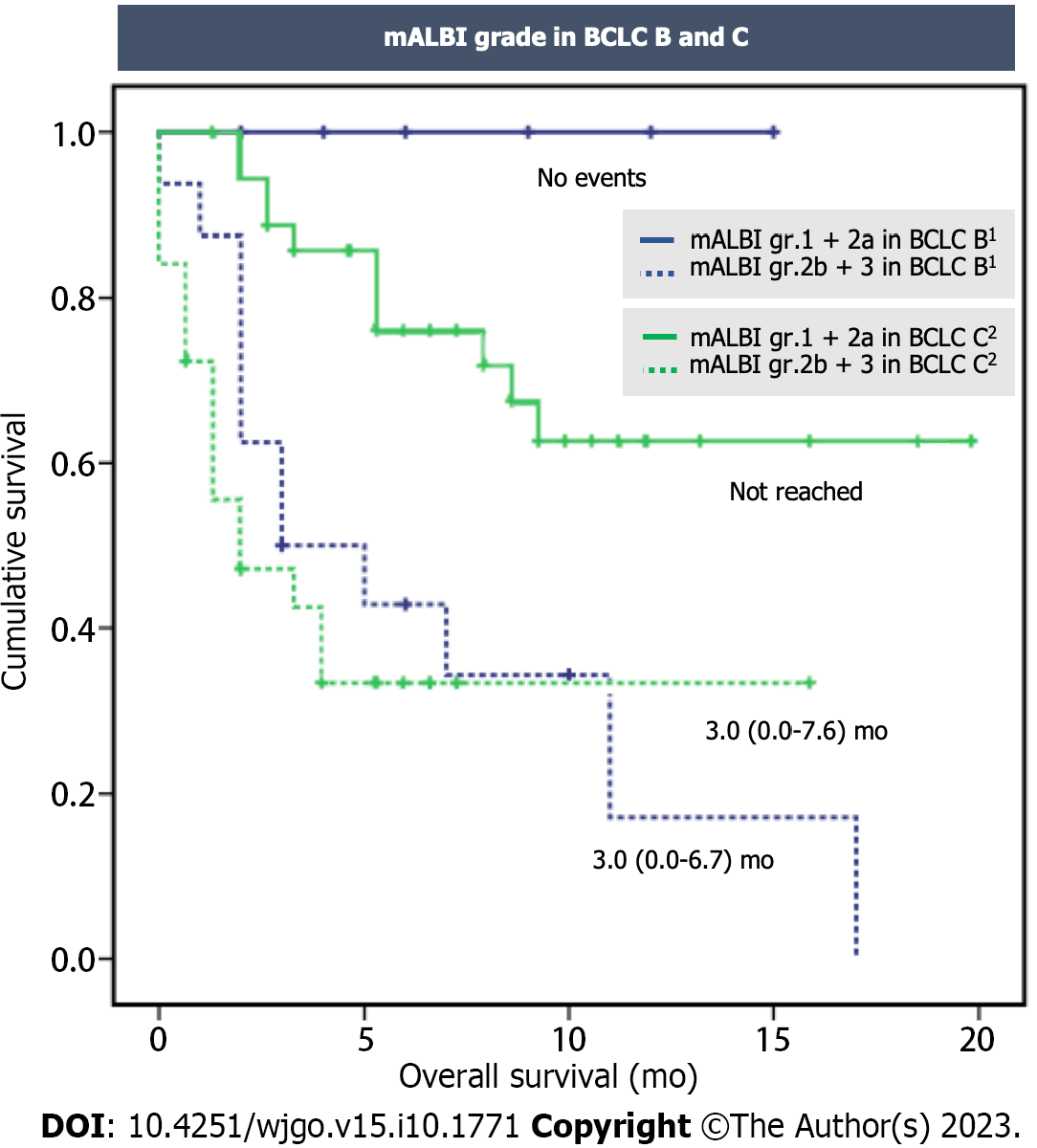
Figure 4 Kaplan-Meier curves for overall survival in patients undergoing atezolizumab plus bevacizumab therapy stratified by modified albumin-bilirubin grade in Barcelona Clinic Liver Cancer B and C.
1P = 0.014; 2P < 0.001. BCLC: Barcelona Clinic Liver Cancer; gr: Group; mALBI: Modified albumin-bilirubin.
- Citation: Navadurong H, Prasoppokakorn T, Siriwong N, Phathong C, Teeyapun N, Tanasanvimon S, Thanapirom K, Komolmit P, Tangkijvanich P, Treeprasertsuk S, Chaiteerakij R. Modified albumin-bilirubin predicted survival of unresectable hepatocellular carcinoma patients treated with immunotherapy. World J Gastrointest Oncol 2023; 15(10): 1771-1783
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1771.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1771