INTRODUCTION
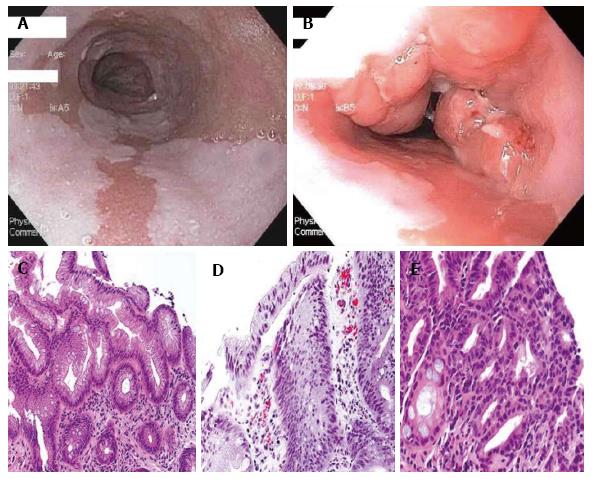
Barrett’s esophagus (BE) is defined by the “American Gastroenterological Association” (AGA) as “a condition in which any extent of metaplastic columnar epithelium that predisposes to cancer development replaces the stratified squamous epithelium that normally lines the distal esophagus”, (Figure 1)[1]. The existence of intestinal metaplasia (IM) in the esophagus predisposes to development of esophageal adenocarcinoma and BE has become a well-recognized and treatable condition. The estimates of progression of non-dysplastic BE to adenocarcinoma are variable but uniformly low, ranging from 0.12% to as high as 2.9% per year, with more recent studies reporting lower rates of progression, generally less than 0.5% per year[2,3]. However, the incidence of progression to adenocarcinoma in patients with BE with dysplasia is up to five times as high as in non-dysplastic BE[2]. The presence of high-grade dysplasia (HGD, Figure 1) in BE portends a significant risk of progression to adenocarcinoma, calculated to be up to a 6% annual risk in one meta-analysis[3].
Figure 1 Histopathology pictures.
A: White-light endoscopic image of long segment BE; B: White-light endoscopic image of BE with nodular mucosa found to be HGD; C: Hematoxylin and eosin (HE) stain of Barrett’s mucosa; D: HE of Barrett’s mucosa with LGD; E: Barrett’s mucosa with HGD. Histopathology pictures courtesy of Purva Gopal, MD, Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas. HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BE: Barrett’s esophagus.
The need for non-invasive strategies to treat dysplasia in patients with BE has become an impetus for gastrointestinal endoscopists to develop new and effective endoscopic techniques. In this paper, we review the different options for treatment of dysplasia in BE, with a focus on endoscopic treatment of HGD.
SURGICAL TREATMENTS
In the past, the gold standard of therapy for HGD was esophagectomy, a procedure with well-recognized morbidity and perioperative mortality as high as 10%[4,5]. More recently, laparoscopic approaches and techniques such as the transhiatal esophagectomy have become more common. These techniques have lower morbidity than some of the older surgical techniques, including reduced hospital length of stay, fewer major complications, and less post-operative dumping syndrome[6,7]. Surgical therapy is a valid curative option for patients in whom there is suspicion of cancer invading the submucosa or if lymph node metastases are present. In patients with early esophageal adenocarcinoma, up to 20% of patients with cancer involving the submucosa will have lymph node metastases, with the risk increasing further with growth of the tumor into the deeper submucosa. In contrast, the risk of lymph node metastases in patients with intramucosal adenocarcinoma (i.e., not invading the submucosa) is much lower at less than 2%[8].
While endoscopic therapy of HGD has become increasingly common, esophagectomy is still an option for patients. The AGA and American Society of Gastrointestinal Endoscopists (ASGE) still acknowledge esophagectomy as a therapeutic option in appropriate patients with BE and HGD, while the American College of Gastroenterology (ACG) guidelines on BE state that esophagectomy is no longer the necessary treatment response to HGD[1,9,10].
ENDOSCOPIC TREATMENTS
For patients with HGD limited to the esophageal mucosa, endoscopic eradication has become the mainstay of therapy. Multiple modalities compatible with endoscopy have been studied including both mechanical removal of tissue and ablative techniques. Methods that involve tissue resection include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). The ablative techniques include several older techniques such as photodynamic therapy (PDT), laser therapy with Nd:YAG (neodymium-doped yttrium aluminum garnet; Nd:Y3Al5O12) or potassium titanyl phosphate (KTP) lasers, multipolar electrocoagulation (MPEC), argon plasma coagulation (APC), and newer techniques such as cryotherapy and radiofrequency ablation (RFA). These therapies are tailored to the type of HGD present, specifically whether the dysplasia is visible, raised, discolored or nodular; features which have been associated with higher rates of malignancy compared to flat mucosa[11]. It is important to note that all the endoscopic treatments described below require acid suppression therapy for success, namely proton pump inhibitor (PPI) therapy.
