Published online Dec 16, 2016. doi: 10.4253/wjge.v8.i20.777
Peer-review started: July 1, 2016
First decision: August 5, 2016
Revised: August 30, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: December 16, 2016
Processing time: 167 Days and 22.9 Hours
To estimate the efficacy of 2 h post-endoscopic retrograde cholangiopancreatography (ERCP) serum amylase levels and other factors for predicting post-ERCP pancreatitis.
This was a retrospective, single-center cohort study of consecutive patients who underwent ERCP from January 2010 to December 2013. Serum amylase levels were measured 2 h post-procedure, and patient- and procedure-related pancreatitis (PEP) risk factors were analyzed using a logistic model.
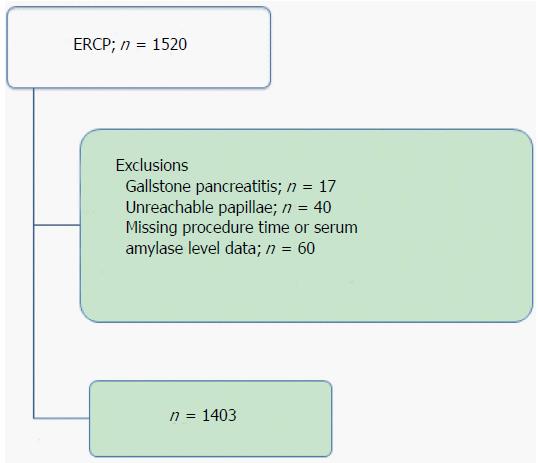
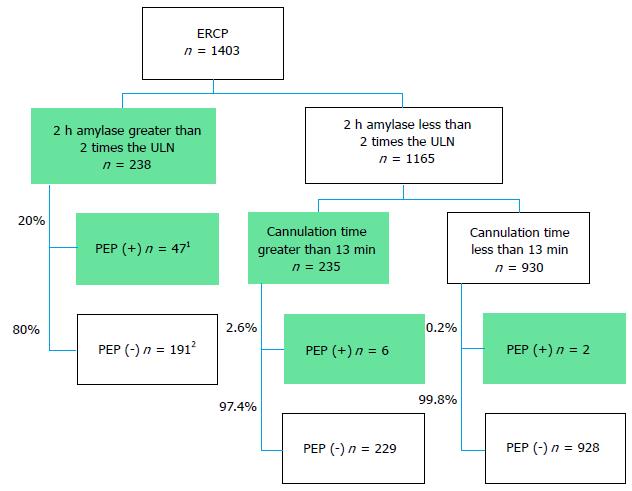
A total of 1520 cases (average age 72 ± 12 years, 60% male) were initially enrolled in this study, and 1403 cases (725 patients) were ultimately analyzed after the exclusion of 117 cases. Fifty-five of these cases developed PEP. We established a 2 h serum amylase cutoff level of two times the upper limit of normal for predicting PEP. Multivariate analysis revealed that a cannulation time of more than 13 min [odds ratio (OR) 2.28, 95%CI: 1.132-4.651, P = 0.0210] and 2 h amylase levels greater than the cutoff level (OR = 24.1, 95%CI: 11.56-57.13, P < 0.0001) were significant predictive factors for PEP. Forty-seven of the 55 patients who developed PEP exhibited 2 h amylase levels greater than the cutoff level (85%), and six of the remaining eight patients who developed PEP (75%) required longer cannulation times. Only 2 of the 1403 patients (0.14%) who developed PEP did not exhibit concerning 2 h amylase levels or require longer cannulation times.
These findings indicate that the combination of 2 h post-ERCP serum amylase levels and cannulation times represents a valuable marker for identifying patients at high risk for PEP.
Core tip: Serum amylase levels have a high negative predictive value (NPV; 95%-100%) and have therefore previously been used to predict post-endoscopic retrograde cholangiopancreatography pancreatitis (PEP) to facilitate patient discharges. However, the positive predictive value (PPV) of serum amylase is highly variable (4%-62%); therefore, a more useful PEP predictor is needed. In this retrospective study, we identified useful predictive factors via multivariate analysis and the combination 2 h amylase levels and cannulation times. The 2 h amylase levels exhibited a good NPV (99%) and a poor PPV (22%) similar to those of previous reports but exhibited a sensitivity of only 86% with respect to PEP detection. However, the combined use of the above two variables increased the sensitivity to 96%; thus, this combination may enable clinicians to detect patients at high risk for PEP during the early phase of treatment.
