Copyright
©The Author(s) 2018.
World J Gastrointest Oncol. Sep 15, 2018; 10(9): 231-243
Published online Sep 15, 2018. doi: 10.4251/wjgo.v10.i9.231
Published online Sep 15, 2018. doi: 10.4251/wjgo.v10.i9.231
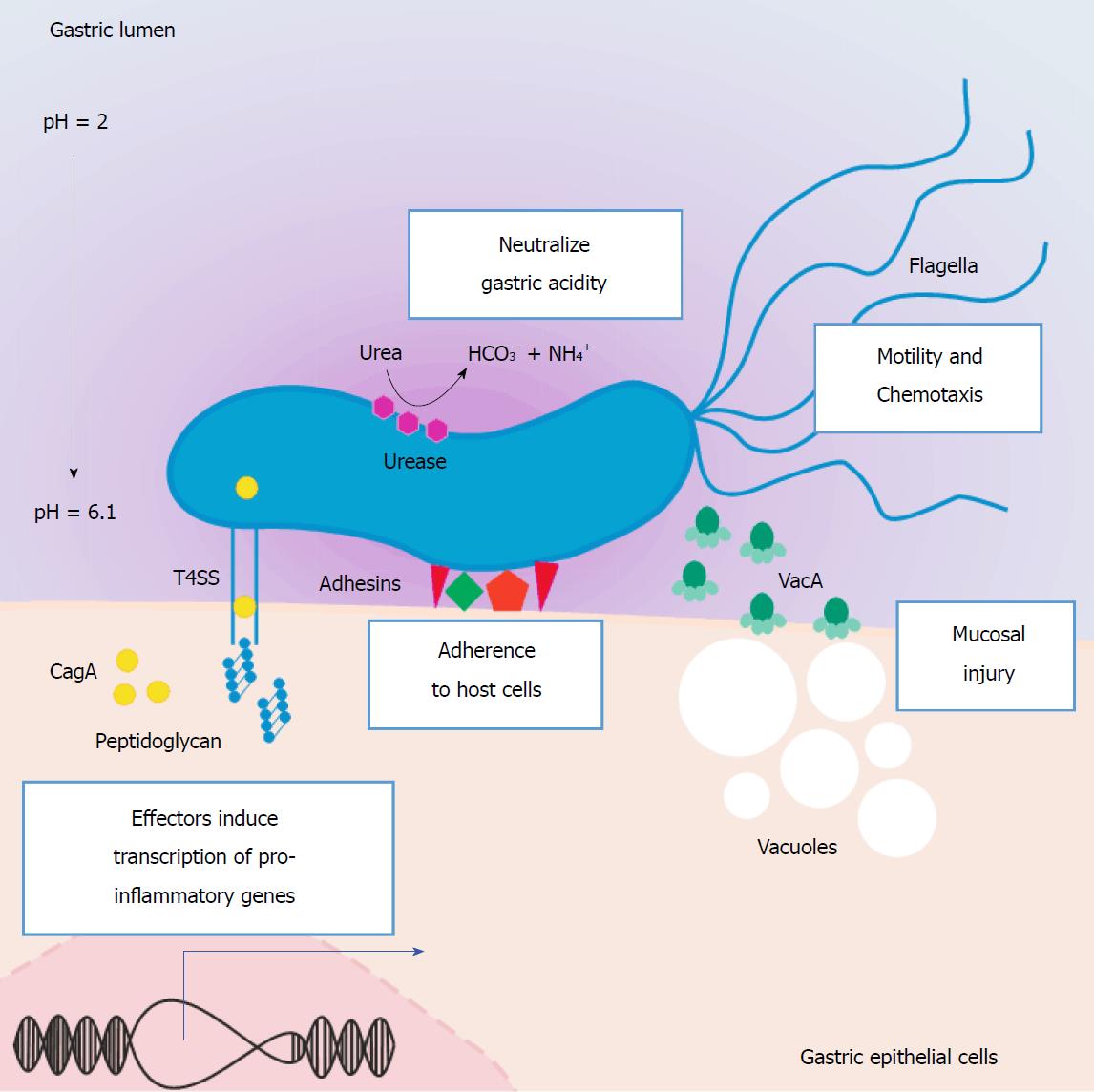
Figure 1 Helicobacter pylori virulence factors associated with gastric pathogenic processes.
