Copyright
©The Author(s) 2020.
World J Gastrointest Oncol. Feb 15, 2020; 12(2): 149-172
Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.149
Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.149
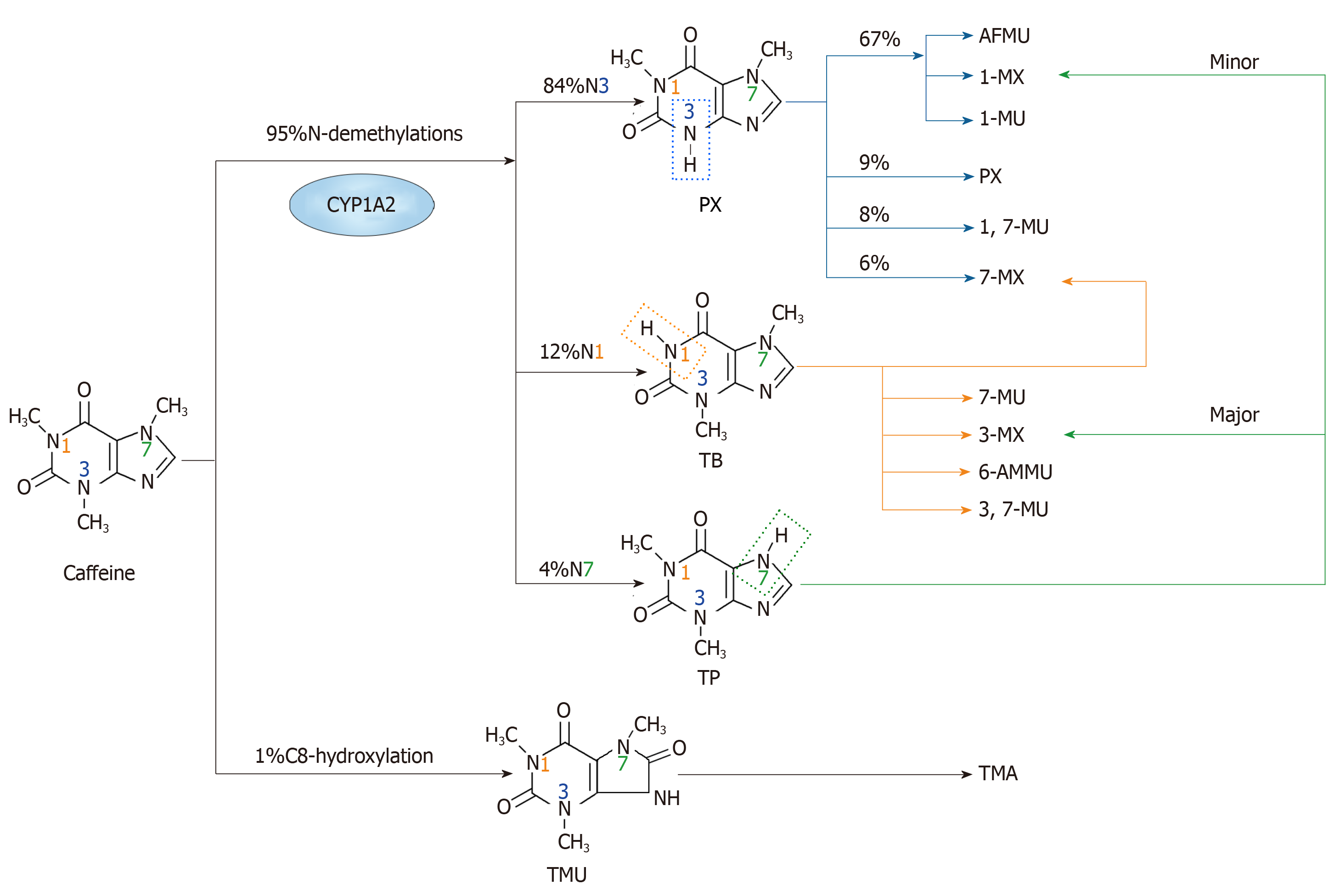
Figure 1 The major metabolism pathways of caffeine.
The primary metabolic action of caffeine involves two main pathways. One is N-demethylation, which includes N1, N3 and N7-demethylations. Through N3-demethylation, caffeine can be degraded to form paraxanthine, which may be further degraded to 5-acetyl-amino-6-formylamino-3-methyluracil, 1-methylxanthine, 1-methyluric acid, 1,7-methyluric acid and 7-methylxanthine. A small amount of paraxanthine can be cleared in its unchanged form. Theobromine can be formed through N1-demethylation of caffeine, and it can be metabolized to form 7-methylxanthine, 7-methyluric acid, 3-methylxanthine, 6-amino-5[N-methylformylamino]-1-methyluracil and 3,7-methyluric acid. Moreover, caffeine can be metabolized to theophylline through N7-demethylation, and then theophylline can be further degraded to 1-methylxanthine and 3-methylxanthine. The other pathway is C8-hydroxylation in which caffeine is metabolized to trimethyluric acid and then 3,6,8-trimethylallantoin. PX: Paraxanthine; AFMU: 5-acetyl-amino-6-formylamino-3-methyluracil; 1-MX: 1-methylxanthine; 1-MU: 1-methyluric acid; 7-MU: 7-methyluric acid; 1,7-MU: 1,7-methyluric acid; 7-MX: 7-methylxanthine; TB: Theobromine; 3-MX: 3-methylxanthine; 6-AMMU: 6-amino-5[N-methylformylamino]-1-methyluracil; 3,7-MU: 3,7-methyluric acid; TP: Theophylline; TMU: Trimethyluric acid; TMA: 3,6,8-trimethylallantoin.
