Published online Aug 10, 2015. doi: 10.4253/wjge.v7.i10.950
Peer-review started: January 29, 2015
First decision: April 13, 2015
Revised: May 7, 2015
Accepted: July 11, 2015
Article in press: July 14, 2015
Published online: August 10, 2015
Processing time: 200 Days and 15.9 Hours
Familial adenomatous polyposis (FAP) is a hereditary disorder caused by Adenomatous Polyposis Gene mutations that lead to the development of colorectal polyps with great malignant risk throughout life. Moreover, numerous extracolonic manifestations incorporate different clinical features to produce varied individual phenotypes. Among them, the occurrence of duodenal adenomatous polyps is considered an almost inevitable event, and their incidence rates increase as a patient’s age advances. Although the majority of patients exhibit different grades of duodenal adenomatosis as they age, only a small proportion (1%-5%) of patients will ultimately develop duodenal carcinoma. Within this context, the aim of the present study was to review the data regarding the epidemiology, classification, genetic features, endoscopic features, carcinogenesis, surveillance and management of duodenal polyps in patients with FAP.
Core tip: The development of duodenal adenomas is considered a very common and important extracolonic manifestation in patients with familial adenomatous polyposis. Results from recently published studies have indicated the need for life-long surveillance of patients presenting with this condition due to a risk of malignization, especially in patients with severe adenomatosis. The present study discusses the incidence, endoscopic features and management of duodenal adenomas and reviews the published data regarding cancer prevention and surveillance.
- Citation: Campos FG, Sulbaran M, Safatle-Ribeiro AV, Martinez CAR. Duodenal adenoma surveillance in patients with familial adenomatous polyposis. World J Gastrointest Endosc 2015; 7(10): 950-959
- URL: https://www.wjgnet.com/1948-5190/full/v7/i10/950.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i10.950
Familial adenomatous polyposis (FAP) is an inherited autosomal dominant syndrome that is caused by germline mutations in one copy of the adenomatous polyposis coli (APC) gene. These mutations lead to the development of a variable number of colorectal polyps during the second and third decade of life[1,2]. APC is a tumor suppressor gene that is located on the long arm of chromosome 5 (5q21-22) and is composed of 15 exons. Exons 1-14 are small compared to the large exon 15, which has 6571 base pairs and accounts for over 70% of the coding portion of the gene[3,4].
As the disease is associated with an almost 100% risk of developing colorectal cancer (CRC) in untreated patients, prophylactic colectomy is considered the cornerstone of FAP management[1,5]. Performing a proctocolectomy before a patient reaches adulthood is associated with a substantial reduction in the incidence of CRC and a better prognosis. Consequently, the extracolonic manifestations (ECM) of the disease have been reported to lead to a relative increase in death[6]. Survival effects associated with screening and prophylactic surgery, life expectancy remains lower than that observed in the general population[7,8].
The majority of ECM have little clinical significance, but some of them may cause serious complications and even lead to death[9-11]. The majority of FAP patients (over 70%) present with some level of ECM during the course of the disease, such as cutaneous lesions (lipomas, fibromas, sebaceous and epidermoid cysts), desmoids tumors, osteomas, dental abnormalities, congenital hypertrophy of retinal pigment epithelium lesions (CHRPE) or upper-gastrointestinal polyps[1]. Moreover, patients with PAF are also at an increased risk for several malignancies, including hepatoblastoma, pancreatic, thyroid, biliary-tree, brain and duodenal cancers[12].
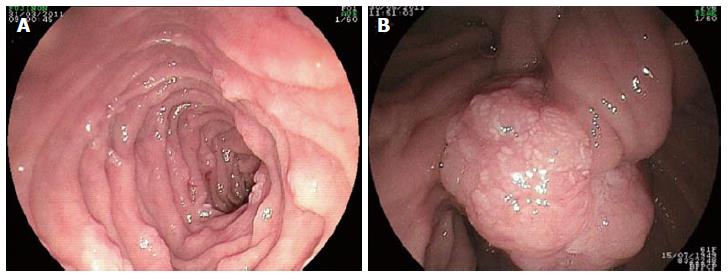
Gastric fundic gland polyps, gastric adenomas, duodenal adenomas and carcinoma represent the most common upper digestive lesions that are diagnosed in FAP patients (Figure 1)[13,14]. As they are an important potential cause of morbidity in FAP patients, duodenal polyps require diagnosis, follow-up and preventive measures to avoid carcinogenesis. Thus, the aim of the present study was to review the data regarding the epidemiology, classification, genetic features, endoscopic features, carcinogenesis, surveillance and management of duodenal polyps in patients with FAP.