- Citation: Hayashi S, Nishida T, Shimakoshi H, Shimoda A, Amano T, Sugimoto A, Takahashi K, Mukai K, Matsubara T, Yamamoto M, Nakajima S, Fukui K, Inada M. Combination of two-hour post-endoscopic retrograde cholangiopancreatography amylase levels and cannulation times is useful for predicting post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastrointest Endosc 2016; 8(20): 777-784
- URL: https://www.wjgnet.com/1948-5190/full/v8/i20/777.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i20.777
Acute pancreatitis is a common post-endoscopic retrograde cholangiopancreatography (ERCP) complication and is therefore known as post-ERCP pancreatitis (PEP). PEP may result in procedure-related death and is often unpreventable. Moreover, no medications appear to be effective with respect to acute pancreatitis treatment[1,2]. Andriulli et al[3] conducted a systematic review of 21 selected surveys involving 16855 patients exhibiting a 3.5% incidence of PEP and observed that 0.11% of those patients died. Although many PEP prophylactic treatments have been reported[4-6], only prompt aggressive intravenous hydration is reportedly effective at reducing morbidity and mortality[7-10]. Therefore, early PEP identification is important, as it facilitates early intervention and may prevent disease progression and death.
Many studies have investigated the factors that increase the risk of PEP[7-10]. Those risk factors can generally be divided into the following two types: Patient-related factors and procedure-related factors. The patient-related risk factors for PEP reportedly include previous PEP, female gender, younger age, normal serum bilirubin levels, and the absence of chronic pancreatitis, whereas the procedure-related risk factors for PEP reportedly include cannulation attempt duration, pancreatic guidewire passage, pancreatic injection, precut sphincterotomy, biliary balloon sphincter dilatation, and failed bile duct stone clearance. No evidence exists indicating that hospital ERCP volume influences PEP occurrence[11,12]. The aforementioned risk factors synergistically increase PEP risk. Serum amylase levels less than 1.5 times the upper limit of normal (ULN) at 2-4 h post-ERCP have a very negative predictive value (NPV) for PEP. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend testing serum amylase or lipase levels 2-6 h after ERCP in patients presenting with pain. Patients exhibiting amylase or lipase values less than 1.5 and 4 times the ULN, respectively, may be discharged on the day of ERCP without concern regarding PEP risk[5]. However, very few tests with good positive predictive values (PPVs) for PEP exist. This study aimed to estimate the efficacy of 2 h post-ERCP serum amylase levels and other risk factors for predicting PEP.
This study was a retrospective single-center cohort study of consecutive hospitalized patients who underwent ERCP or ERCP-related procedures at Toyonaka Municipal Hospital, certified as a teaching hospital by the Japan Gastroenterological Endoscopy Society (JGES) (No. 1239), from January 2010 to December 2013. A total of 1520 procedures were enrolled in this study. Of these cases, 117 procedures with the following conditions were excluded: (1) gallstone pancreatitis, n = 17; (2) unreachable papillae, n = 40; and (3) missing procedure time or serum amylase level data, n = 60 (including cases with pancreatitis before ERCP). A total of 1403 procedures were ultimately analyzed in the present study (Figure 1).
The following demographic and clinical data were collected: Age and sex, ERCP indications, ERCP history, and 2 h post-ERCP serum amylase levels (after scope removal from the patient). The following procedural data were retrospectively collected from patient medical records: Biliary and pancreatic sphincterotomy with and without stent placement, procedure time, cannulation time, and complications. This study was approved by the Institutional Review Board of Toyonaka Municipal Hospital.
Trainees or experts performed ERCP because our hospital is a JGES-certified teaching hospital, and trainees were assisted by experts as needed to avoid complications and ensure procedural quality when performing ERPC. We did not use a strict cannulation protocol. Cannulation was attempted via the wire-loaded cannulation method, which entails the use of contrast and wire-guided cannulation using a side-viewing duodenoscope (JF260 V: Olympus Optical Co. Tokyo, Japan). Procedure times were measured using a stopwatch, and images were recorded at key points and subsequently reviewed. Patients underwent routine blood tests 2 h after the procedure and the following day and received routine protease inhibitor (200 mg gabexate mesilate × 2/d) treatments until the day after the procedure. No patients received rectal diclofenac or indomethacin for PEP prophylaxis during this period.