H. pylori is genetically highly variable, and some strains are more strongly associated with gastric pathologies, including GC. The most prominent are those that express the virulence factors VacA and Cag-PAI. VacA is a cytotoxic protein expressed by the polymorphic gene vacA that induces the formation of vacuoles, thus generating damage in the gastric epithelium. The Cag-PAI-positive strains (approximately 60%) possess a functional genetic region, which contains approximately 30 genes that code for proteins, that together make up a T4SS. The secretion system introduces a number of molecules, including the virulence factor CagA and peptidoglycans, into the cytoplasm of epithelial cells of the gastric mucosa. Once in the cytoplasm, CagA is phosphorylated, and this triggers downstream intracellular events, such as cytoskeletal rearrangement, alterations in cellular polarity, expression of inflammatory mediators, and activation of signaling pathways that promote cellular proliferation. The conversion of urea into ammonium and carbon dioxide by urease is essential to the survival H. pylori in the stomach. Flagella play an important role in the colonization of the gastric mucosa, as they produce differential motility depending on the pH of the stomach lumen and the concentration of compounds such as urea, thus enabling H. pylori bacteria to swim across the mucous layer towards the epithelial lining. Other less well-characterized virulence factors of H. pylori associated with gastric pathology are the adhesins, which include BabA, SabA, and OipA. Cag-PAI: Cag pathogenicity island; GC: Gastric cancer; H. pylori: Helicobacter pylori; T4SS: Type IV secretion system; VacA: Vacuolating cytotoxin A.
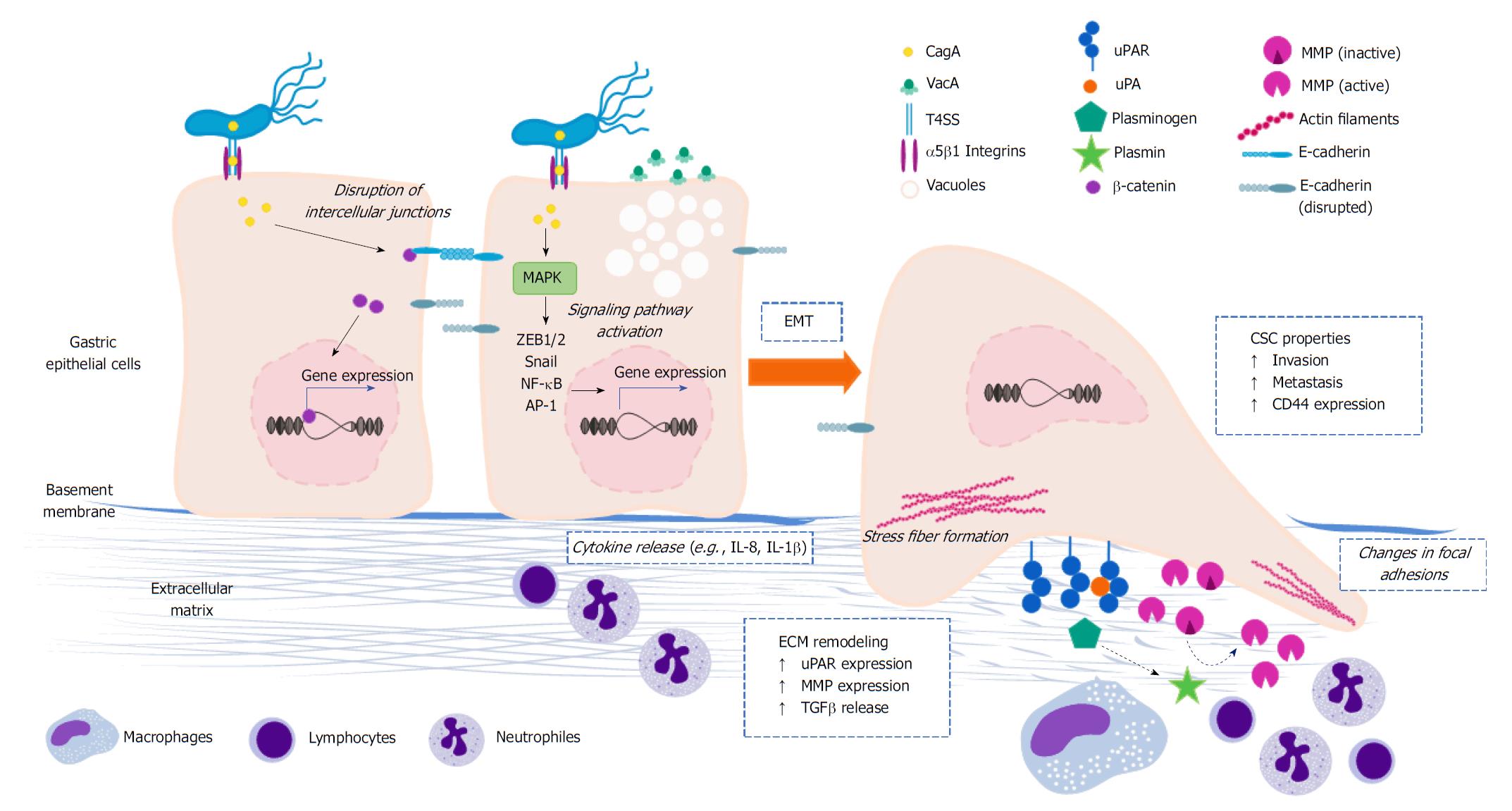
Figure 2 Role of Helicobacter pylori in the reprograming of cellular and molecular programs related to invasive behavior.
Upon infection, H. pylori induces a number of intracellular processes in gastric epithelial cells, some of them can result in the reprogramming of cellular and molecular mechanisms underlying the invasive cell behavior. Several of these events take place after phosphorylation of CagA, others may be independent of the translocation and phosphorylation of CagA, and a portion are not connected to the Cag-PAI at all. Although not exclusively, CagA plays an important role in the activation of transcription factors, such as β-catenin, Snail, ZEB1/2, NF-κB, and AP-1. Some of these transcriptional regulators (e.g., β-catenin, Snail, ZEB1/2) modify the expression of genes encoding for key effectors of the EMT and stem cell programs, including up-regulation of TGFβ and CD44 and down-regulation of E-cadherin. Changing of the gastric epithelial cell polarity is another downstream intracellular event connected to H. pylori, which is primarily attributed to the physical interaction of components of the T4SS (especially CagL) and β1 integrins. In addition to this translocation-independent mechanism, alteration of the cell polarity can also result from CagA translocation and phosphorylation. Another consequence of the manipulation of the invasive properties by H. pylori is the induction of the expression of ECM remodeling effectors, namely uPAR, uPA, MMP7, MMP2, MMP3, and MMP9. This is presumably linked to the activation of the MAPK signaling pathway, which in turn leads to the activation of NF-κB and AP-1. It is not yet clear whether plasminogen and MMP activation (dashed arrows) takes place, or what are the functional implications of the enhanced expression of these ECM-related molecules in this particular context. Cag-PAI: Cag pathogenicity island; ECM: Extracellular matrix; EMT: Epithelial-to-mesenchymal transition; H. pylori: Helicobacter pylori; MMP: Matrix metalloproteinase; NF-κB: Nuclear factor-kappa B; T4SS: Type IV secretion system; uPA: Plasminogen activator; uPAR: Plasminogen activator receptor.