After the colon and rectum, the duodenum is the second most common site of polyp development in patients with FAP[12-14]. The existence of gastric and duodenal polyps in these patients was established more than a century ago, and Cabot described the first case of duodenal cancer in 1935[12-17]. In a different study, it was found that a considerable number of stomach and duodenum polyps develop at an early age in the majority of pediatric patients, which led to the recommendation of periodic screening of the upper gastrointestinal in the 1960s[18]. The malignant potential of duodenal lesions was gradually established over the next decade, primarily following the introduction of flexible endoscopes during the 1970s[18-21]. During the 1970s and 1980s, numerous additional studies described high numbers of gastroduodenal polyps being identified during endoscopic screenings, providing definitive support for the inclusion of upper digestive endoscopy during routine evaluation and surveillance of FAP patients[22,23].
Duodenal adenomas tend to occur approximately 15 years after the appearance of colonic adenomas[20,21,24]. Duodenal adenomas have been found in 30%-92% of FAP patients, with a lifetime risk approaching 100%[7,14,22-24]. The frequency of detecting duodenal adenomas in FAP patients may vary depending on endoscopic technique and the method of tissue sampling[7,23-27]. Employing side-viewing endoscopes and random biopsies, exceptional detection rates of 70% and above may be achieved for duodenal and periampullary adenomas[22,26,28]. Biopsies of periampullary regions and duodenal papilla revealed numerous microadenomas that were not detected in normal duodenal mucosa[22,26,27].
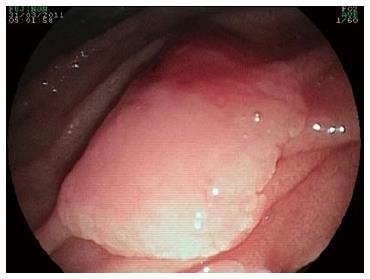
The macroscopic appearance of duodenal adenomas in patients with FAP varies widely[21,29-31]. These lesions are usually white, numerous and sessile flat. Due to their small size, they may easily be missed or even entirely overlooked during upper endoscopy. With the aid of chromoscopic techniques, such as sprinkling indigo-carmine or methylene blue over the mucosa, the number of detected polyps may increase considerably. In any given patient, using such techniques can identify anywhere from no visible microadenomas to the existence of over 100 microadenomas of varying diameters (1-10 mm)[7,21,22]. The use of side-viewing endoscopes may eventually enable the detection of a prominent papilla of Vater within a solitary adenoma (Figure 2).
The distribution pattern throughout the duodenum and the upper part of the small bowel reveals that the majority of polyps are found in clusters around and mainly distal to the ampulla of Vater (second and third part of the duodenum)[32,33].
In 1989, Spigelman et al[22] proposed a five-stage classification (0-IV) system to evaluate polyp severity that has become widely adopted. Classification is based on points that are accumulated according to the number, size, histology and dysplasia of polyps. Following this, disease stages are categorized as mild (I), moderate (II), or severe (III and IV) (Table 1).
| Criterion | Points | ||
| 1 | 2 | 3 | |
| Polyp number | 1-4 | 5-20 | > 20 |
| Polyp size (mm) | 1-4 | 5-10 | > 10 |
| Histology | Tubular | Tubulo-villous | Villous |
| Dysplasia | Mild | Moderate | Severe |
| Stage 0: 0 points; stage I: 1-4 points; stage II: 5-6 points; stage III: 7-8 points; stage IV: 9-12 points | |||
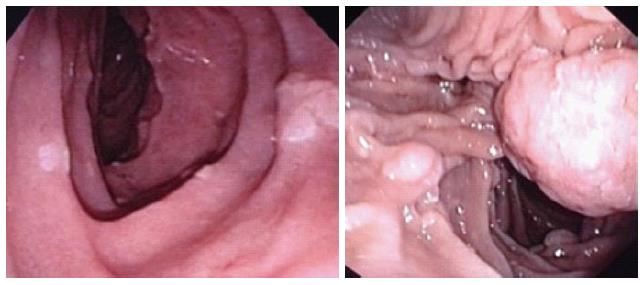
Previous reports have indicated that approximately 70%-80% of FAP patients have stage II or III duodenal disease and 20%-30% have stage I or IV disease[22,33] (Figure 3). In a retrospective Swedish study that evaluated 180 patients with FAP, 134 (74%) of the patients exhibited duodenal adenomas, of which only 14 (7.8%) were classified as stage IV periampullary adenomas. The authors estimated a time course of 7.1 (range: 5.3-9.8) years for the development of stage IV periampullary adenomas from normal duodenum. Periampullary adenocarcinomas were diagnosed in 5 (2.7%) patients, of whom 3 had a previous diagnosis of stage IV disease based on endoscopic screening and 2 had less severe periampullary adenomatosis[34].
In an interesting, large multicentric study that analyzed 368 upper endoscopies, Bülow et al[14] detected duodenal polyps in 228 (61.9%) patients, with adenomas in 209 (91%) and normal mucosa in 19 (9%). Moreover, random duodenal biopsies revealed adenomatous tissue in 28 patients who did not have visible polyps at endoscopy. Based on Spigelman classification, 34%, 15%, 27%, 17% and 7% of patients had stage I, II, III, IV and V disease, respectively. Two of the patients in this series presented with duodenal carcinoma during screening. The estimated cumulative lifetime risks were 88% for duodenal adenomatosis and 35% for stage IV disease. The authors also measured a cumulative cancer incidence of 18% at 75 years of age. Groves et al[33] followed 99 patients over a course of 10 years and reported a progression in the incidence of stage IV disease from 9.6% to 14% in patients with a mean age of 42 years. These prospective studies showed that adenomas progress slowly in the duodenum and that adenomatosis is usually diagnosed at a premalignant stage.
In addition to the above, it must be emphasized that although Spigelman classification correlates well with duodenal cancer risk, it focuses primarily on non-ampullary duodenal disease. Therefore, a separate evaluation of ampullary disease is required to establish an accurate individual risk assessment[35].
The distribution of adenomas within the duodenum probably reflects the exposure of duodenal mucosa to bile acids, suggesting a role for these compounds in duodenal carcinogenesis[22]. Duodenal cancer is one of the two leading causes of death (the other being desmoid tumors) in patients with FAP after they receive prophylactic colectomy[10,12]. When compared to the general population (in whom duodenal carcinoma is rare), the relative risks of developing duodenal adenocarcinoma and ampullary carcinoma were respectively 331 and 124 times higher in FAP patients[36]. Similarly, another study estimated these risks as being 100- to 330-fold higher[27]. The absolute lifetime risk was estimated to be approximately 3%-5%[32-35].
In contrast to colorectal polyps, duodenal polyps do not inevitably transform into cancer[14]. Dysplastic duodenal polyps in FAP patients generally occur 10-20 years after the development of colorectal polyps, and the risk of malignant transformation ranges from 1% to 5%[14,33,37,38].
Stage IV patients have the greatest risk of developing duodenal cancer, with rates of 7%-36% having been described in 7.6- to 10-year follow-up periods[14,33]. Alternatively, this risk is low (0.7%) among stage 0 to stage III patients[12] . Mortality rates from duodenal cancer vary from 1.7% to 8.2%[10,39-43].
Several genotype-phenotype correlations have been described for colonic polyposis and ECM in FAP patients, including those related to CHRPE and desmoids[44-46]. Aside from the identification of genetic hot spots that are associated with the severity of duodenal adenomatosis, a genotype-phenotype correlation for the disease has not been well defined[12,47].