PEP was diagnosed based on consensus criteria[13]. Briefly, PEP was defined as the combination of abdominal pain persisting for at least 24 h after the procedure and a high serum amylase level equivalent to 3 times the ULN at 24 h after the procedure. Bleeding was defined as blood loss requiring emergency endoscopic hemostasis or a transfusion or a hemoglobin level decrease greater than 2 g/dL following ERCP. Perforation was diagnosed endoscopically during ERCP or based on the observation of free air on post-ERCP plain radiography or computed tomography. Procedure-related mortality was defined as any death within 30 d of ERCP.
Patient- and procedure-related PEP risk factors were analyzed via logistic regression using the following factors: Sex, native papilla, cannulation time, total procedure time, endoscopic nasobiliary drainage, endoscopic biliary stent (EBS) placement, precut sphincterotomy, endoscopic sphincterotomy (EST), endoscopic papillary balloon dilation (EPBD), pancreatic duct brush cytology, and 2 h amylase levels. Cannulation time was defined as the time from papilla identification until successful biliary cannulation, and procedure time was defined as the time from papilla identification until the scope was removed from the patient. PEP development was analyzed in relation to the following factors via univariate logistic regression: Patient-related factors (sex, age, and native papilla), procedure-related factors (cannulation time, total procedure time, endoscopic nasal pancreatic drainage, EBS, endoscopic metallic stent, endoscopic pancreatic stent, precut sphincterotomy, EST, EPBD, and pancreatic duct brush cytology), and 2 h post-ERCP amylase levels.
All continuous variables are expressed as the mean ± SD, except for the nonparametric variables, which are expressed as the median and range. Categorical variables are expressed as the number in each category or the frequency. Continuous variables were compared using student’s t test, whereas categorical variables were compared using a χ2 test or Fisher’s exact test when appropriate. Receiver operating characteristic (ROC) curve analysis was used to determine the 2 h amylase level cutoff, the cannulation times, and the procedure times for predicting PEP. Univariate and multivariate logistic regression analyses were performed to identify complication-related factors. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (ver. 11.1.1, SAS Institute Inc., Cary, NC, United States).
Patient characteristics are summarized in Table 1. A total of 1403 procedures (725 patients) were analyzed in the present study. The median age of the study population was 73 years, and 846 patients were male (60%). A total of 688 patients (59%) exhibited naive papillae. ERCP was performed for choledocholithiasis (n = 771); biliary malignancies from pancreatic cancer (n = 203); biliary malignancies from common bile duct cancer (n = 161); other biliary malignancies, including gallbladder cancer, intrahepatic bile duct cancer and other metastatic cancers (n = 158); and other conditions (n = 110). The median cannulation time was 5 min (range 1-185), and the median procedure time was 37 min (range 3-185 min). Primary cannulation was successful in 97.7% of cases. The median 2 h post-ERCP amylase level was 97 IU/L.