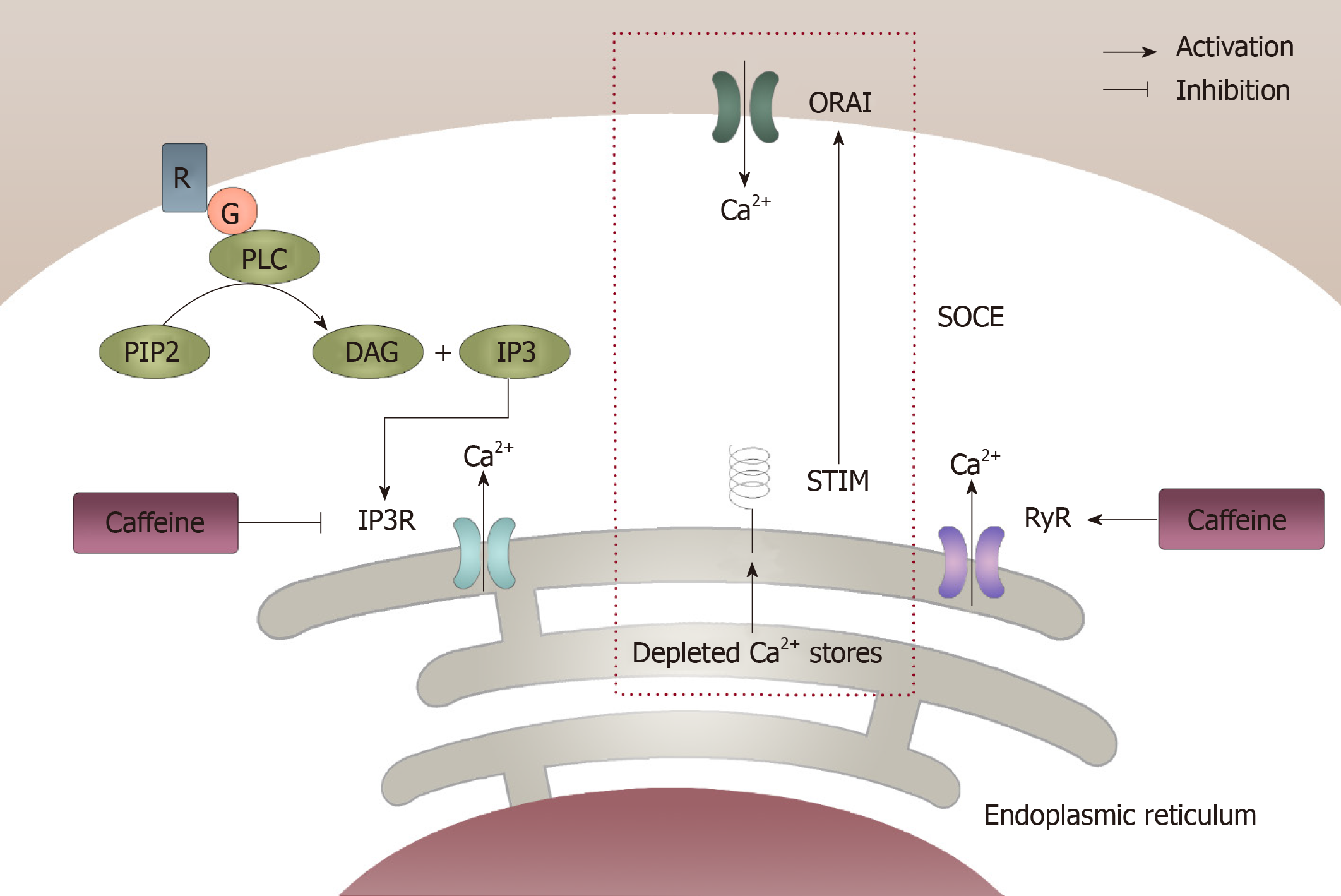
Figure 2 Modulation of intracellular calcium homeostasis by caffeine.
Caffeine can modulate intracellular calcium homeostasis of inexcitable cells in two different ways. One way is by inhibiting the inositol 1,4,5-trisphosphate receptor through which Ca2+ released from the endoplasmic reticulum can be decreased, which results in a decrease in intracellular calcium levels. However, caffeine can increase the intracellular calcium levels by activating the ryanodine receptor, which causes an increase in Ca2+ being released from the endoplasmic reticulum. In this case, the store-operated Ca2+ entry, which was initiated from the depletion of Ca2+ from the endoplasmic reticulum can be activated and then result in an increase in extracellular Ca2+ getting into the intracellular compartment. IP3: 1,4,5-trisphosphate; IP3R: 1,4,5-trisphosphate receptor; ER: Endoplasmic reticulum; RyR: Ryanodine receptor; SOCE: Store-operated Ca2+ entry.