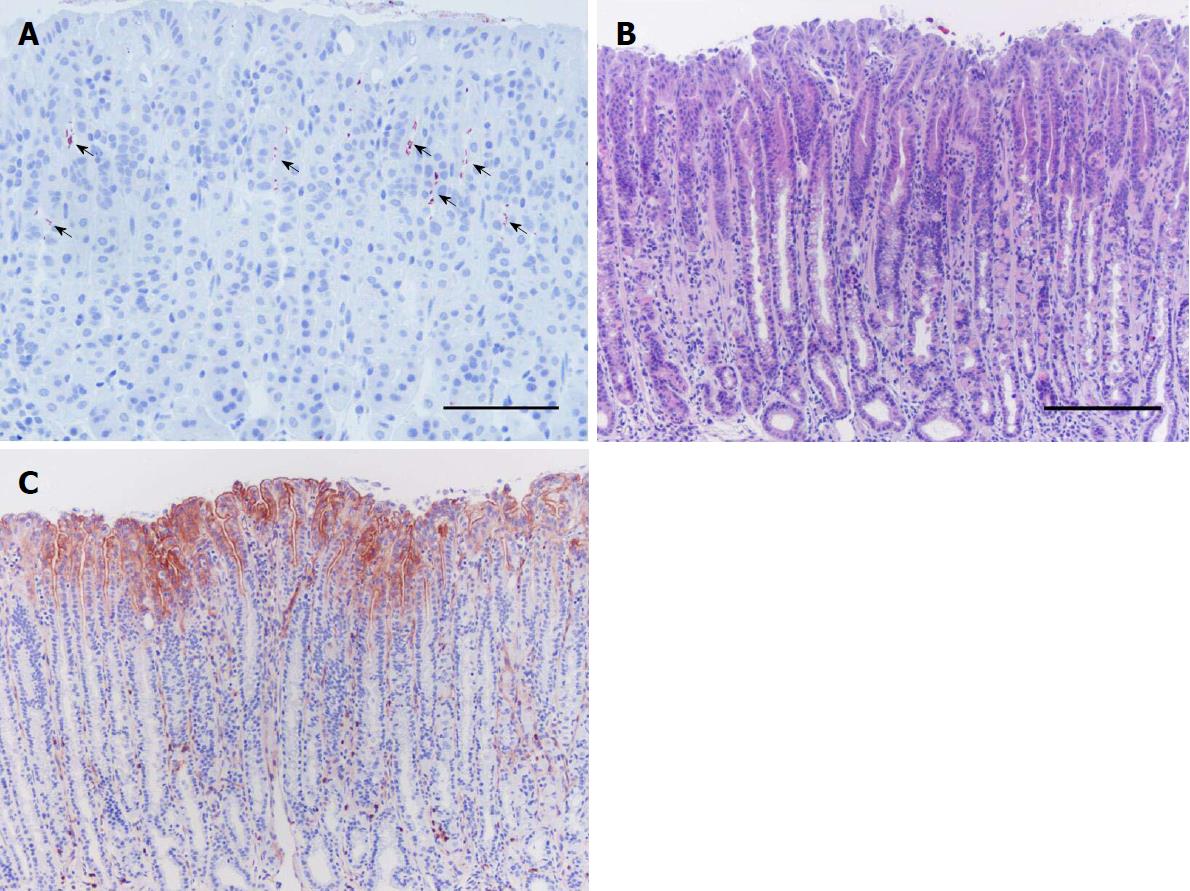
Figure 3 Plasminogen activator receptor induction in gastric epithelial cells in response to Helicobacter pylori infection.
Stomach tissue sections of a mouse infected with H. pylori and euthanized 14 wk after inoculation processed for immunohistochemistry against H. pylori (A) and uPAR (C), and with H&E staining (B). Clusters of H. pylori bacteria (arrows) are observed in the upper third of the gastric glands along the gastric epithelium of the mouse stomach (A). Histopathological alterations are seen, including inflammation and mucous metaplasia (B). uPAR expression becomes evident at the apical membrane of foveolar epithelial cells in the corpus epithelium of H. pylori-colonized mice, such as the representative immunohistochemistry staining shown here (C). uPAR-positive scattered neutrophils are seen in the microphotograph (C) since they constitutively express this molecule. Scale bars: A: 100 μm; B and C: 200 μm. H. pylori: Helicobacter pylori; H&E: Hematoxylin and eosin; uPAR: Plasminogen activator receptor.
- Citation: Molina-Castro S, Ramírez-Mayorga V, Alpízar-Alpízar W. Priming the seed: Helicobacter pylori alters epithelial cell invasiveness in early gastric carcinogenesis. World J Gastrointest Oncol 2018; 10(9): 231-243
- URL: https://www.wjgnet.com/1948-5204/full/v10/i9/231.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i9.231