EMR AND ESD
EMR, initially developed in Japan for treatment of superficial squamous cell esophageal carcinoma, is now the treatment of choice for nodular HGD in the esophagus[12]. It is also considered helpful diagnostic tool to evaluate for adenocarcinoma invading the submucosa, as well as to determine whether mucosal nodules harbor dysplasia. EMR is useful in staging, as illustrated by Wani et al[13]’s study which found that in patients with BE and dysplasia or early cancer, EMR resulted in upstaging of the diagnosis in 10% of patients and downgrading of the diagnosis in 21%. The two main EMR techniques are use of an endoscopic resection cap (ER-cap) (Olympus, Tokyo, Japan), which varies in terms of shape and texture and a multi-band ligator (Wilson-Cook, Indianapolis, United States) used for multiband mucosectomy (MBM). A diathermic snare is used for resection in both techniques. A submucosal lift with saline or a more viscous solution such as hydroxypropyl methylcellulose (artificial tears) can also be employed prior to resection when using the ER-cap method and is sometimes used with MBM. Pouw et al[14] performed a randomized controlled trial comparing of ER-cap and MBM and found that MBM was less costly and resulted in fewer acute complications without any significant difference in the depth of tissue resected.
EMR has been shown to be safe and effective as monotherapy for eradication of HGD in several studies. The reported rates of remission from HGD after EMR range from 87%-96% with median follow-up of 22-28 mo[15-17]. The long term remission rate and the durability of EMR as a solo modality for treatment are not currently known; therefore, these patients should be maintained in a surveillance endoscopy program. Complications of EMR include bleeding, perforation, and most commonly stricture formation. The frequency of stricture development reported in EMR studies varies widely, from 12.5% to 88%, depending on the extent of EMR and number of sessions[15-17]. For the majority of patients, post-EMR strictures are easily treated with endoscopic dilation techniques. In general, the smaller the area of resection, the lower the likelihood of stricture formation[17].
ESD is a safe and effective therapy for early gastric cancers and large dysplastic colon polyps[18,19]. Technically, the procedure differs from EMR in that a specialized ESD knife is used to access the submucosal space and dissect the superficial lesion away from the submucosa. As with EMR, a cushion of fluid is first injected to lift the lesion of interest and protect the esophageal wall from deeper penetration of the ESD knife. This fluid typically contains a viscous agent to allow for a sustained lift and a dye to help identify tissue planes for appropriate dissection[20]. The rationale for using ESD is that this technique can allow for a larger and more precise area of dysplastic tissue removal than EMR can safely target.
ESD has recently been evaluated in the management of BE with HGD and early adenocarcinoma. A German group reported a 77% curative resection rate in a small group of patients with a recurrence rate of 5.9% in two years follow-up. The complication rate was 27% for this group of patients and included one perforation and three strictures[21]. A retrospective analysis of 70 Belgian patients who underwent ESD reported a curative resection rate of 64% for patients with HGD and 85% for patients with early adenocarcinoma. At a median follow-up of 20 mo, 92% of patients retained remission from neoplasia. Strictures formed in 60% of patients and these were managed endoscopically[22]. The technique of ESD requires specific training and is only safe in qualified hands in high volume centers. At this time, the ASGE is the only major United States GI society that recognizes ESD as a potential treatment for visible HGD[10].
PDT
PDT is a technique for endoscopic ablation using either 5-aminolevulinic acid or porfimer sodium as a photosensitizing agent followed by exposure to laser light, which causes a photochemical reaction, damaging both mucosal and deeper tissues. The largest study of PDT was a randomized clinical trial evaluating PDT plus omeprazole vs omeprazole alone, which showed that patients treated with PDT had a HGD eradication rate of 77% compared to 39% in the omeprazole-alone group. With 5-year follow-up 15% of patients treated with PDT had progressed to cancer, compared to 29% in the omeprazole group[23]. In one longer-term follow-up study of 66 patients with HGD and early adenocarcinoma who underwent PDT, in the calculated 5-year survival was 97% in patients with HGD and 80% in those with early adenocarcinoma without significant long-term complications[24]. Currently, all three major United States societies mention PDT as an option for ablating HGD in BE[1,9,10].
