Copyright
©The Author(s) 2021.
World J Gastrointest Oncol. Nov 15, 2021; 13(11): 1725-1740
Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1725
Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1725
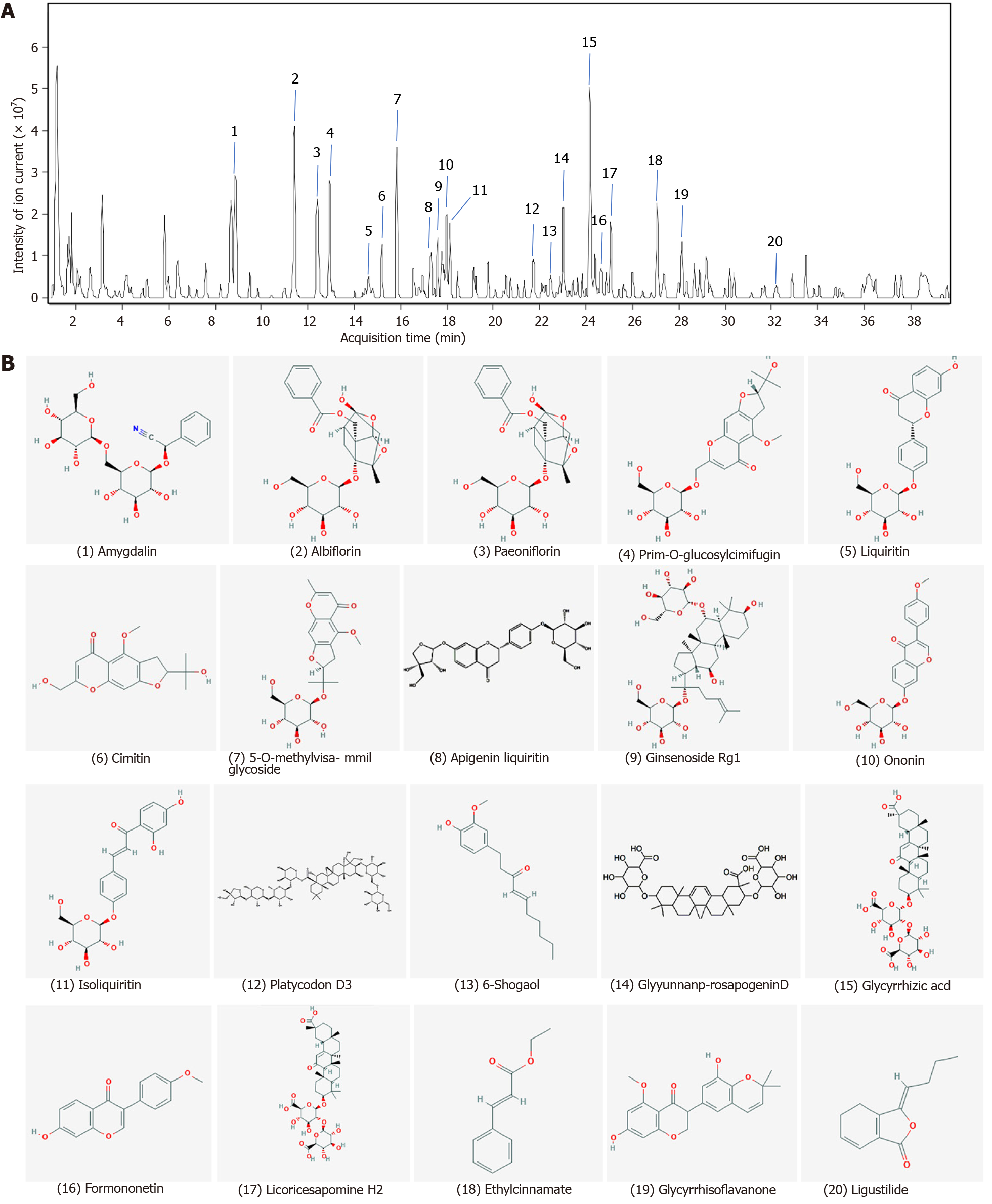
Figure 1 Finger-print of Shuyu pills.