| Patients | n |
| Male, % | 846, 60% |
| Age, median (range) | 73 (12-99) |
| Native papilla | 668, 47.6% |
| Indication | |
| Malignancy | 522 |
| Choledocholithiasis | 771 |
| Others | 110 |
| Cannulation time, median (range) | 5 min (1-185) |
| Procedure time, median | 37 min (3-185) |
| 2 h amylase | 97 IU/mL (10-3502) |
| median (range) | |
| ERCP and related procedures | |
| Total ERCP | 1403 |
| ENBD | 362 |
| EBS | 380 |
| EMS | 42 |
| EPS | 124 |
| Precut | 35 |
| EST | 505 |
| EPBD | 20 |
| EPLBD | 38 |
| Pancreatic duct brush | 15 |
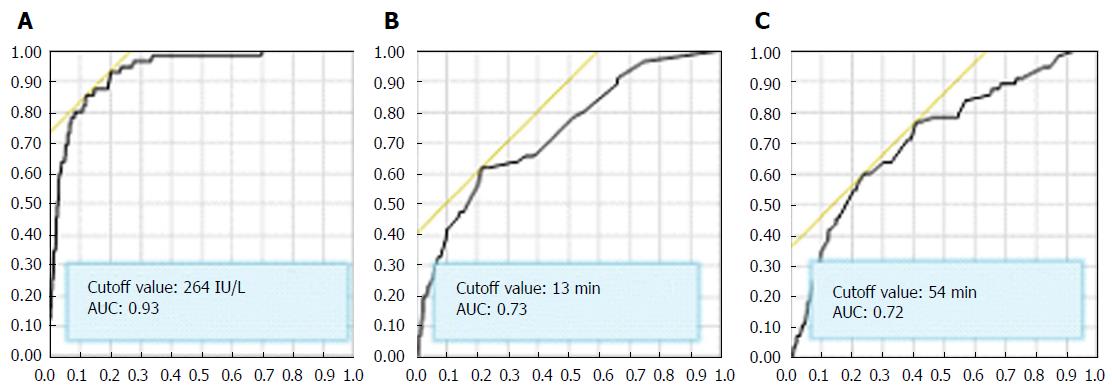
The overall complication rate was 4.8%. PEP developed in 55 patients (4.5%, 95%CI: 3.02-5.07), and perforation and bleeding occurred in 5 (0.35%, 95%CI: 0.15-0.83) and 8 patients (0.57%, 95%CI: 0.28-1.12), respectively (Table 2). All the patients who developed PEP improved with conservative therapy. The 2 h amylase cutoff value for predicting PEP was 264 IU/L (AUC: 0.93) (Figure 2) and remained 264 IU/L when limited to naïve papilla cases (n = 688). This cutoff level was 2.2 times the ULN at our hospital; thus, we established a serum amylase cutoff level of 2 times the ULN (240 U/L) for predicting PEP. Patients with an amylase level greater than 2 times the ULN (47/238, 19.8%) exhibited a significantly higher PEP rate than patients with a lower amylase level (8/1165, 0.7%) (P < 0.0001). Two-hour post-ERCP amylase levels greater than 2 times the ULN exhibited an NPV and a PPV for PEP of 99.3% and 19.8%, respectively.
| Complications | n, % (95%CI) |
| Bleeding | 8, 0.57 (0.28-1.12) |
| Perforation | 5, 0.35 (0.15-0.83) |
| Pancreatitis (severe pancreatitis) | 55, 3.9 (3.02-5.07) [3, 0.2 (0.073-0.64)] |
| Procedure-related death | 0, 0 |
The cannulation and procedure time cutoff values for predicting PEP were 13 (AUC: 0.93) and 54 min (AUC: 0.72), respectively (Figure 2), and similar results (13 and 55 min) were observed in naïve cases. Patients with cannulation times ≥ 13 min exhibited a significantly higher PEP rate (34/327, 10.4%) than patients with shorter cannulation times (21/1075, 2.0%) (P < 0.0001), and patients with procedure times ≥ 54 min exhibited a significantly higher PEP rate (33/359, 9.2%) than patients with shorter procedure times (22/1044, 2.1%) (P < 0.0001).
We analyzed the ability of patient- and procedure-related risk factors to predict PEP. Univariate analysis identified 10 significant predictive factors for PEP: Female sex, native papillae, cannulation time, total procedure time, EBSs, precut sphincterotomy, EST, EPBD, pancreatic duct brush cytology, and 2 h amylase levels (Table 3).
| Predictors | Odds ratio | 95%CI | P value |
| Sex (female) | 0.53 | 0.31-0.92 | 0.0245 |
| Native papilla | 5.62 | 2.73-11.6 | < 0.0001 |
| ENBD | 0.77 | 0.43-1.38 | 0.4313 |
| EBS1 | 2.62 | 1.18-5.85 | 0.0129 |
| EMS | 0.37 | 0.13-1.08 | 0.0784 |
| EPS | 0.47 | 0.22-1.00 | 0.0528 |
| Precut | 0.23 | 0.08-0.61 | 0.0102 |
| EST | 0.49 | 0.28-0.84 | 0.0099 |
| EPBD | 0.22 | 0.06-0.78 | 0.0405 |
| EPLBD | - | - | 0.3983 |
| Pancreatic duct brush | 6.42 | 1.75-23.5 | 0.0186 |
| 2-h amylase ≥ 2 times ULN | 36.6 | 17.6-76.3 | < 0.0001 |
| Cannulation time ≥ 13 min | 5.82 | 3.33-10.2 | < 0.0001 |
| Procedure time ≥ 54 min | 4.70 | 2.70-8.18 | < 0.0001 |
Multivariate analysis adjusted for age revealed that cannulation times longer than 13 min (OR = 2.28, 95%CI: 1.132-4.651, P = 0.0210) and 2 h amylase levels 2 times the ULN (OR = 24.1, 95%CI: 11.56-57.13, P < 0.0001) were significant predictive factors for PEP (Table 4).