LASER THERAPIES
Nd:YAG and KTP laser-derived thermal therapies have also been evaluated as a treatment tool for HGD in BE. Both Nd:YAG and KTP are crystals that when used in lasers produce wavelengths of light that can damage tissue, such as dysplastic BE. These lasers have typically been studied in tandem with one another or combined with another mode of therapy. Sharma et al[25] reported a series of seven patients with BE and HGD who were not surgical candidates who underwent combination therapy with Nd:YAG laser and monopolar electrocautery. The dysplasia was eradicated in all seven with only residual metaplasia in three patients over a mean follow-up of 3.4 years. Nd:YAG-enhanced KTP laser was also shown to be safe and effective in pilot study of 10 patients with 100% eradication of dysplasia on follow-up esophageal biopsies and no recurrence on average follow-up of 10 mo[26]. Laser treatment is rarely used at this time as other therapies have become more popular.
APC AND MULTIPOLAR ELECTROCOAGULATION
APC is another form of endoscopic thermal therapy using the medium of argon gas to conduct electrical current leading to tissue destruction. The therapy is performed via a catheter that fits through the endoscope working channel. MPEC utilizes electrical current through an endoscopic catheter to cause localized tissue destruction. One prospective trial compared APC and MPEC for treatment of dysplastic BE and found no statistical difference in either endoscopic or histologic eradication of dysplasia[27]. However, MPEC required significantly fewer endoscopic therapy sessions with a trend toward better histologic eradication. There were no serious adverse events but 8% of patients treated with MPEC and 13% of patients treated with APC experienced transient upper GI symptoms. While APC is not typically used as a solo modality for treatment of BE and dysplasia, APC can be used to treat small areas of residual BE. In one study of patient with BE and HGD who underwent mucosectomy, treatment of residual disease with APC was found to prolong recurrence-free survival[28].
CRYOTHERAPY
The goal of this endoscopic therapy is to use freeze-thaw cycles for the destruction of tissue. Cryotherapy is performed using low-pressure liquid nitrogen (CSA Medical, Maryland, United States) or carbon dioxide (GI Supply, Pennsylvania, United States) delivered via spray catheter. One of the earlier prospective studies of cryotherapy found a 94% eradication rate for HGD with complications including chest pain and dysphagia, as well as one gastric perforation[29]. Recently, a prospective cohort study of 96 patients (two-thirds of whom had HGD) underwent cryotherapy, resulting in a complete eradication rate of 81% for HGD. Only three patients developed a stricture in the 37 mo of follow-up[30]. The durability of cryotherapy in preventing disease recurrence has come into question. Halsey et al[31] published data suggesting that up to 30% of patients treated with cryotherapy experienced disease recurrence at a median of 6.5 mo, and 10% had a second recurrence. However, a more recent single center retrospective cohort reported a HGD eradication rate of 100% with sustained remission in 97% of patients with previous HGD over a range of 24-57 mo[32]. At this time, only in the ASGE guidelines is cryotherapy specifically mentioned as a treatment option for dysplasia in BE[10].
RFA
RFA has emerged as the ablative technique of choice for BE with HGD because of the quality of evidence to support the ease of its administration, its efficacy, and safety profile. The procedure involves the direct application of radiofrequency energy to the esophageal mucosa, using either a balloon for circumferential treatment and more focal treatment through an attachment to the end of the endoscope or a small catheter that can pass through the working channel (Barxx/Covidien, Dublin, Ireland). With these tools, RFA can be applied to the mucosa circumferentially or focally. In the landmark multicenter sham-controlled randomized controlled trial by Shaheen et al[33], RFA resulted in eradication of dysplasia in 81% of patients with HGD. The treatment also decreased the progression of dysplasia to cancer. Complications were rare in this study, with only a 6% rate of stricture formation over 12 mo of follow-up[33]. RFA has also been shown to be successful in eradicating persistent dysplasia after initial therapy with PDT. In one study, RFA used as rescue therapy after PDT treatment successfully eradicated residual HGD in 86% of patients[34].
For some patients with BE, multiple endoscopic therapies are required for treatment. RFA is most effective on smooth BE mucosa, and is not adequate treatment for nodular dysplasia. As a result, endoscopists have been combining endoscopic eradication therapies, most commonly EMR and RFA. With combination therapy, visible or nodular dysplasia can be precisely removed with EMR, and any residual dysplasia or metaplasia can be systematically treated with RFA, typically performed after the EMR site has healed. One retrospective study of combination therapy reported an 86% complete eradication rate of HGD, but complete eradication of only 62% of nondysplastic intestinal metaplasia[35]. More recently, a multicenter prospective trial in Europe (EURO II) evaluated the efficacy and safety of such a treatment strategy. EMR was performed on visible abnormalities within the BE segment and the remaining visible Barrett’s mucosa was treated with RFA 6 wk later. Patients underwent a median of two RFA sessions. This combination of procedures achieved a 92% complete eradication rate for HGD and neoplasia and complete eradication of intestinal metaplasia in 87% of patients. At 36 mo of follow-up, only 4% of patients had recurrence of neoplasia. There were no major complications from the procedures and the rate of esophageal stenosis rate was 6%[36].