The finger-print of Shuyu pills was determined by high resolution mass spectrometry. A: The 20 pharmaceutical ingredients were labeled according to the chromatographic retention time and their structures were analyzed by mass spectrometry; B: Chemical structure formulae of 20 compounds. Chemical structures and formulae of 20 compounds from PubChem (https://pubchem.ncbi.nlm.nih.gov/), numbered according to the compounds information in Table 1.
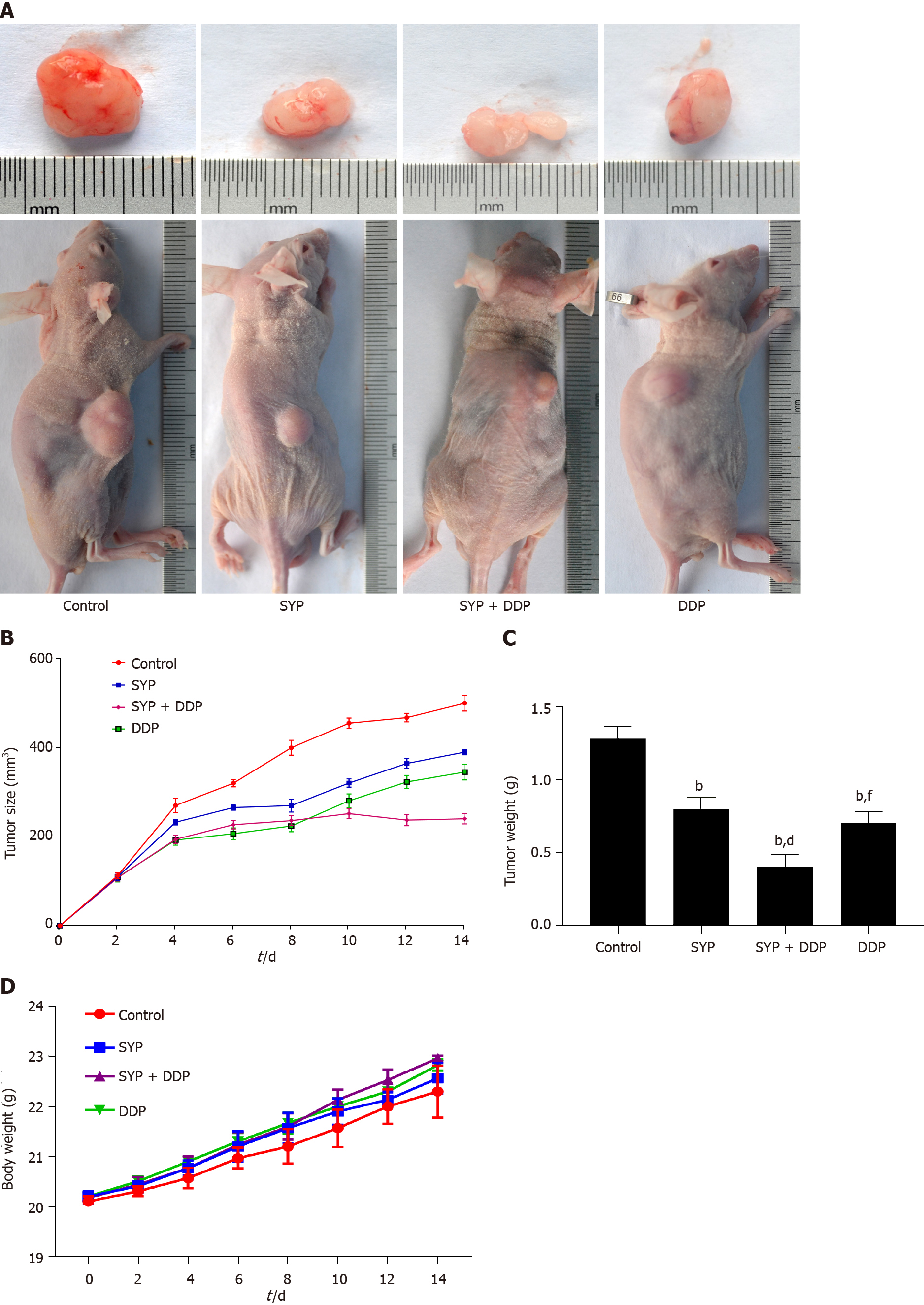
Figure 2 Shuyu pills inhibited the growth of hepatocellular carcinoma in vivo.
