Copyright
©The Author(s) 2021.
World J Gastrointest Oncol. Nov 15, 2021; 13(11): 1561-1598
Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1561
Published online Nov 15, 2021. doi: 10.4251/wjgo.v13.i11.1561
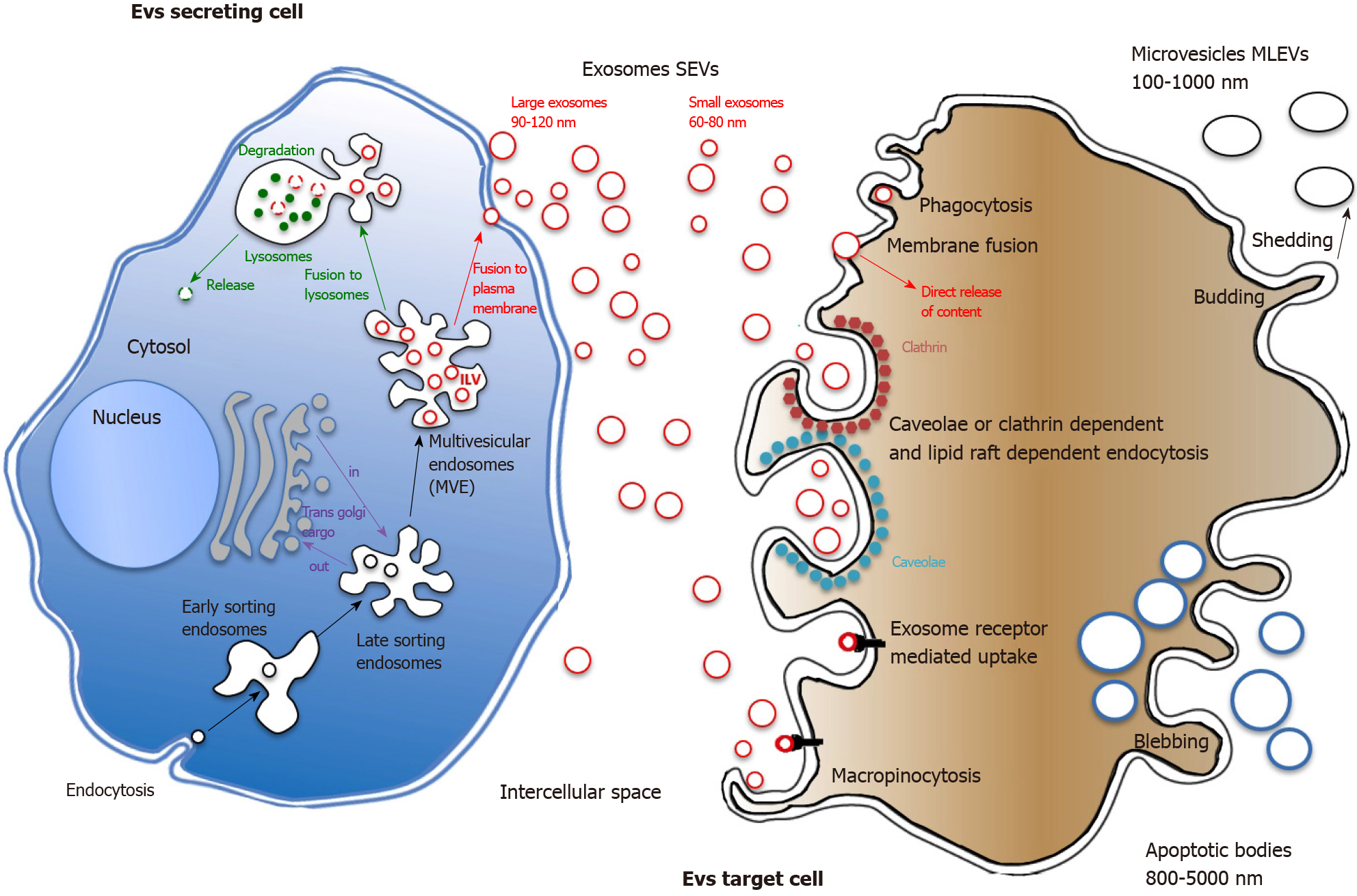
Figure 1 Extracellular vesicles biogenesis and interaction with recipient cells.