| Predictors | Odds ratio | 95%CI | P value |
| Sex (female) | 1.46 | 0.77-2.75 | 0.2431 |
| Native papilla | 1.78 | 0.75-4.48 | 0.1908 |
| Endoscopic biliary stent | 0.61 | 0.23-1.45 | 0.2810 |
| Precut | 1.71 | 0.43-6.00 | 0.4288 |
| EST | 1.18 | 0.60-2.35 | 0.6278 |
| EPBD | 1.94 | 0.34-8.91 | 0.4296 |
| Pancreatic duct brush | 3.15 | 0.54-15.5 | 0.1870 |
| 2 h amylase ≥ 2 times ULN | 25.4 | 12.2-59.9 | < 0.0001 |
| Cannulation time ≥ 13 min | 2.63 | 1.34-5.23 | 0.0051 |
| Procedure time ≥ 54 min | 1.23 | 0.389-3.67 | 0.7183 |
The consensus PEP definition and severity grading system developed by Cotton et al[13] has been used for more than 20 years, but PEP remains a primary concern for endoscopists performing ERCP, as it is the most frequent post-ERCP complication, with an incidence of 3.5% in unselected patients[3,5]. Approximately 90% of cases are of mild-to-moderate in severity; however, PEP results procedure-related death in 3% of PEP cases[3]. Many prophylactic treatments have been reported, and the most recent ESGE guidelines recommend rectal NSAID administration for PEP prophylaxis[5]. However, PEP is difficult to prevent, and few medications are effective at treating PEP once it develops. Only prompt aggressive intravenous hydration is reportedly effective with respect to decreasing morbidity and mortality[2,7,8,10]. Appropriate and early fluid therapy can mitigate PEP severity[14]; therefore, PEP must be diagnosed, and treatment must be initiated during the early phase of the disease to prevent severe acute pancreatitis development and progression.
Numerous studies have identified factors that increase PEP risk. Among these factors, the measured amylase levels after ERCP have been evaluated for the prediction of PEP[15-17]. Many reports have shown the effectiveness of the 2-8 h amylase measurement. Generally, the NPVs are 95%-100%, the PPVs are 4%-62%, the sensitivity values are 23%-100% and the specificities are 63%-98%, although some differences in the definition of PEP and amylase cutoff levels exist across studies (Table 5).
| Ref. | Year | n | Time1(h) | Amylasecut off | Sensitivity | Specificity | PPV | NPV | Definition of PEP |
| LaFerla et al[23] | 1986 | 20 | 2 | 800 | n.d. | n.d. | n.d. | Unlikely | Amy > 1200 |
| Gottlieb et al[24] | 1996 | 231 | 2 | 276 | 82 | 76 | 15 | 98 | Consensus criteria |
| Testoni et al[25] | 1999 | 409 | 2 | 5 × | 23.1 | 98.2 | 46.2 | 94.9 | Amy > 5 × ULN |
| 4 | 5 × | 53.8 | 95 | 42.4 | 96.8 | ||||
| 8 | 5 × | 76.9 | 96.9 | 62.5 | 98.4 | ||||
| Testoni et al[26] | 2001 | 1185 | 6-8 | 3 × | n.d. | n.d. | n.d. | 100 | Pancreatic type pain |
| Thomas et al[27] | 2001 | 263 | 4 | 2 × | 90 | 92.9 | 24.3 | 99.6 | Consensus criteria |
| 4 | 3 × | 70 | 95.3 | 36.8 | 98.8 | ||||
| Kapetanos et al[28] | 2007 | 97 | 2 | 3 × | 72 | 79 | 32 | 95 | Consensus criteria |
| 6 | 3 × | 82 | 75 | 30 | 97 | ||||
| Ito et al[16] | 2007 | 1291 | 3 | 3 × | 77 | n.d. | 29 | n.d. | Amy > 1 × ULN, |
| 6 | 3 × | 85 | n.d. | 24 | n.d. | with pain at 24 h | |||
| Nishino et al[29] | 2009 | 1631 | 4 | 3 × | 89.8 | 72.9 | 12.7 | 99.4 | Consensus criteria |
| 4 | 4 × | 84.7 | 80.4 | 16 | 99.2 | ||||
| Artifon et al[30] | 2010 | 300 | 4 | 1.5 × | 77 | 63 | 26 | 94 | Consensus criteria |
| Sutton et al[15] | 2011 | 959 | 4 | 2.5 ×2 | 80 | 80.4 | 11.1 | 99.2 | Consensus criteria |
| 4 | 2.5 ×3 | 100 | 91.8 | 4.3 | 100 | (mod/severe only) | |||
| Our study | 2015 | 1403 | 2 | 2 × | 85.5 | 85.8 | 19.8 | 99.3 | Consensus criteria |
| 2 | 2 ×4 | 96.4 | 68.8 | 11.2 | 99.8 |