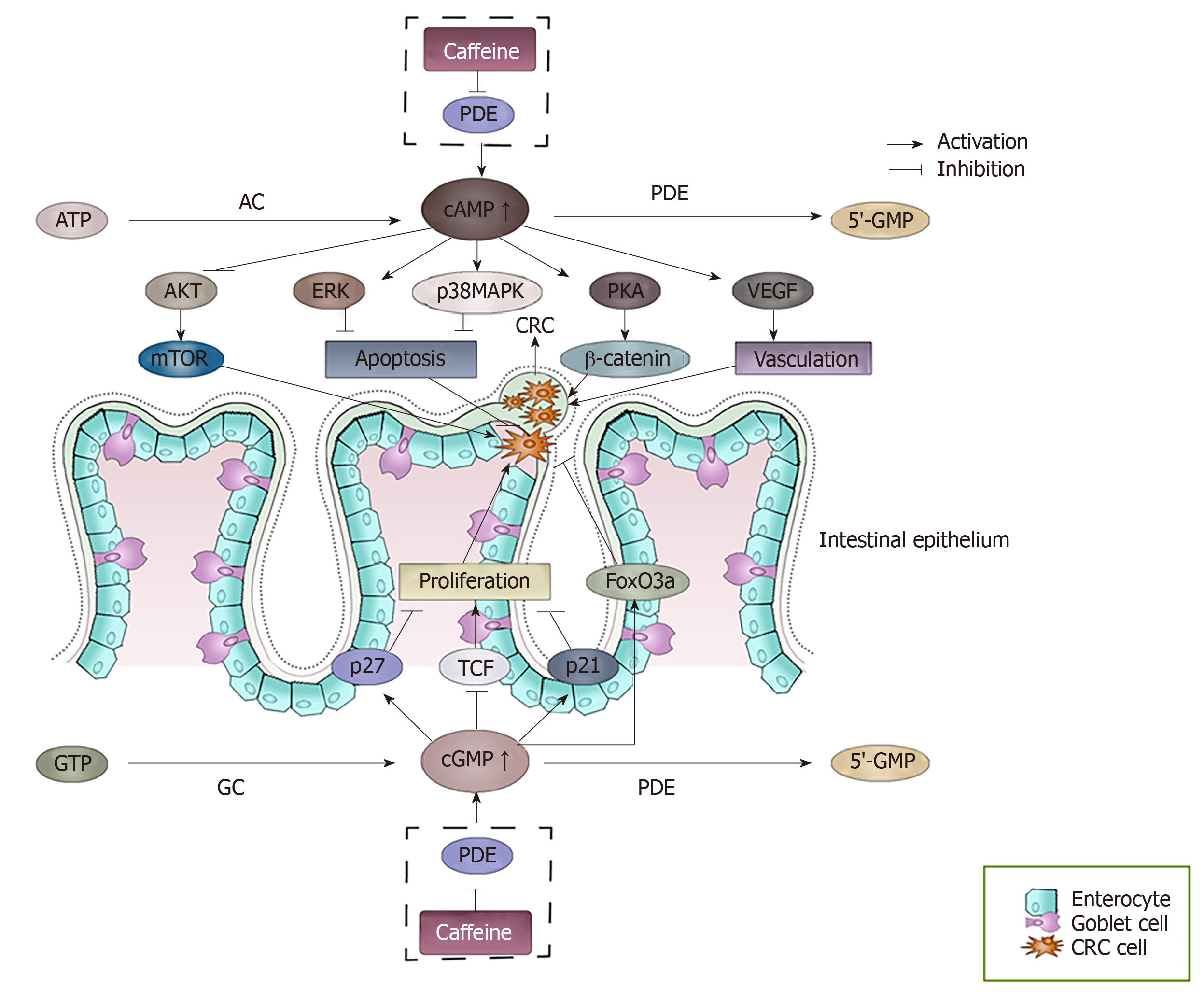
Figure 3 The effect of accumulated cAMP and cGMP caused by the inhibition of phosphodiesterase by caffeine.
cAMP and cGMP levels are regulated by the balance between the activities of two types of enzymes, the generating enzymes (adenylate cyclase/guanylyl cyclase) and the degrading enzymes (phosphodiesterase). Caffeine can inhibit the degrading enzymes, which causes the accumulation of cAMP and cGMP. The accumulated cAMP and cGMP can interact with many factors and finally may result in a series of events of colorectal cancer cells. cAMP: Cyclic adenosine 3′,5′-monophosphate; cGMP: Cyclic guanosine 3′-5′ monophosphate; AC: Adenylate cyclases; GC: Guanylyl cyclase; PDEs: Phosphodiesterase; PKA: Protein kinase A; VEGF: Vascular endothelial growth factor; TCF: T cell factor.
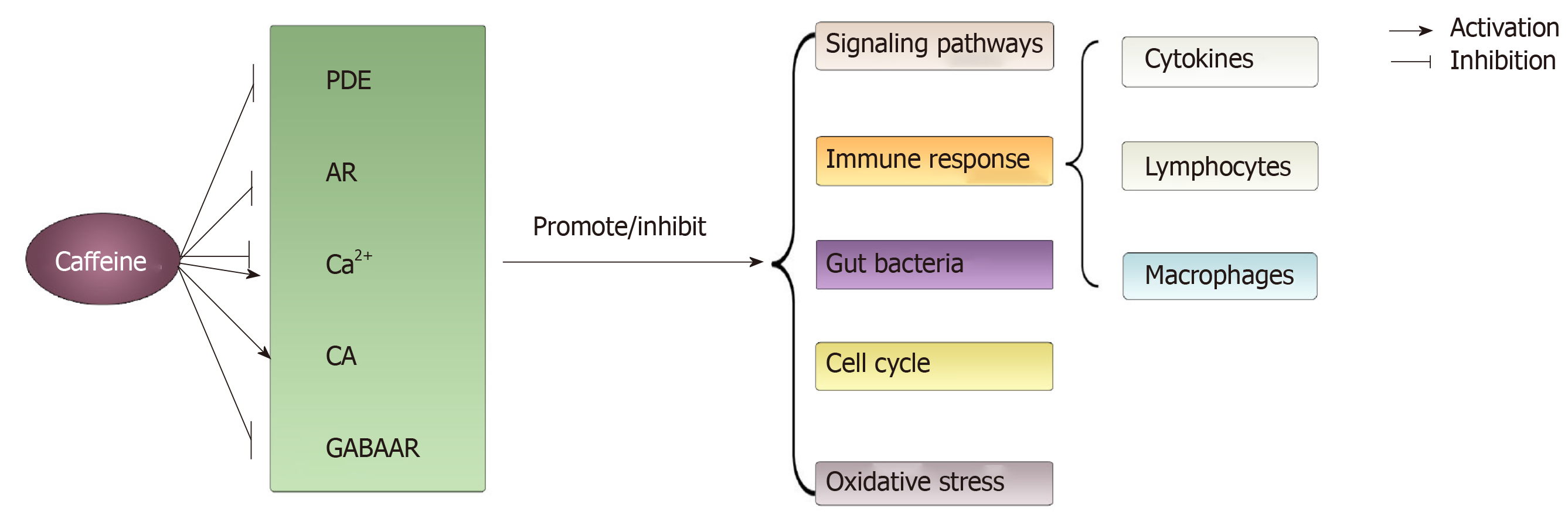
Figure 4 The main acting sites and physiological processes modulated by caffeine on colorectal cancer involved in this article.
Through modulating acting sites such as phosphodiesterase, adenosine, Ca2+, catecholamines and γ-aminobutyric acid receptor, caffeine can exert varied effects (promote or inhibit) on physiological processes such as signal pathways, immune response, gut bacteria, cell cycle and oxidative stress. PDE: Phosphodiesterase; AR: Adenosine receptor; CA: Catecholamines; GABAAR: γ-aminobutyric acid receptor.
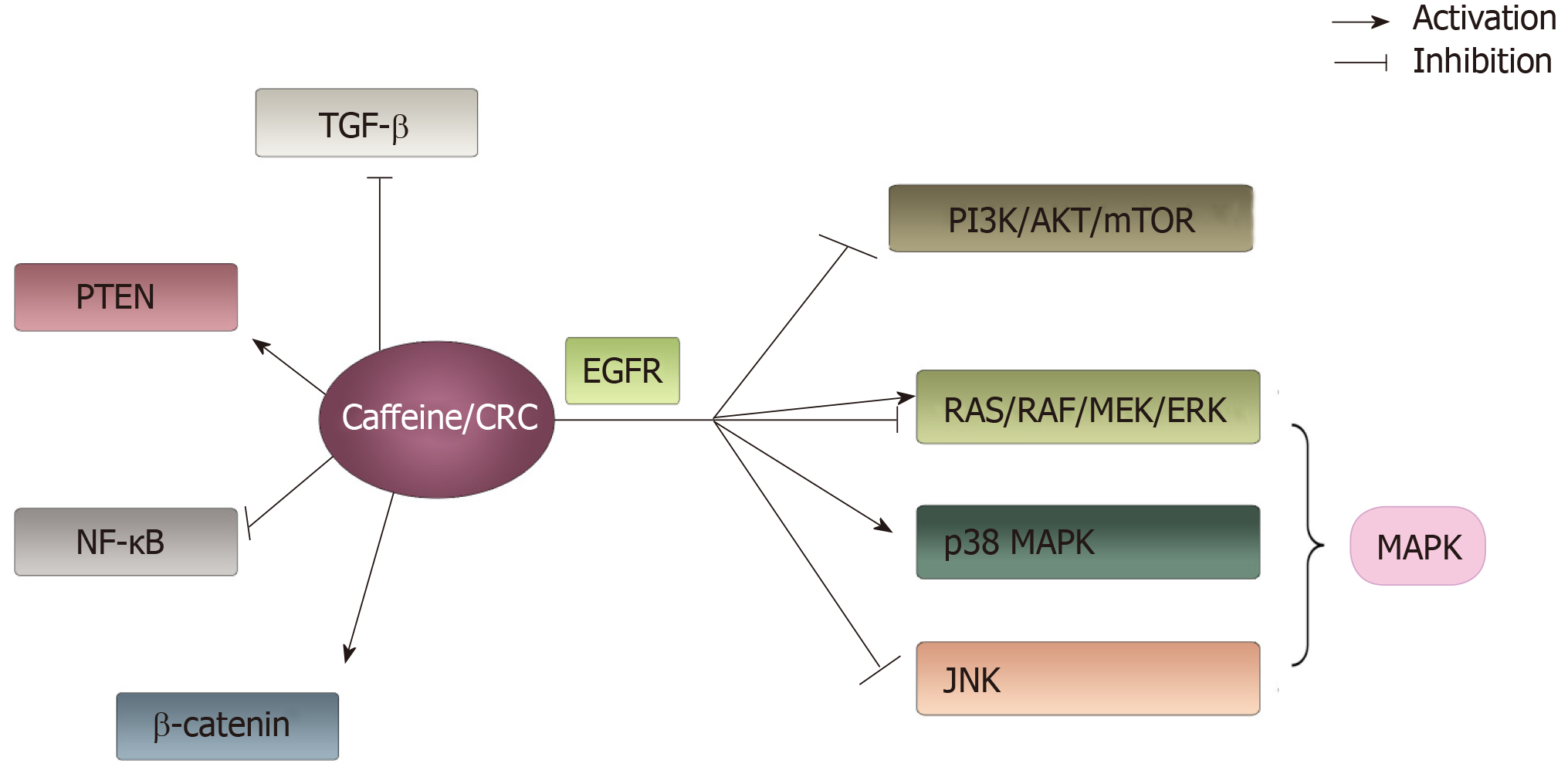
Figure 5 The main signaling pathways associated with colorectal cancer that can be influenced by caffeine involved in this article.
Caffeine can interact with a number of signaling pathways. It shows an active effect on phosphatase and tensin homolog, β-catenin and p38 mitogen-activated protein kinase pathways. Additionally, it has negative effects on transforming growth factor β, NF-ΚB, phosphoinositide-3-kinase/AKT/mammalian target of rapamycin and c-Jun N-terminal kinase pathways. Moreover, caffeine showed a dual function on RAS/RAF/MEK/ERK pathways depending on concentration of caffeine. PTEN: Phosphatase and tensin homolog; MAPK: Mitogen-activated protein kinase; TGF-β: Transforming growth factor β; PI3K: Phosphoinositide-3-kinase; mTOR: Mammalian target of rapamycin; JNK: c-Jun N-terminal kinase.
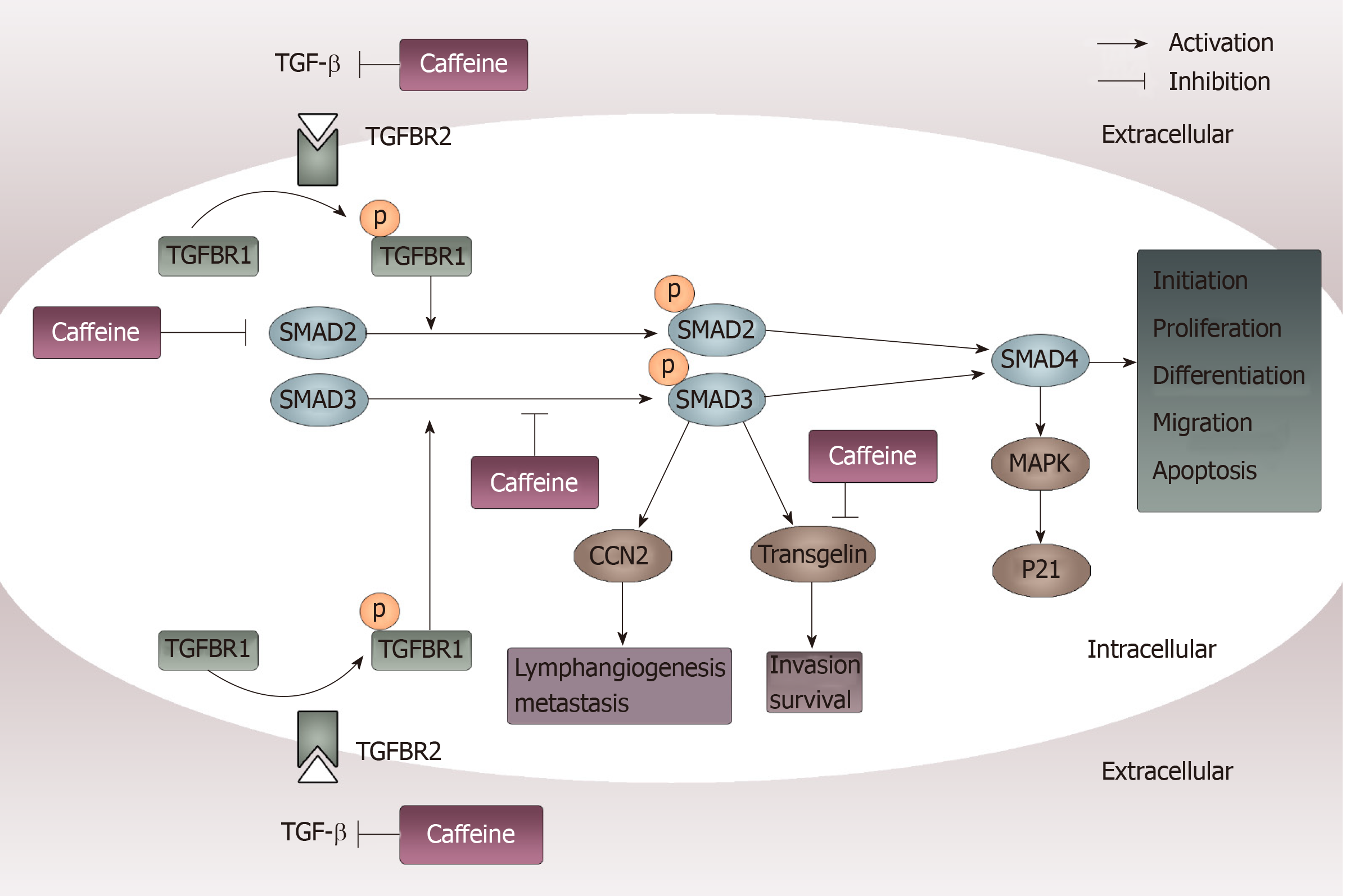
Figure 6 Inhibition of transforming growth factor β pathways caused by caffeine.
When transforming growth factor (TGF-β) acts on its type II receptor, the type I receptor can be recruited and phosphorylated. Then, mediated by the activated TGF-β type I receptor, SMAD2 and SMAD3 are phosphorylated and exert their functions by modulating downstream factors such as CCN-family protein 2 and transgelin or by binding to SMAD4. Caffeine may influence this pathway not only through inhibiting TGF-β at the beginning of the pathway but also by reducing the steady state concentration of total SMAD2 and decreasing phosphorylation of SMAD3. Furthermore, caffeine can directly inhibit transgelin, which plays an important part in invasion and survival. TGF-β: Transforming growth factor β; CCN2: CCN-family protein 2; TGFBR2: Transforming growth factor binding to the type II receptor; TGFBR1: Transforming growth factor binding to the type I receptor; MAPK: Mitogen-activated protein kinase.
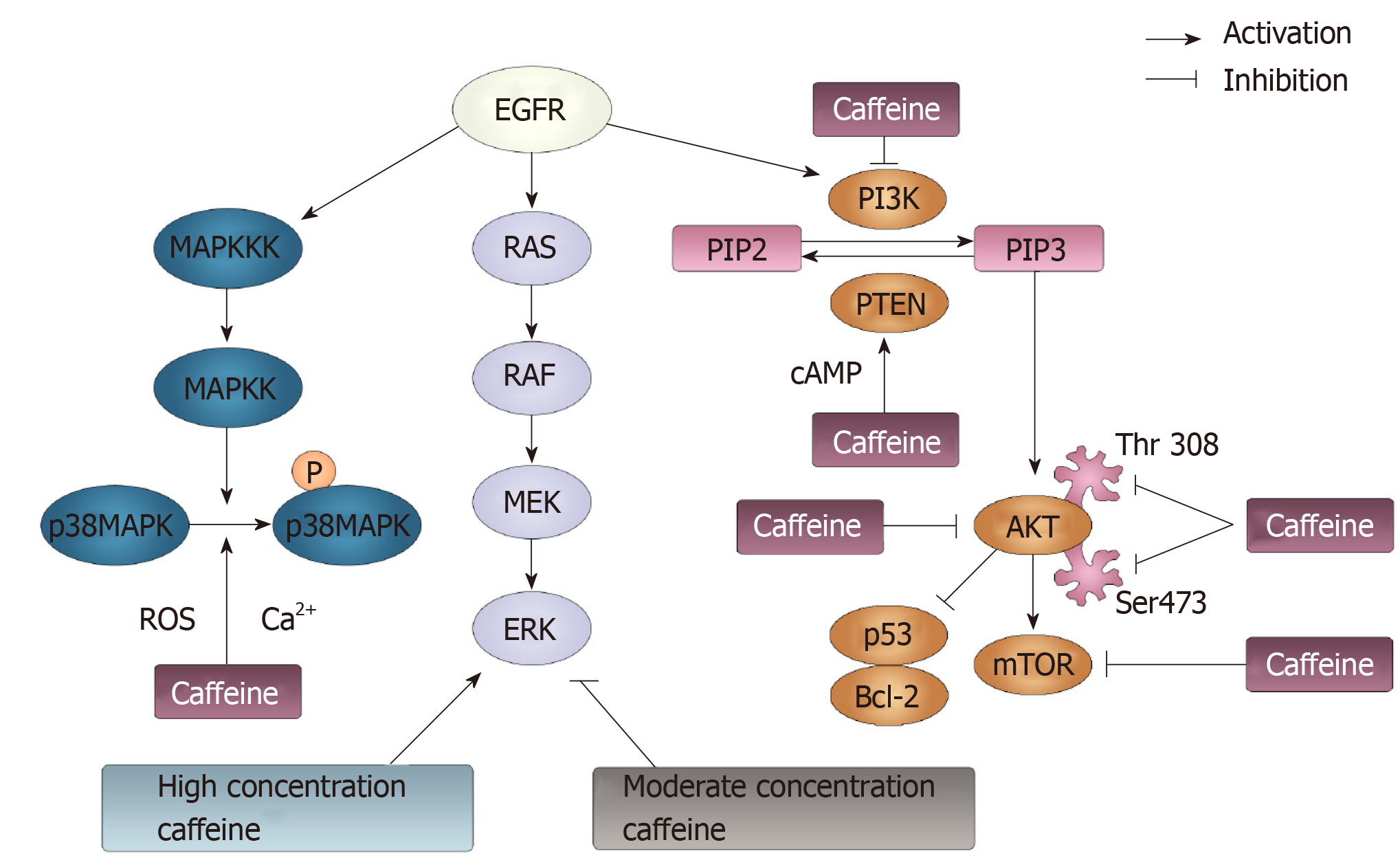
Figure 7 Phosphoinositide-3-kinase, phosphatase and tensin homolog, p38 mitogen-activated protein kinase and RAS pathways modulated by caffeine.
Three signaling pathways can be initiated by epidermal growth factor receptor: p38 mitogen-activated protein kinase (MAPK), RAS/RAF/MEK/ERK and PI3K/AKT/ mTOR pathways. p38 MAPK can be phosphorylated following the activation of MAPK kinase kinases and MAPK kinases. Caffeine can inhibit the activation of p38 MAPK though modulating the Ca2+ concentration and reactive oxygen species generation. Additionally, caffeine can exert a dual function on ERK, the downstream factor of the RAS/RAF/MEK pathway. At a high concentration, caffeine activates the ERK pathway. At a moderate concentration, caffeine shows an inhibitory effect on the ERK pathway. PI3K can convert phosphatidylinositol-4-phosphate (PIP) 2 to PIP3, and PTEN can reverse this conversion. Caffeine can activate PTEN and inhibit PI3K, in which case PIP3 can be reduced and PIP2 can be increased. Then, PIP3 can activate AKT, through which p53 and Bcl-2 can be activated, and the mTOR pathway can be inhibited. Caffeine can inhibit the phosphorylation of AKT by suppressing its residues Thr308 and Ser473. Moreover, caffeine can directly inhibit mTOR pathways. EGFR: Epidermal growth factor receptor; ERK: Extracellular-signal regulated kinases; mTOR: Mammalian target of rapamycin; MAPK: Mitogen-activated protein kinase; MAPKK: MAPK kinases; MAPKKKs: MAPK kinase kinases; ROS: Reactive oxygen species; PI3K: Phosphoinositide-3-kinase; PIP: Phosphatidylinositol-4-phosphate; PTEN: Phosphatase and tensin homolog.
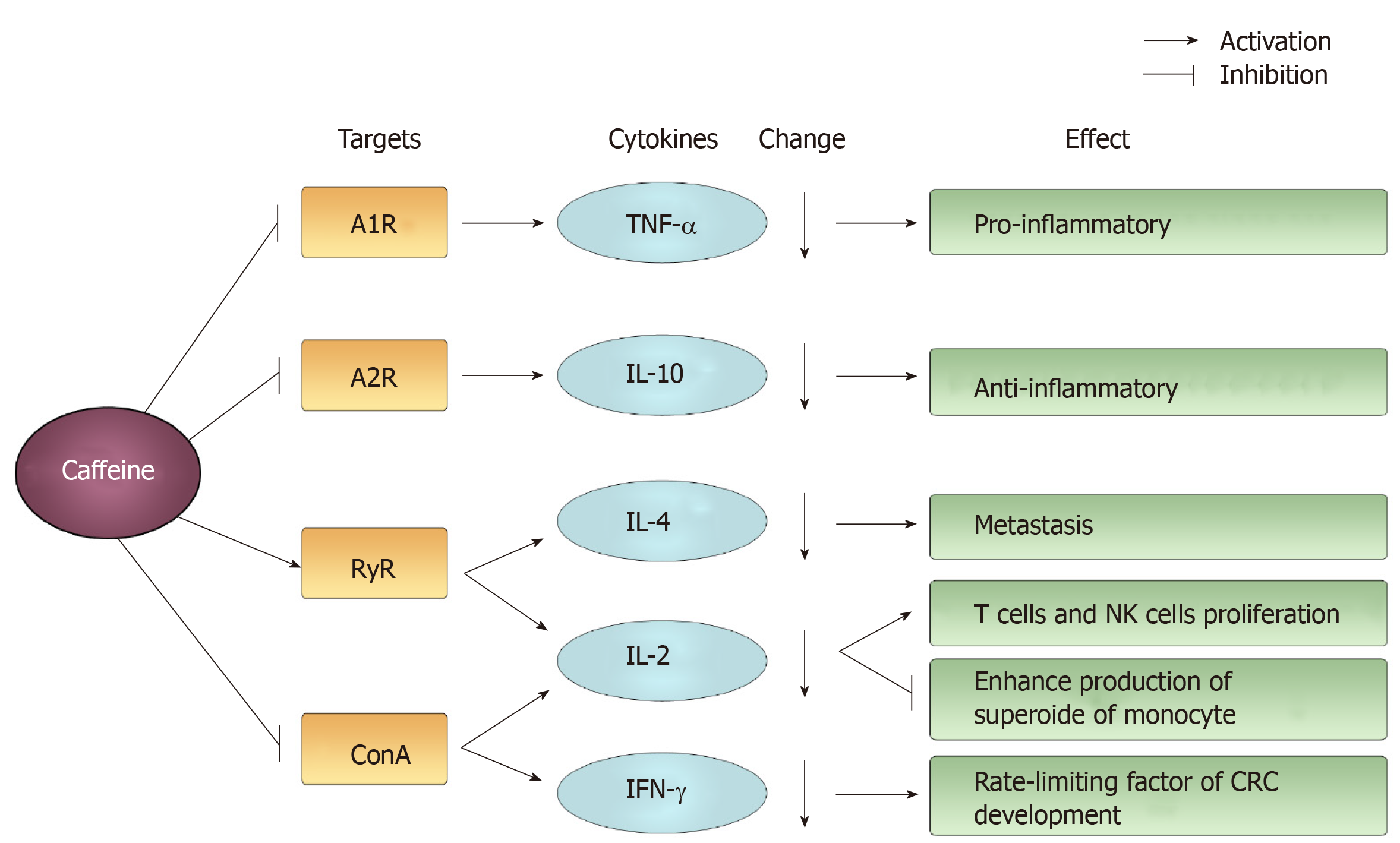
Figure 8 The main targets and changes induced by caffeine acting on cytokines and their effects.
Examples of mediated targets are A1R, A2R, ryanodine receptor and concanavalin A. Caffeine induces a decline in cytokines, such as tumor necrosis factor-alpha, interleukin (IL)-10, IL-4, IL-2, interferon-γ and exerts a differential effect. RyR: Ryanodine receptor; ConA: Concanavalin A; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; IFN: Interferon.
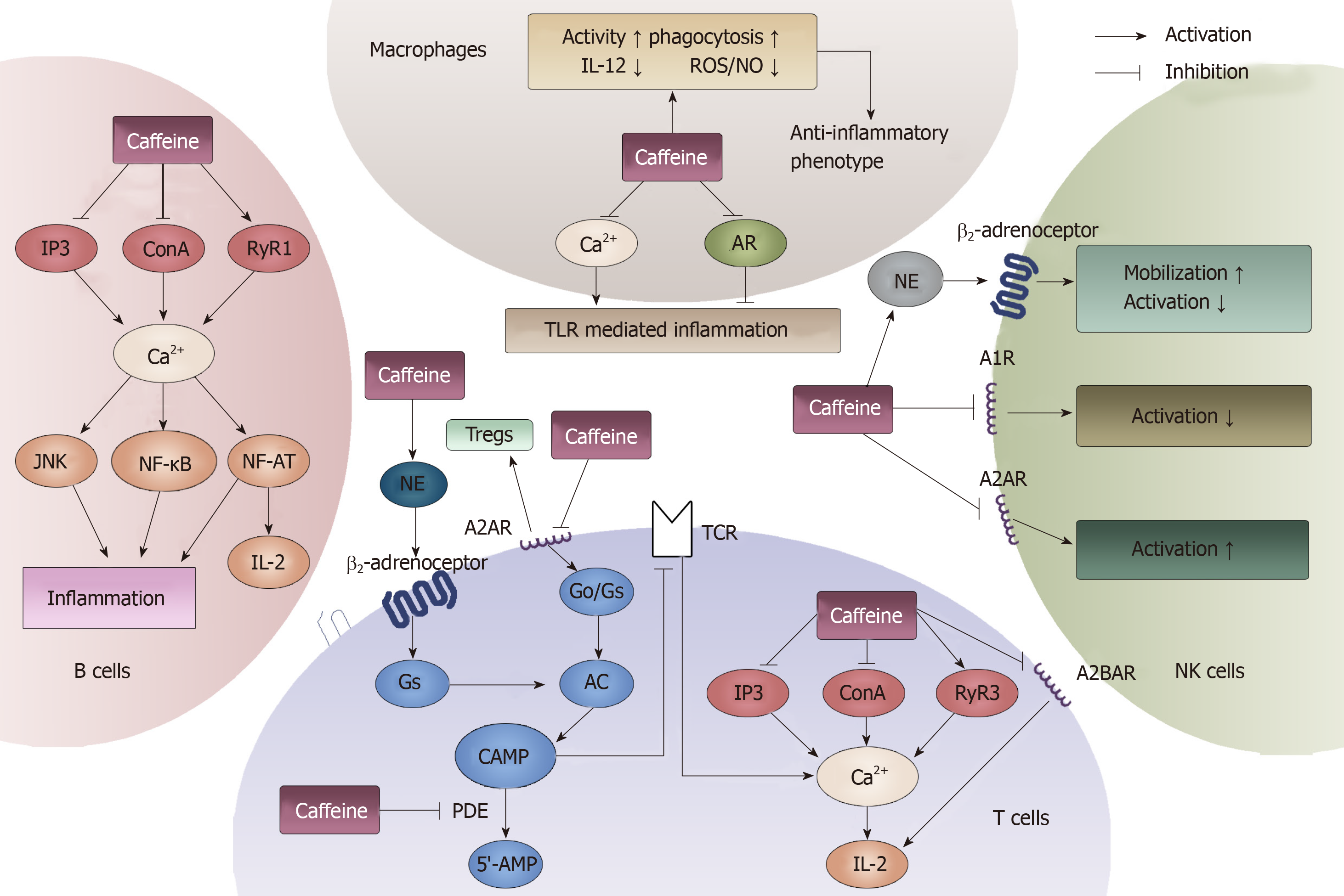
Figure 9 General effects of caffeine on lymphocytes and macrophages.