Extracellular vesicles (EVs) may have multiple origins. They can originate from plasma membrane blebbing during the apoptotic process giving rise to large apoptotic bodies or by membrane budding that leads to heterogeneous membranous EVs shedding. Small EVs (SEVs, exosomes) originate from internal budding of plasma membrane giving rise to early endosomes. By complex maturating interactions with the Golgi apparatus, early become late endosomes. The membranes of late endosomes form intraluminal vesicles (ILVs), small cargos containing proteins from plasma membrane and Golgi as well as nucleic acids. ILVs are contained in multivesicular endosomes that will fuse with either plasma membrane, releasing SEVs in the extracellular space or with lysosomes for further internal degradation. The endosomal sorting complex required for transport is the key machinery of protein sorting into SEVs. Once recognized, strongly depending on recipient cell type, EVs will enter through a variety of endocytic routes, either through clathrin-dependent or independent pathways (caveolin-mediated uptake, lipid raft-mediated internalization, etc.). Phagocytosis, macropinocytosis and simple membrane fusion can also be involved in EVs uptake. MLEVs: Medium large extracellular vesicles; SEVs: Small extracellular vesicles; MVEs: Multivesicular endosomes; ILVs: Intraluminal vesicles; MVP: Multivesicular particles; ESCRT: Endosomal sorting complex required for transport.
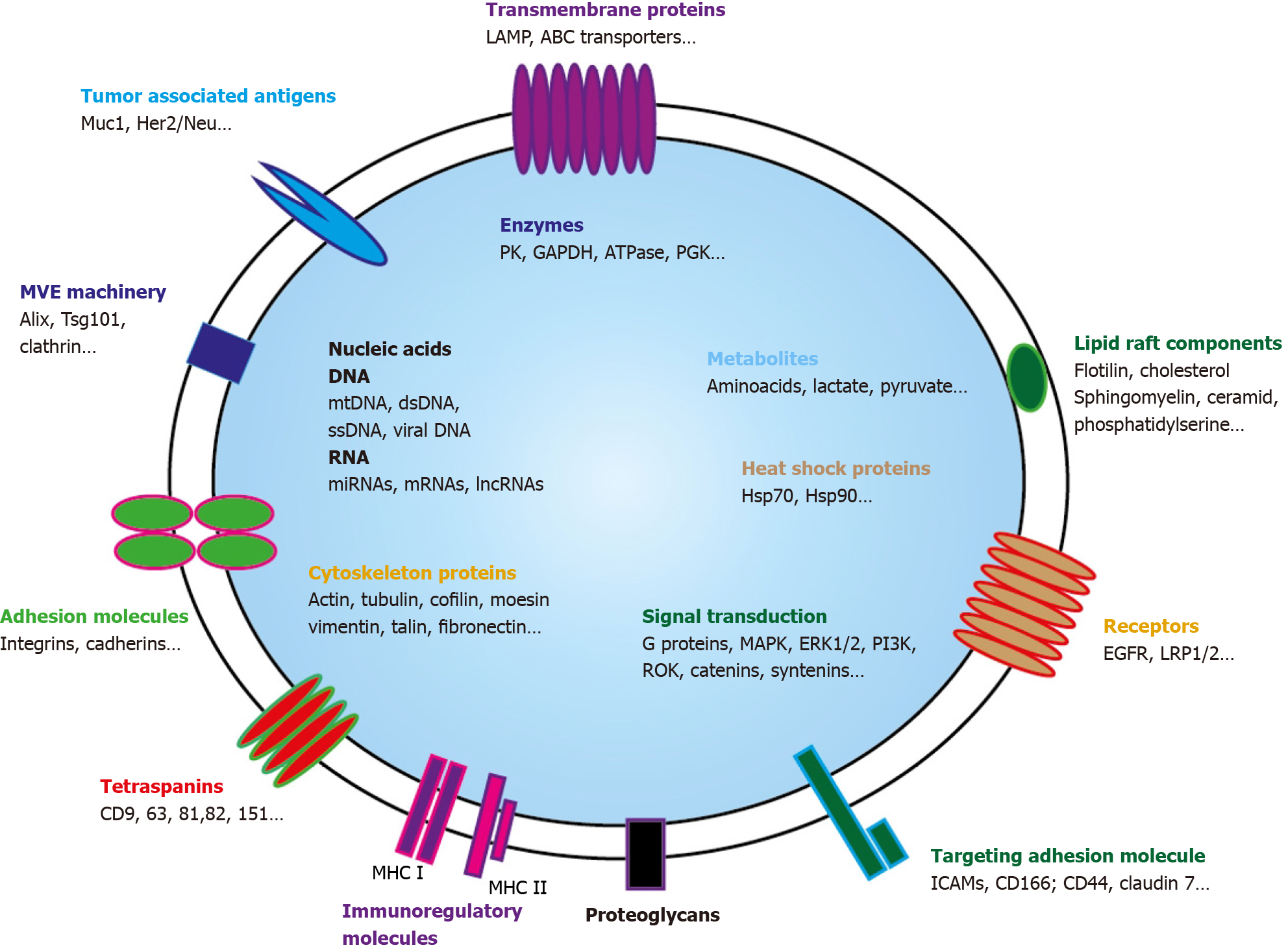
Figure 2 Exosome and its cargo content.