Consequently, the ESGE guidelines indicate that 2-4 h amylase levels have very high NPVs but do not demonstrate sufficient PPVs (evidence level 2+)[4] and therefore recommend measuring serum amylase or lipase levels 2-6 h after ERCP in patients presenting with pain who are to be discharged on the day of their ERCP procedure (recommendation grade B). In this study, 2 h amylase levels exhibited a good NPV of 99% and a poor PPV of 20%, findings consistent with the above results, as well as a good sensitivity (84%) for the diagnosis of PEP. Previous studies have reported values of 70%-90%, particularly studies using the Consensus Criteria PEP definition. A PPV of 20% is not sufficient to identify PEP but may be suitable for identifying patients at high risk for developing PEP. Moreover, 2 h amylase levels may enable clinicians to identify high-risk patients requiring early acute PEP treatments, such as infusion therapy.
Previous studies have demonstrated that difficult cannulation is a risk factor for PEP[12,18,19]. Tian et al[20] reported that cannulation time is a more accurate measure of cannulation difficulty in ERCP than other parameters. Moreover, Halttunen et al[21] reported that cannulation attempts lasting > 5 min may increase the incidence of PEP and that procedures lasting less than 5 min had a lower PEP rate (2.6%) than longer procedures (11.8%). The most recent ESGE guidelines state that PEP risk factor analyses have demonstrated that cannulation attempts lasting > 10 min had an odds ratio (OR) of 1.76 (1.13-2.74) with respect to PEP development and that the pooled incidences of PEP in patients with and without this risk factor were 10.8% and 3.8%, respectively. ROC curve analysis was performed in the present study and demonstrated that the cannulation and the procedure time cutoff values for predicting PEP were 13 (AUC: 0.93) and 54 min (AUC: 0.72), respectively. The incidences of PEP in patients with and without cannulation attempts lasting > 13 min were 10.4% and 2.0%, respectively, and the incidences of PEP in patients with and without cannulation times lasting > 10 min were 9.6% and 2.1%, respectively (data not shown), findings similar to those reported by Halttunen et al[21]. Multivariate analysis indicated that cannulation time is another significant PEP risk factor; therefore, we propose that cannulation time is a reliable marker for predicting PEP, in addition to 2 h post-ERCP amylase levels.
Based on above findings, we used the following markers to predict PEP development: 2 h post-ERCP amylase levels greater than 2 times the ULN and cannulation times greater than 13 min. Figure 3 includes a flowchart depicting these markers. A total of 238 patients (17%) in the present study exhibited 2 h post-ERCP amylase levels greater than 2 times the ULN, 47 of whom (20%) developed PEP, whereas a total of 1165 patients (83%) exhibited 2 h post-ERCP amylase levels less than 2 times the ULN. Eight patients (0.7%) in the latter group developed PEP; however, six of these patients required more than 13 min for cannulation. Thus, only 2 of the 1403 patients (0.14%) who developed PEP did not exhibit concerning 2 h post-ERCP amylase levels or require longer cannulation times. This study demonstrated that cannulation time inclusion may rescue 75% (6/8) of patients with non-concerning 2 h amylase levels and that the combination of 2 h post-ERCP levels and cannulation times exhibited a 96% sensitivity and an 11.2% PPV for the identification of PEP. The latter percentage is not sufficient to identify PEP but may be useful for identifying high-risk patients in whom early treatments, such as aggressive infusions, are necessary.