The existing evidence for treatment of low-grade dysplasia (LGD, Figure 1) in BE (most often with RFA) is less abundant than studies of patients with BE and HGD. However, a recent randomized clinical trial (the SURF trial) showed a significantly lower rate of progression of LGD to either HGD or adenocarcinoma over three years after RFA[37]. Complicating the decision to ablate LGD is the fact that there is significant disagreement between pathologists on the definition of LGD. Several studies have highlighted the discrepancy in pathologist interobserver agreement when evaluating specimens with LGD. In one such study, expert pathologist confirmed only 15% of previously diagnosed LGD[38]. The AGA recommends RFA as therapy for BE with LGD based on high quality evidence while the ASGE dictates that RFA should be considered as therapy for LGD, and ACG acknowledges the effectiveness of RFA for LGD[1,9,10].
RISK OF RECURRENCE AFTER ENDOSCOPIC THERAPY
Recurrence after endoscopic therapy is a concern for gastroenterologists treating patients with dysplastic BE and the rates of recurrence vary widely depending on the study. Gupta et al[39] noted that IM returned in up to 33% of patients at 2 years after endoscopic therapy including RFA. A smaller percentage of recurrent IM was dysplastic (22%). The investigators were unable to identify any predictors for recurrence in this particular population of patients[39]. Other groups have tried to define predictors for recurrence of IM after definitive ablative therapy. In one recent large retrospective analysis, researchers found a slightly lower recurrence rate of 20% at 2.4 years for either IM or dysplasia. These investigators were able to identify risk factors for recurrence of BE and neoplasia, which included a worse pre-treatment histology, older age, and longer BE segments[40]. A single-center retrospective analysis of patients who achieved complete eradication of both IM and dysplasia with RFA found the one-year recurrence rate of IM to be 25% while dysplasia recurred in 8.5% of patients[41]. In contrast, a systematic review and meta-analysis of prospective and retrospective studies of RFA found that recurrence of dysplasia and IM was much lower after RFA treatment, with a 0.9% pooled recurrence rate for dysplasia and a 13% rate of recurrence for IM with an average follow-up of 1.5 years. There was wide range of IM recurrence rates reported in this study, ranging from 8% to 21%[42].
ENDOSCOPIC THERAPY OF NON-DYSPLASTIC BE
The debate rages on in the world of BE whether ablation of non-dysplastic Barrett’s esophagus (NDBE) should be performed. Endoscopists advocating ablation of NDBE extrapolate the success of RFA in patients with HGD and LGD, applying these findings to non-dysplastic metaplasia. Another argument favoring ablation of NDBE is the lack of randomized controlled trials showing that surveillance of BE reduces mortality from esophageal adenocarcinoma, and thus other interventions should be considered[43,44]. Endoscopists who argue against ablation of NDBE focus on the lack of high quality evidence available to support such a notion and the very low rates of progression to cancer reported for non-dysplastic BE. Other issues proposed in the argument against ablation of nondysplastic BE include issues related to subjecting large numbers of patients to multiple endoscopic procedures, and the associated costs of the procedures and risk of complications[45]. One other argument against ablation of non-dysplastic BE is the possibility of missing subtle nodularity or mucosal changes that would be optimally treated with EMR, and instead burying it with suboptimal RFA therapy[45,46]. More prospective randomized controlled trials are needed to study the utility of RFA and other endoscopic therapies to treat NDBE. The AGA and ASGE mention that RFA could be considered for selected patients with NDBE thought to be at increased risk of progression to HGD and cancer[1,10].
CONCLUSION
The treatment options for HGD in BE have evolved into less-invasive therapies. There are now highly effective endoscopic therapies that are less morbid than esophagectomy. Most patients are treated with a combination of endoscopic resection and RFA with good outcomes. However, it is the job of the gastrointestinal endoscopist to be vigilant in surveillance for possible dysplasia recurrence in these patients. We have not yet reached the point where a patient can be told he or she has experienced complete eradication with no possibility of recurrence, and all patients should remain in surveillance. Until that time comes, we will continue to sharpen the endoscopic tools that will help us along the way to a durable cure.
P- Reviewer: Anadol Z, dos Santos JS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