The xenograft mouse model was established in BALB/c nude mice that were then randomly divided into four groups (n = 6): Control group (0.9% normal saline, daily), Shuyu pills (SYP) (200 mg/kg, daily), Cisplatin (DDP) (5 mg/kg, once a week), and SYP (200 mg/kg, daily) + DDP (5 mg/kg, once a week). The body weight of each mouse and the tumor volume were measured every 2 d, with the latter calculated as follows: Maximum tumor length × width (2 × 0.5). A: Representative images of the tumors at the end of treatment; B: Average tumor volumes, measured every 2 d; C: Tumor weights at the end of treatment; D: Average body weights of the mice, measured every 2 d. bP < 0.01 vs Control group; dP < 0.01 vs SYP group;
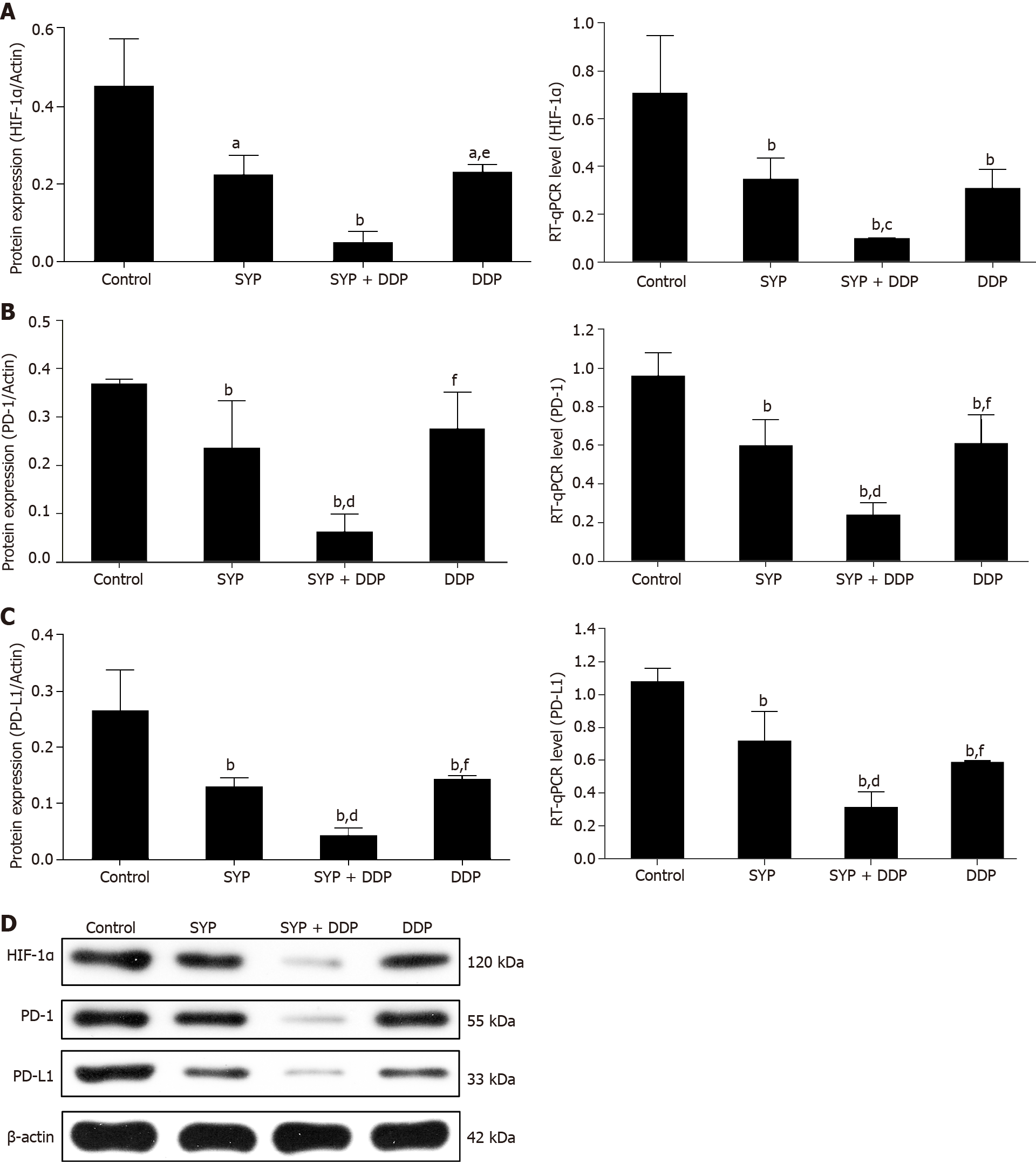
Figure 3 Shuyu pills inhibited the expression of hypoxia-inducible factor-1 alpha, programmed cell death 1, and programmed cell death 1 ligand 1.
Nude mice injected subcutaneously with human hepatocellular carcinoma cells were treated with Shuyu pills (SYP), Cisplatin (DDP), or a combination of the two for 14 d, following which the tumor tissues were harvested as indicated. A–C: Western blot and quantitative reverse transcription polymerase chain reaction assays were used to respectively detect the protein and mRNA expression levels of hypoxia-inducible factor-1 alpha (HIF-1α), programmed cell death protein 1 (PD-1), and programmed cell death 1 ligand 1 (PD-L1) in the tumor tissue; D: Representative protein expression patterns of HIF-1α, PD-1, and PD-L1 as measured by western blot assay. Data are presented as the mean ± standard error of the mean, and comparisons between two groups were performed using the least significant difference test or Dunnett’s T3 method. aP < 0.05 and bP < 0.01 vs Control group; cP < 0.05 and dP < 0.01 vs SYP group; eP < 0.05 and fP < 0.01 vs SYP + DDP group. SYP: Shuyu pills; DDP: Cisplatin; HIF-1α: Hypoxia-inducible factor-1 alpha; PD-1: Programmed cell death 1; PD-L1: Programmed cell death 1 ligand 1.
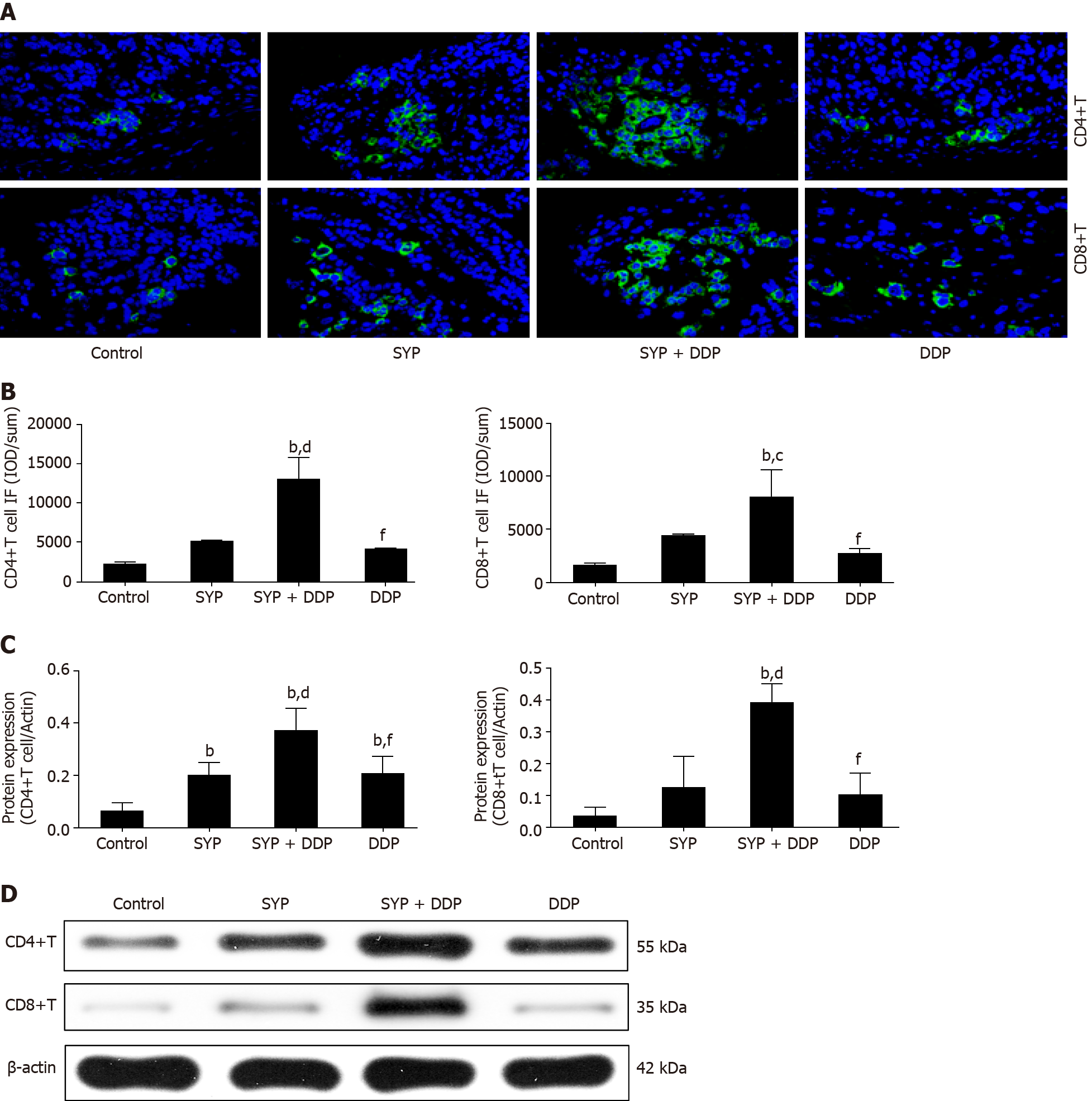
Figure 4 Effects of Shuyu pills on the expression of CD4+ T cells and CD8+ T cells in subcutaneous hepatocellular carcinoma xenografts.