The present study had several limitations because of its retrospective design. Routine protease inhibitor administration without rectal diclofenac or indomethacin administration may have influenced the frequency of PEP. However, nonsteroidal anti-inflammatory drugs (NSAIDs) were reportedly used infrequently for PEP prevention in clinical practice in Japan until the publication of the 2015 Japanese Guideline[22], which recommends prophylactic NSAID administration to prevent PEP. In addition, we did not strictly evaluate certain PEP risk factors, such as the number of cannulation attempts, pancreatic guidewire, and pancreatic injection, because of the retrospective design of this study. The number of cannulation attempts represents the degree of cannulation difficulty; the most recent ESGE guidelines recommend keeping this number as low as possible[21]. The degree of cannulation difficulty during ERCP is positively correlated with PEP[18]. The degree of cannulation difficulty during ERCP procedures may differ when different methods are used (total cannulation time vs number of attempts); thus, grading scales used to evaluate the difficulty of performing ERCP via different methods should not be used interchangeably. Tian et al[20] reported that cannulation time is a more objective and accurate means of grading cannulation difficulty than the number of papilla cannulation attempts. The ESGE guidelines categorize pancreatic guidewire use and pancreatic injection as definite PEP risk factors. However, it is sometimes difficult to establish if either procedure has been performed, particularly cannulation, which is performed via contrast and wire-guided methods at our institution. In addition, the ESGE guidelines recommend that prophylactic pancreatic stent placement should be strongly considered in patients at high risk for PEP. Prophylactic pancreatic stents were placed in 124 patients in the present study, 9 of whom (7.3%) developed PEP. However, multivariate analysis demonstrated that stent placement did not significantly prevent PEP, perhaps because pancreatic stents tend to be used in patients at high risk for PEP, in accordance with the above guidelines. Therefore, we must target patients at high risk for PEP to evaluate the efficacy of prophylactic pancreatic stent placement. Because of the above limitations, in the present study, we evaluated cannulation time and procedure time as surrogate markers of procedure-related risk factors in the present study. Despite these limitations, we believe that this study has effectively demonstrated that Two-hour post-ERCP amylase levels and cannulation times are useful PEP predictors.
In conclusion, 2 h post-ERCP serum amylase levels and cannulation times may be useful markers for predicting PEP development. We plan to conduct prophylactic interventions to reduce the incidence of PEP in high-risk patients exhibiting 2 h post-ERCP amylase levels greater than 2 times the ULN or requiring cannulation times greater than 13 min.
Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) may result in procedure-related death and is often unpreventable. So it is important to predict and treat in early phase.
Post-ERCP serum amylase levels are known as a predictor of PEP, which have good negative predictive value (NPV) and poor positive predictive value (PPV). The aim of this study was to estimate the efficacy of post-ERCP 2 h serum amylase levels and other factors for predicting PEP.
The 2-h amylase levels exhibited a good NPV (99%) and a poor PPV (22%) similar to previous reports but exhibited a sensitivity of 86%, and the combined use with cannulation time increased the sensitivity to 96%.
Combination of Two-hour post-ERCP amylase levels and cannulation times may be simple useful markers for predicting PEP development in early phase.
PEP is one of the major adverse events of ERCP. It is most frequent and sometimes results in death, so that it has been the most concern still now.