Four types of immune cells can be influenced by caffeine. In T cells, cAMP and Ca2+ can be modulated by caffeine through the binding of adenosine receptors, modulating norepinephrine and inhibiting phosphodiesterase. In B cells, caffeine can influence the immune response through interacting with Ca2+. In macrophages, caffeine can both inhibit and activate toll-like receptor-mediated inflammation by modulating Ca2+ and binding to adenosine. Furthermore, in natural killer cells, caffeine exerts a dual function on its activation. When it acts on A1R, it inhibits. When it acts on A2R, it induces. Moreover, by stimulating norepinephrine , caffeine can promote the mobilization of natural killer cells. AR: Adenosine receptors; NE: Norepinephrine; PDE: Phosphodiesterase; TLR: Toll-like receptor; NK: Natural killer; IL: Interleukin.
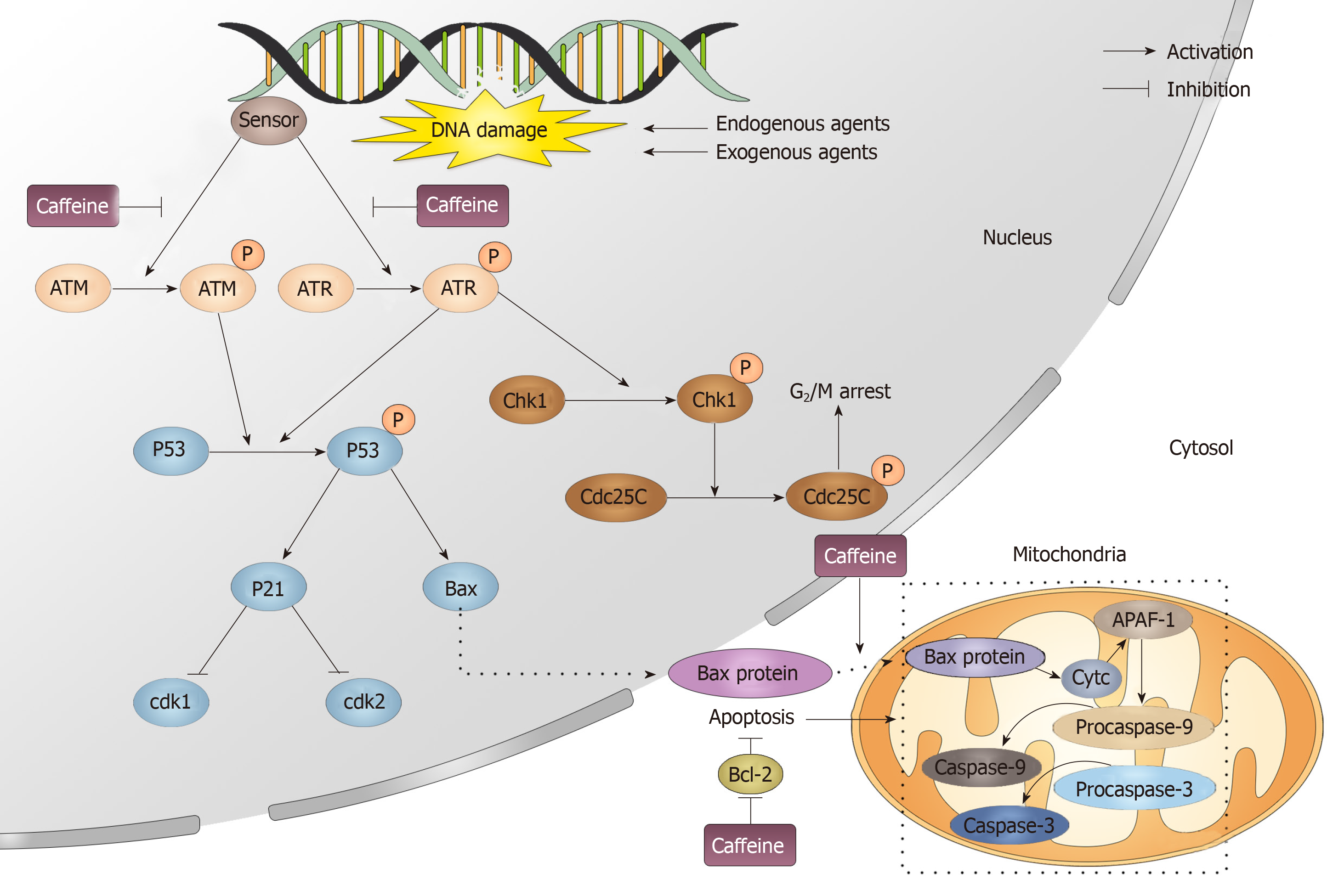
Figure 10 Caffeine influences the DNA repair process.
When exogenous and endogenous agents attack DNA, DNA can be damaged, which immediately activates the DNA repair process. In this process, a sensor detects the damage and then causes the phosphorylation of ataxia-telangiectasia mutated and ataxia telangiectasia and Rad3-related. Caffeine can inhibit the activation of both ataxia-telangiectasia mutated and ataxia telangiectasia and Rad3-related. Phosphorylated ataxia telangiectasia and Rad3-related and ataxia-telangiectasia mutated can activate cyclin-dependent kinase 1, which induces G2/M arrest and p53. p53 then modulates its downstream target p21, which can influence the cell cycle by inhibiting Cdk1 and Cdk2. Moreover, Bax is also downstream of p53, and when it enters the cytosol, it can initiate the apoptosis process. Caffeine can promote apoptosis by inhibiting the translocation of Bax from the nucleus to the mitochondria and also by promoting the apoptosis inhibitor Bcl-2. ATM: Ataxia-telangiectasia mutated; ATR: Ataxia telangiectasia and Rad3-related; Chk: Cyclin-dependent kinase.
- Citation: Cui WQ, Wang ST, Pan D, Chang B, Sang LX. Caffeine and its main targets of colorectal cancer. World J Gastrointest Oncol 2020; 12(2): 149-172
- URL: https://www.wjgnet.com/1948-5204/full/v12/i2/149.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i2.149