Nude mice injected subcutaneously with human hepatocellular carcinoma cells were treated with Shuyu pills (SYP), Cisplatin (DDP), or SYP + DDP for 14 d. The tumor tissues were then collected and their expression of CD4+ T cells and CD8+ T cells was measured using the immunofluorescence assay. A: Immunofluorescence images of CD4+ T cells and CD8+ T cells; B: Quantitative analysis of the CD4+ T-cell and CD8+ T-cell immunofluorescence intensities; C and D: Protein expression levels of CD4+ T cells and CD8+ T cells as measured by western blot assay. Data are presented as the mean ± standard error of the mean, and comparisons between two groups were performed using the least significant difference test or Dunnett’s T3 method. bP < 0.01 vs Control group; cP < 0.05 and dP < 0.01 vs SYP group; fP < 0.01 vs SYP + DDP group. SYP: Shuyu pills; DDP: Cisplatin.
- Citation: Deng Z, Teng YJ, Zhou Q, Ouyang ZG, Hu YX, Long HP, Hu MJ, Mei S, Lin FX, Dai XJ, Zhang BY, Feng T, Tian XF. Shuyu pills inhibit immune escape and enhance chemosensitization in hepatocellular carcinoma. World J Gastrointest Oncol 2021; 13(11): 1725-1740
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1725.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1725