This retrospective study was performed to identify the risk factors for PEP, and the authors revealed that two factors of serum amylase levels 2 h after ERCP and cannulation time were significant independent factor. This is well designed study which revealed interesting results.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Altonbary AY, Ikeuchi N, Isaji S, Kitamura K, Kikuyama M, Paduani GF S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 2. | Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1148] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 3. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 770] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 4. | Dumonceau JM, Andriulli A, Deviere J, Mariani A, Rigaux J, Baron TH, Testoni PA. European Society of Gastrointestinal Endoscopy (ESGE) Guideline: prophylaxis of post-ERCP pancreatitis. Endoscopy. 2010;42:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Dumonceau JM, Andriulli A, Elmunzer BJ, Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C, Testoni PA. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy. 2014;46:799-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 398] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 6. | Wong LL, Tsai HH. Prevention of post-ERCP pancreatitis. World J Gastrointest Pathophysiol. 2014;5:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Sagi SV, Schmidt S, Fogel E, Lehman GA, McHenry L, Sherman S, Watkins J, Coté GA. Association of greater intravenous volume infusion with shorter hospitalization for patients with post-ERCP pancreatitis. J Gastroenterol Hepatol. 2014;29:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Gardner TB, Vege SS, Chari ST, Petersen BT, Topazian MD, Clain JE, Pearson RK, Levy MJ, Sarr MG. Faster rate of initial fluid resuscitation in severe acute pancreatitis diminishes in-hospital mortality. Pancreatology. 2009;9:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1415; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1377] [Article Influence: 114.8] [Reference Citation Analysis (3)] |
| 10. | Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, Burchard PR, Gordon SR, Gardner TB. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 11. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 779] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 12. | Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, Veitch P, Wilkinson ML, Williamson PR. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy. 2007;39:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2035] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 14. | DiMagno MJ, Wamsteker EJ, Maratt J, Rivera MA, Spaete JP, Ballard DD, Elmunzer J, Saini SD. Do larger periprocedural fluid volumes reduce the severity of post-endoscopic retrograde cholangiopancreatography pancreatitis? Pancreas. 2014;43:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Sutton VR, Hong MK, Thomas PR. Using the 4-hour Post-ERCP amylase level to predict post-ERCP pancreatitis. JOP. 2011;12:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J, Takasawa O, Obana T. Relationship between post-ERCP pancreatitis and the change of serum amylase level after the procedure. World J Gastroenterol. 2007;13:3855-3860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Sultan S, Baillie J. What are the predictors of post-ERCP pancreatitis, and how useful are they? JOP. 2002;3:188-194. [PubMed] |
| 18. | Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 835] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 19. | Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 329] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 20. | Tian C, Gamboa A, Chaudhury B, Willingham FF, Keilin S, Cai Q. Cannulation time is a more accurate measure of cannulation difficulty in endoscopic retrograde cholangiopancreatography than the number of attempts. Gastroenterol Rep (Oxf). 2013;1:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Halttunen J, Meisner S, Aabakken L, Arnelo U, Grönroos J, Hauge T, Kleveland PM, Nordblad Schmidt P, Saarela A, Swahn F. Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand J Gastroenterol. 2014;49:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22:405-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | LaFerla G, Gordon S, Archibald M, Murray WR. Hyperamylasaemia and acute pancreatitis following endoscopic retrograde cholangiopancreatography. Pancreas. 1986;1:160-163. [PubMed] |
| 24. | Gottlieb K, Sherman S, Pezzi J, Esber E, Lehman GA. Early recognition of post-ERCP pancreatitis by clinical assessment and serum pancreatic enzymes. Am J Gastroenterol. 1996;91:1553-1557. [PubMed] |
| 25. | Testoni PA, Caporuscio S, Bagnolo F, Lella F. Twenty-four-hour serum amylase predicting pancreatic reaction after endoscopic sphincterotomy. Endoscopy. 1999;31:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Testoni PA, Bagnolo F. Pain at 24 hours associated with amylase levels greater than 5 times the upper normal limit as the most reliable indicator of post-ERCP pancreatitis. Gastrointest Endosc. 2001;53:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Thomas PR, Sengupta S. Prediction of pancreatitis following endoscopic retrograde cholangiopancreatography by the 4-h post procedure amylase level. J Gastroenterol Hepatol. 2001;16:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Kapetanos D, Kokozidis G, Kinigopoulou P, Xiarchos P, Antonopoulos Z, Progia E, Kitis G. The value of serum amylase and elastase measurements in the prediction of post-ERCP acute pancreatitis. Hepatogastroenterology. 2007;54:556-560. [PubMed] |
| 29. | Nishino T, Toki F, Oyama H, Shiratori K. More accurate prediction of post-ERCP pancreatitis by 4-h serum lipase levels than amylase levels. Digest Endosc. 2008;20:169-177. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Artifon EL, Chu A, Freeman M, Sakai P, Usmani A, Kumar A. A comparison of the consensus and clinical definitions of pancreatitis with a proposal to redefine post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2010;39:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |