Copyright
©The Author(s) 2021.
World J Gastrointest Oncol. May 15, 2021; 13(5): 312-331
Published online May 15, 2021. doi: 10.4251/wjgo.v13.i5.312
Published online May 15, 2021. doi: 10.4251/wjgo.v13.i5.312
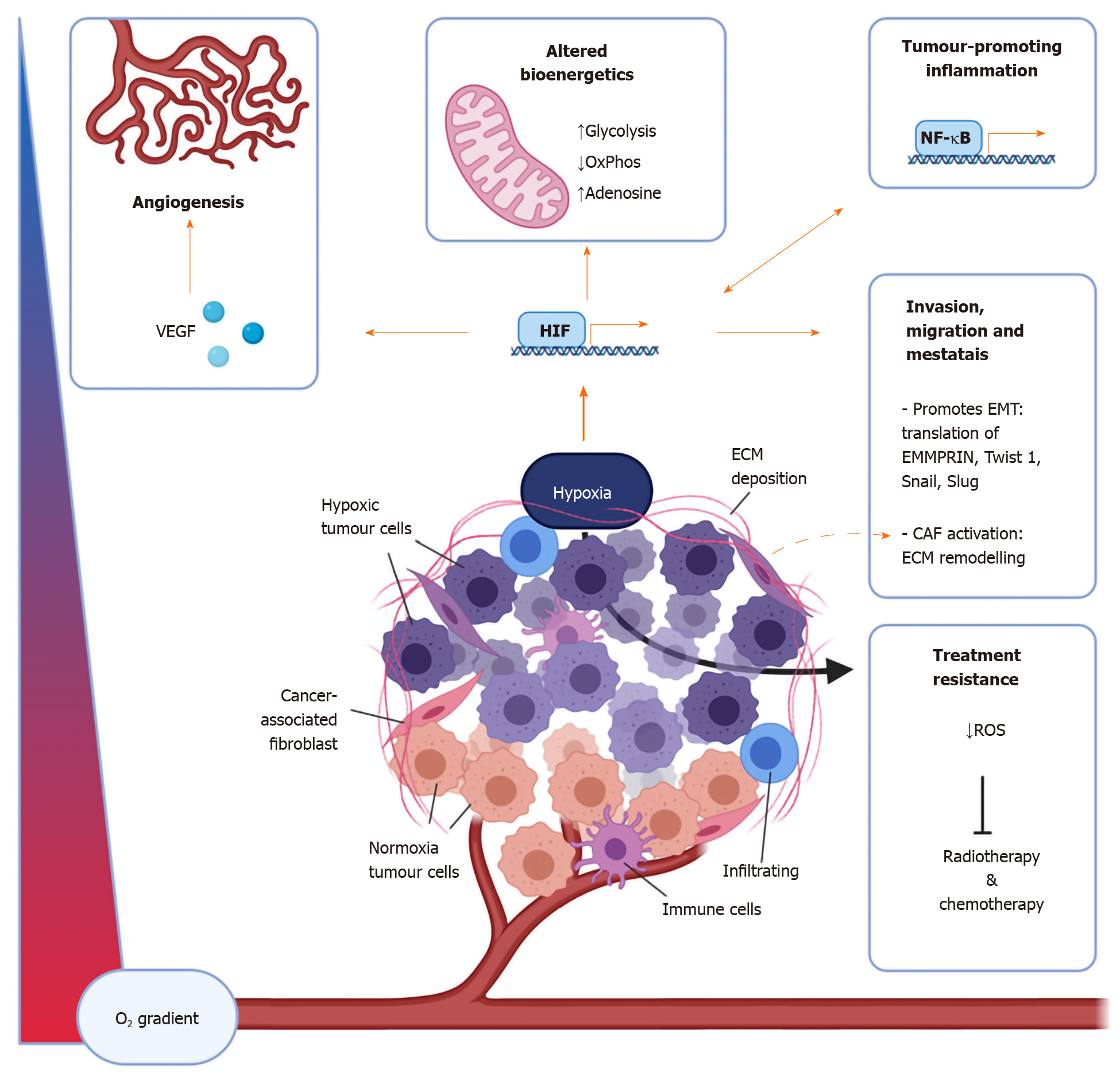
Figure 1 The components of the tumour microenvironment are affected by hypoxia in numerous ways.
Important cellular components of the tumour microenvironment include immune cells including macrophages, dendritic cells, myeloid-derived suppressor cells, T cells, natural killer cells, as well as cancer-associated fibroblasts. Non-cellular aspects include the extracellular matrix and signalling molecules such as vascular endothelial growth factor, adenosine, and cytokines and chemokines including interleukin-6, interferon-γ, CXCL1, CXCL3, CCL28[12-14,40]. CAF: Cancer associated fibroblasts; OxPhos: Oxidative phosphorylation; ROS: Reactive oxygen species; VEGF: Vascular endothelial growth factor; NFκB: Nuclear factor-kappa light chain enhancer of activated B cells; HIF: Hypoxia inducible factor; ECM: Extracellular matrix; EMT: Epithelial-mesenchymal transition.
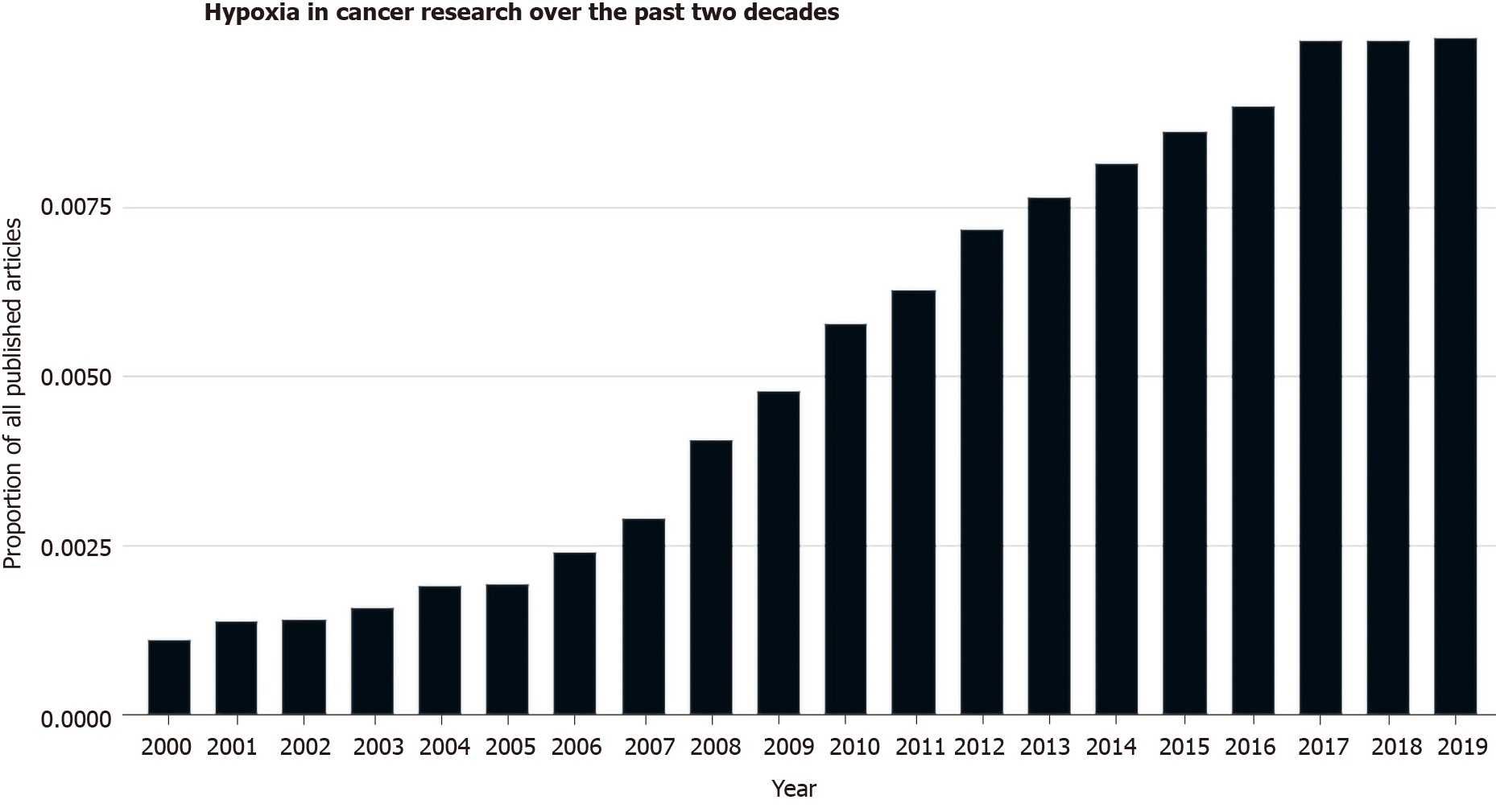
Figure 2
The amount of research investigating the role of hypoxia in cancer has increased over the past 20 yr as seen as a proportion of PubMed listed articles[177].
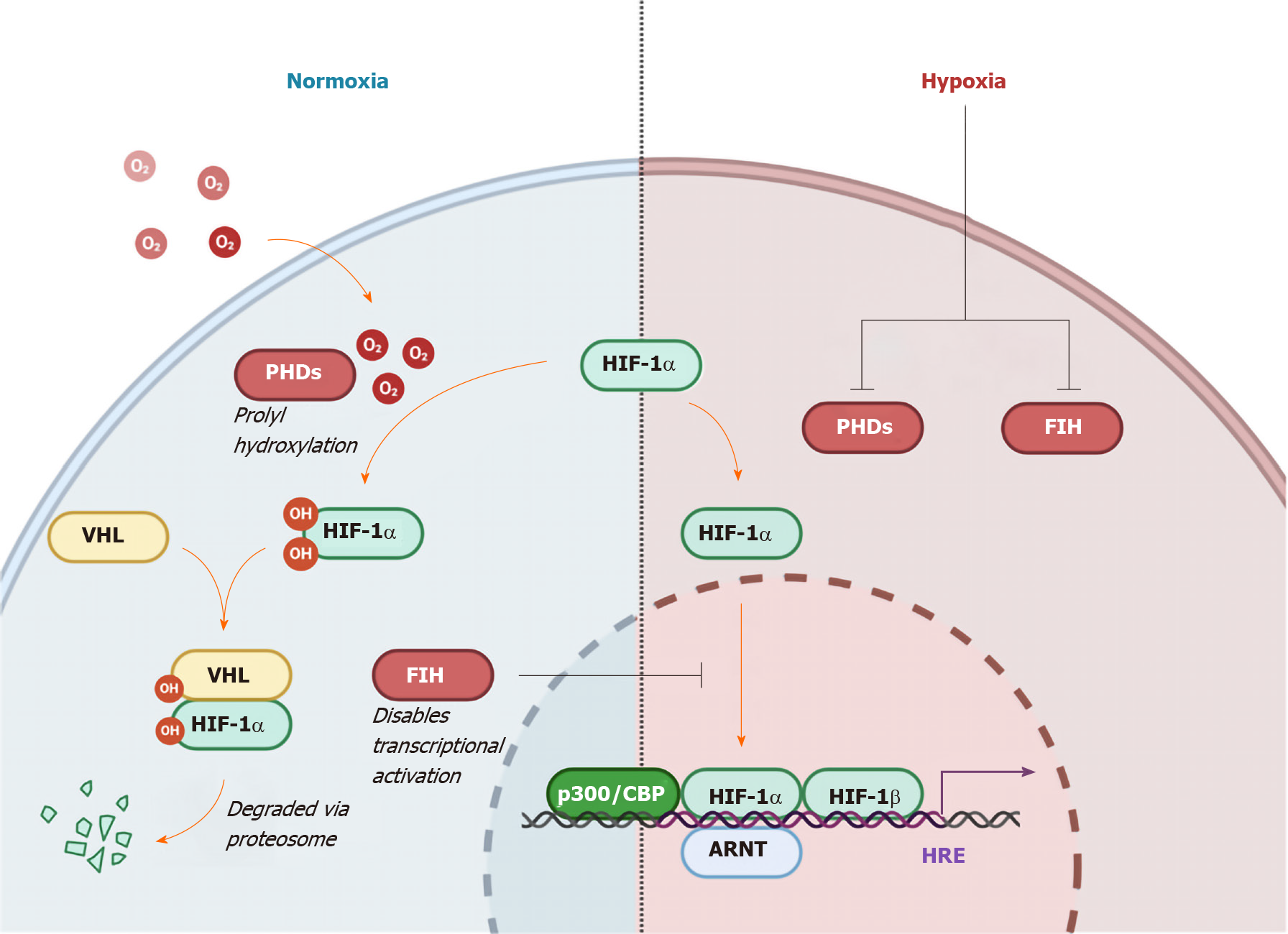
Figure 3 Regulation of hypoxia-inducible factor 1-α by oxygen levels and von Hippel Lindau protein.
Hydroxylation by oxygen-dependent prolyl hydroxylase domain enzymes triggers recognition by the E3 ubiquitin ligase von Hippel Lindau, ensuring proteasomal degradation. In the non-von Hippel Lindau protein dependent pathway, induction of Factor Inhibiting hypoxia-inducible factor (HIF) leads to hydroxylation of an asparagine residue preventing HIF1-α from localizing with the co-activators p300 and CBP, hence disabling transcriptional activation[30]. The HIF pathway functions to conduct and orchestrate the cellular response to low oxygen availability[24,25]. HRE: Hypoxia response element; ARNT: Aryl hydrocarbon receptor nuclear translocator; PHD: Prolyl hydroxylase domain enzymes; VHL: Von Hippel Lindau; HIF1-α: Hypoxia-inducible factor 1-α; FIH: Factor inhibiting hypoxia-inducible factor.
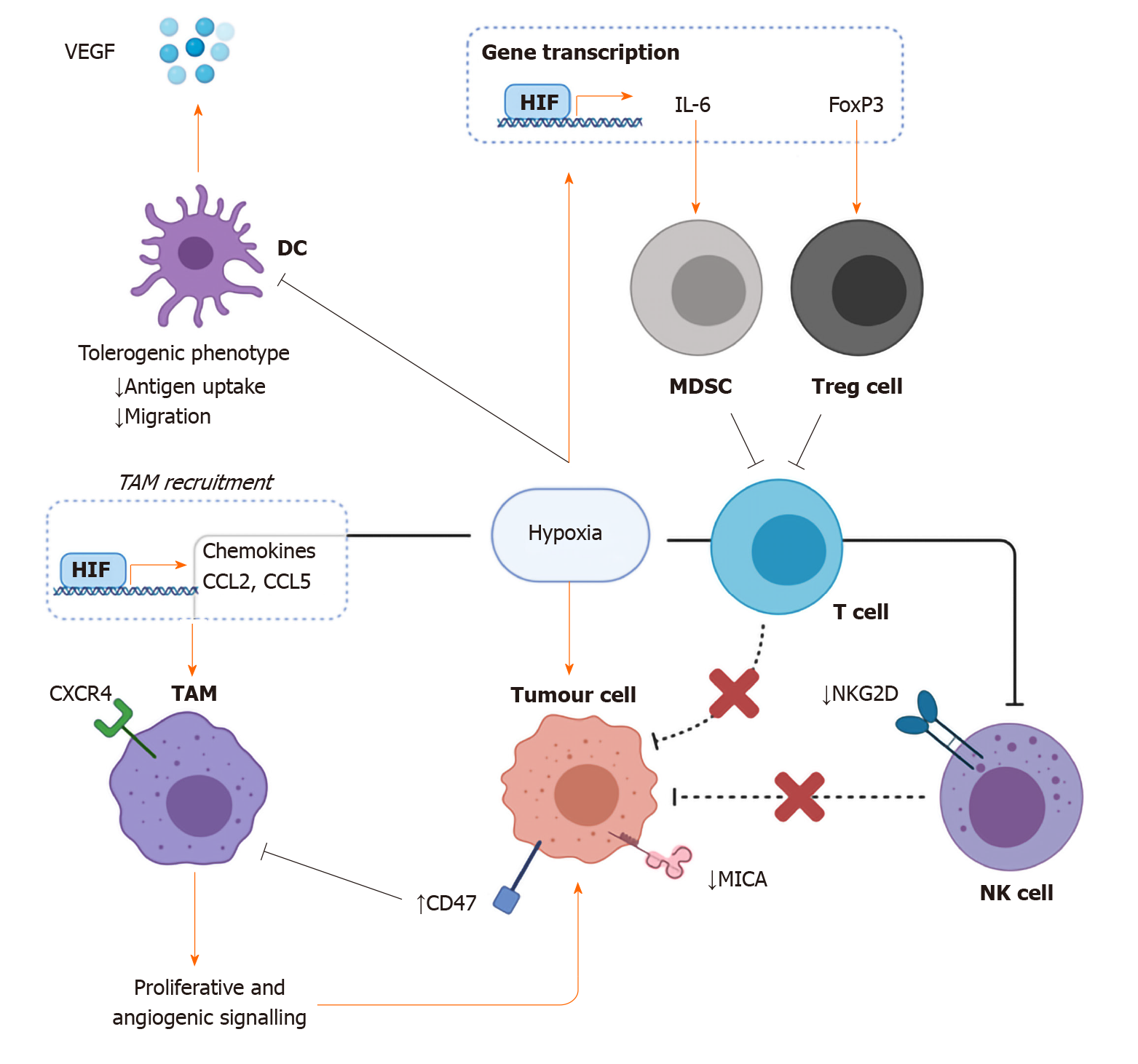
Figure 4 The effects of hypoxia on immune evasion.
Hypoxia has been shown to impair antigen uptake and migration in dendritic cells while at the same time increasing vascular endothelial growth factor production thus impairing the bridge between the innate anticancer immune response and the adaptive response while also enhancing angiogenic signalling. Hypoxia-inducible factor-mediated transcription of the cytokine interleukin-6 and FoxP3 results in the subsequent recruitment of immunosuppressive myeloid derived suppressor cells and in increased proportion of protumourigenic Tregs respectively. Low oxygen status is also linked with decreased tumour expression of the natural killer (NK) cell receptor ligand MHC class I chain-related molecule A, as well as its receptor NKG2D on NK cells. Hypoxia-dependent transcription of chemokines such as CCL2 and CCL5 enhance the recruitment of tumour associated macrophages through receptors such as CXCR4. DC: Dendritic cell; MDSC: Myeloid derived suppressor cell; NK cell: Natural killer cell; TAM: Tumour associated macrophage; Treg cell: T regulatory cell; VEGF: Vascular endothelial growth factor; HIF: Hypoxia inducible factor; IL: Interleukin; MICA: MHC class I chain-related molecule A.
- Citation: King R, Hayes C, Donohoe CL, Dunne MR, Davern M, Donlon NE. Hypoxia and its impact on the tumour microenvironment of gastroesophageal cancers. World J Gastrointest Oncol 2021; 13(5): 312-331
- URL: https://www.wjgnet.com/1948-5204/full/v13/i5/312.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i5.312