Small extravesicles (SEVs) are nano-sized membrane vesicles released by a variety of cell types and are thought to play important roles in intercellular communications. SEVs contain many kinds of proteins, either cytosolic or plasma membrane ones. Transporters, receptors, signaling proteins… but also enzymes can be evidenced. Metabolites are also present as well as nucleic acids. Genomic and mitochondrial DNAs, and multiple RNAs (mRNAs, miRNA, lncRNA, circRNA…) can be detected. Through horizontal transfer of these bioactive molecules, SEVS are emerging as local and systemic cell-to-cell mediators of oncogenic information. MHC: Major histocompatibility complex; MVE: Multivesicular endosomes.
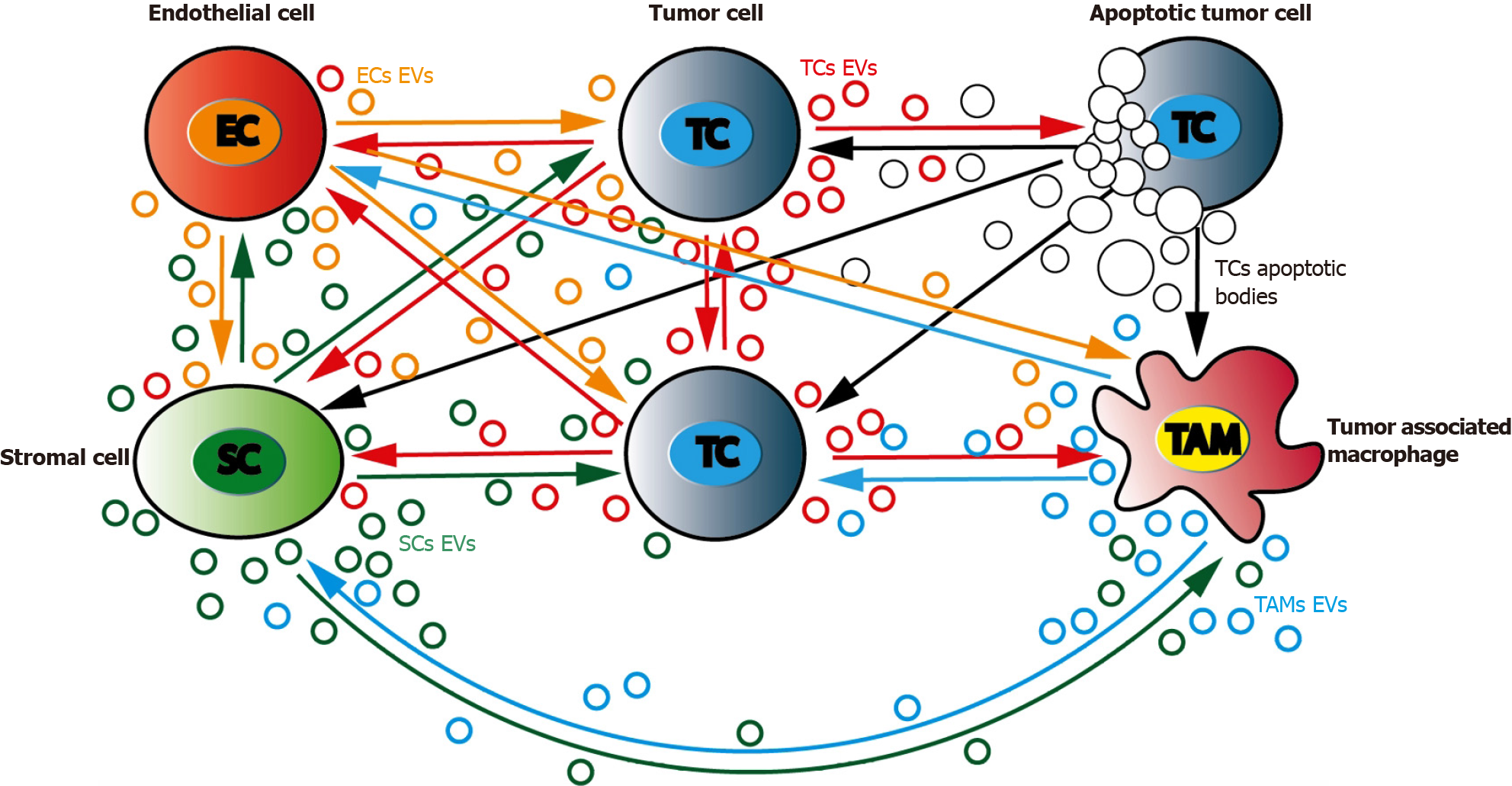
Figure 3 Bidirectional communications between tumor cells and their surrounding environment.
Tumor microenvironment is a complex and dynamic network that include tumor (TC), stromal (SC), immune (tumor associated macrophages, TAM) and endothelial cells (EC). TC can bidirectionally signal to each other through extracellular vesicles (EVs) production. TC can produce EVs that will regulate SCs and TAMs differentiation and activity. SCs as well as TCs can regulate ECs activity, especially in hypoxic situations. TAMs and ECs can cooperate to promote angiogenesis. TC: Tumor cells; EC: Endothelial cells; SC: Stromal cells; TAMs: Tumor associated macrophages.
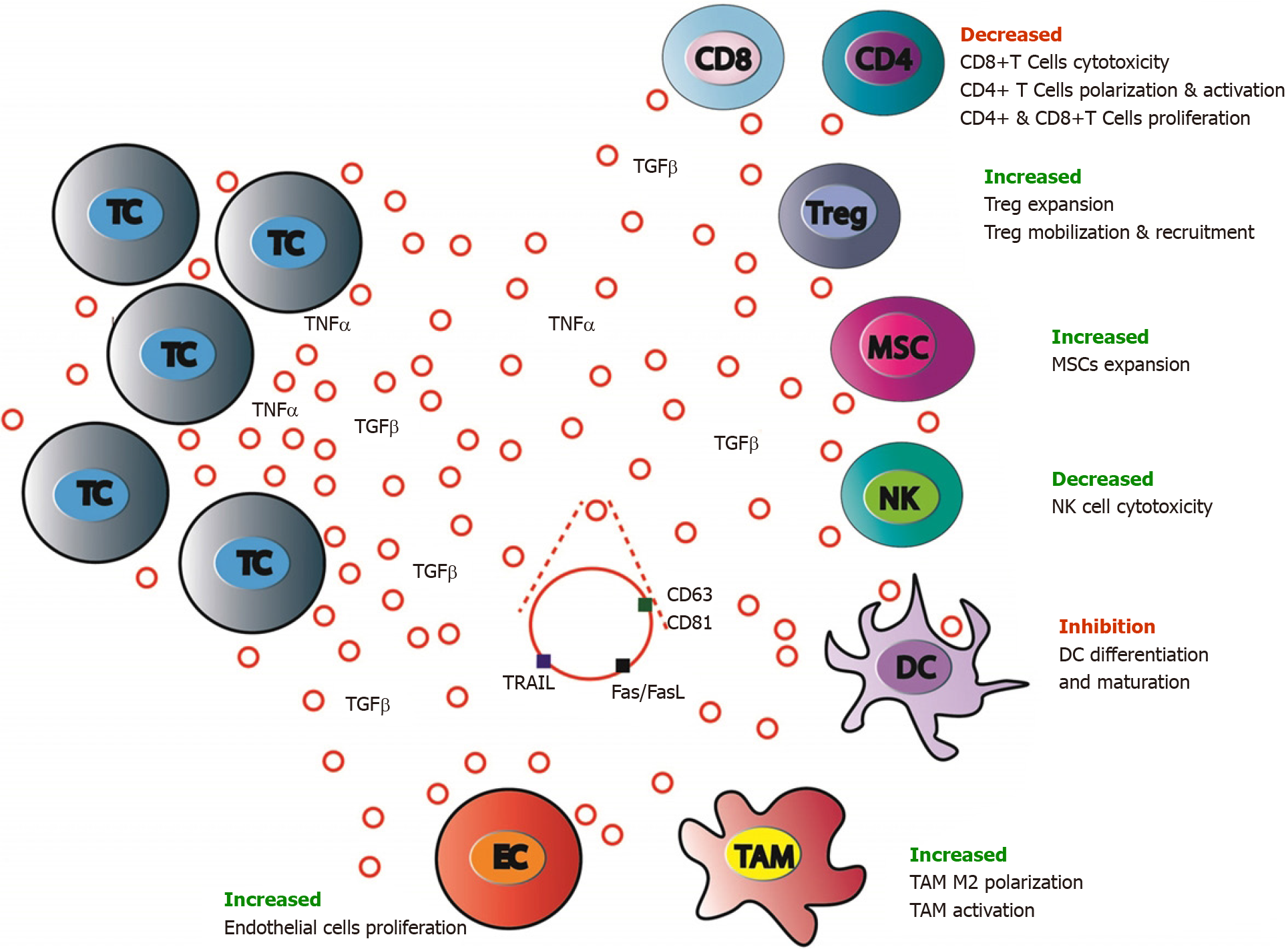
Figure 4 Antitumor immune system balance modulation by colorectal cancer cells extracellular vesicles.
Antitumor immune response is largely modulated by colorectal cancer (CRC) cells through either extracellular signaling molecules (cytokines, etc.) secretion or extracellular vesicles (EVs) production and release. CRC cells EVs contain inhibiting or activating molecules that favor target cells expansion, mobilization, and recruitment (regulatory T cells and mesenchymal stem cells), polarization and activation (tumor associated macrophages M2) and block others (CD8+ T-cells, dendritic cells, and natural killer cells). MSC: Mesenchymal stromal cells; CD4: CD4 positive T cells; CD8: CD8 positive T cells; EC: Endothelial cells; TC: Tumor cells; TAM: Tumor associated macrophages; NK: Natural killer cells; Treg: Regulatory T cells.
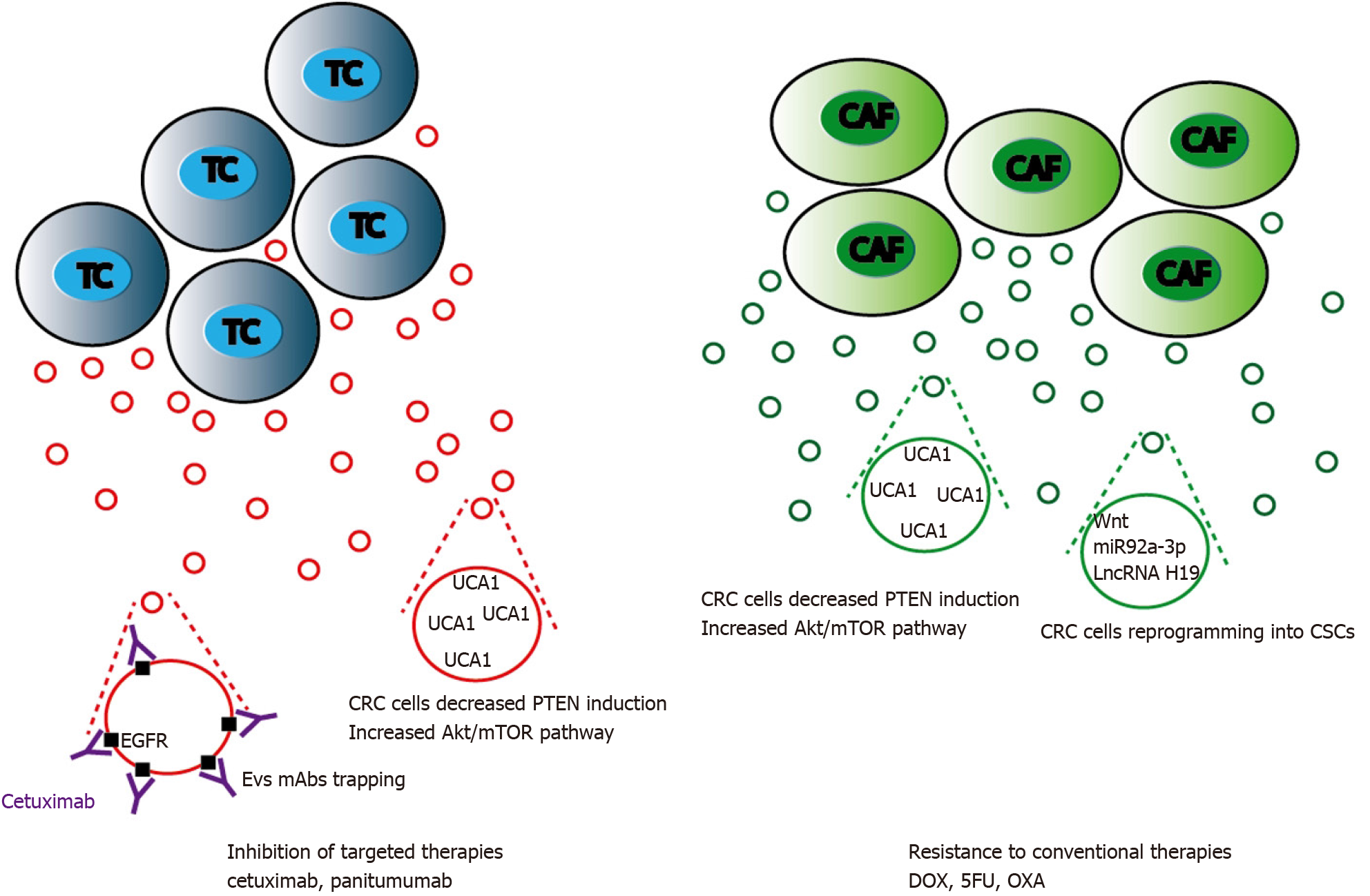
Figure 5 Mechanisms of extracellular vesicles-mediated chemoresistance in colorectal cancer treatment.
Extracellular vesicles (EVs) released either by colorectal cancer (CRC) or cancer activated fibroblasts cells can cooperate to promote cytotoxic drugs or targeted therapies resistance. These processes are mainly mediated by lncRNAs such as urothelial carcinoma-associated 1 that stimulate mTOR and STAT3 signaling, and by Wnt proteins or miRNAs targeting Wnt signaling pathway leading to CRC cell acquisition of stemness features. EVs can also trap targeted anti-epidermal growth factor receptor antibodies reducing their bioavailability and further action on CRC cells. TC: Tumor cells; CAF: Cancer activated fibroblasts; DOX: Doxycycline; 5-FU: 5-fluorouracil; OXA: Oxaliplatin.
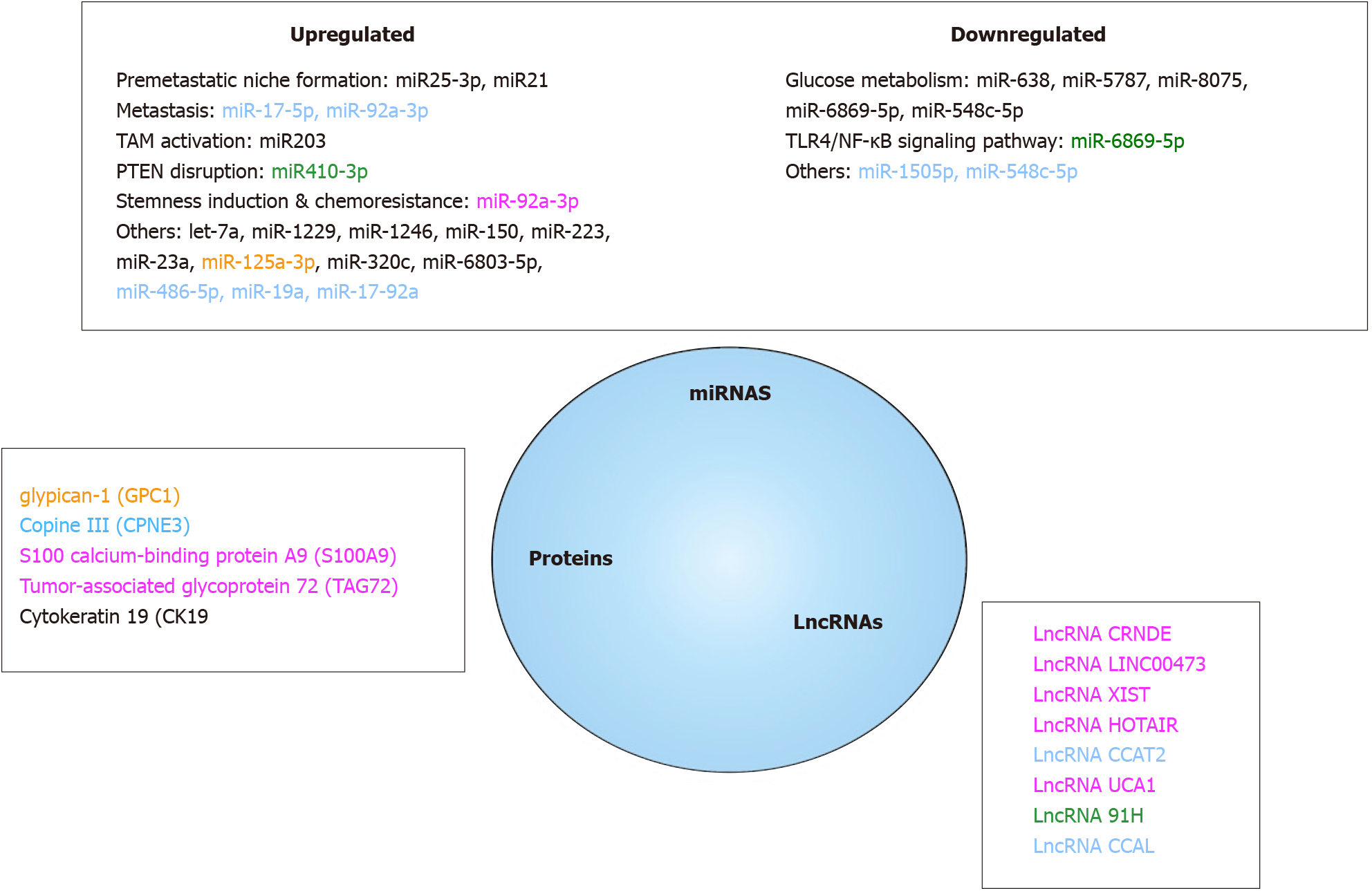
Figure 6 Colorectal cancer cells extracellular vesicles molecules as relevant cancer biomarkers.
Among all the molecules present in extracellular vesicles, only a subset (proteins, miRNAs, lncRNAs) have been shown to be of potential clinical value on colorectal cancer detection, diagnosis, prognosis and treatment response evaluation. All referenced markers were found to be differentially expressed in cancer patients and in healthy people. The yellow ones were useful for diagnosis, the green ones for progression, the blue ones for prognosis and the pink ones were associated with chemoresistance. TAM: Tumor associated macrophages.
- Citation: Mammes A, Pasquier J, Mammes O, Conti M, Douard R, Loric S. Extracellular vesicles: General features and usefulness in diagnosis and therapeutic management of colorectal cancer. World J Gastrointest Oncol 2021; 13(11): 1561-1598
- URL: https://www.wjgnet.com/1948-5204/full/v13/i11/1561.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i11.1561