In a study conducted by Friedl et al[48], no correlation was detected between the locations of mutations and the severity of duodenal polyposis. Conversely, Soravia et al[49] described severe duodenal polyposis in patients with 5’ mutations. Additional reports have suggested that mutations in the central part of the APC gene and in exon 15 (particularly distal to codon 1400) may predispose an individual to a severe duodenal phenotype[50].
In patients with FAP, small bowel polyps are predominantly found in the duodenum and ampulla, although they may also develop in ileostomies and ileal pouches[51]. Within the duodenum, the cumulative incidence of polyp development increases with age (65% at 38 years and 90%-95% by 70 years)[14].
Recognition of the problem is essential toward establishing recommendations for surveillance of the upper gastrointestinal tract. The risk of malignancy increases with size, location (ampullary) and adenomatosis severity[35,52]. Thus, between the existence of an almost 100% lifetime risk of developing duodenal adenomatosis and the cumulative incidence rates of Spigelman stage IV disease and carcinoma (4% to 10%), there is a clear need for careful follow-up and surveillance of this population[14,52-54].
Improving prognosis through early detection of neoplastic changes is the basis for endoscopic surveillance, and decision analysis has shown that surveillance increases life expectancy by seven months[40]. Moreover, a surveillance program that was based on endoscopic/histological findings and associated with early diagnosis and resection of cancer was shown to improve the prognosis of selected patients[55].
Adequate evaluation of the duodenum can be obtained with the use of frontal and side viewing (lateral) endoscopes, which facilitate evaluation of the Vater Papilla. Additionally, indigo carmine chromoendoscopy and electronic imaging techniques may improve the efficacy of detecting lesions. As periampullary carcinomas represent a leading cause of death in FAP patients, biopsies of this region should be performed regardless of whether mucosa appears normal, as approximately 7.6% of patients with normal endoscopic results exhibit adenomatous tissue on random biopsy[10,14,33].
When to begin surveillance of FAP patients is a controversial issue, with some clinicians supporting that surveillance begin when FAP is diagnosed and others proposing that it should not begin until patients reach 25-30 years in age, as a diagnosis of duodenal cancer before age 30 is rare[12,33,56,57]. Post-baseline evaluations should be planned according to Spigelman disease stage. This classification is widely accepted as the best option for stratifying the risk of duodenal cancer[54]. Surveillance is the most advantageous in stage IV patients, as their risk of duodenal carcinoma ranges from 7%-36% compared to non-stage IV patients, who have an overall risk of 5%.
Although published recommendations differ, in general, early stage patients are advised to undergo endoscopy either every 4-5 years (stages 0-I) or every 3-5 years (stage II)[33,57-60]. In stage II patients, Groves et al[33] have suggested that endoscopic therapy include endoscopic mucosal resection (EMR). However, the above intervals may be reduced to 1-3 years for patients with mild polyposis (stage III) or to 6-12 mo for patients with severe polyposis, large adenomas or dysplasia[12,14,58]. It has been suggested that stage III patients undergo EMR to reduce duodenal adenomatosis[33,57]. As one third of stage IV patients may experience malignant transformation if they are not treated, these patients should also undergo endoscopic ultrasonography and computed tomography for staging during initial evaluation[14,61].
For patients with periampullary lesions, a different protocol has been proposed due to the greater associated risks[35,62]. This protocol recommends that patients with ampullary polyposis should be examined annually, irrespective of disease severity in other regions of the duodenum. Progression of the disease may be evaluated with magnetic resonance imaging and/or endoscopic ultrasound.
The majority of large studies have shown that the risk of advanced duodenal adenomatosis (stage IV) increases with age. Bulow et al[63] found a 52% cumulative risk at 70 years, which was similar to the 50% risk reported by Saurin et al[14] and the 20%-30% risk found by studies conducted in Sweden and Finland[14,34,63,64] .
Therefore, endoscopic surveillance programs should be performed according to the following published recommendations (Table 2).
| Spigelmanstage | Suggested interval (yr) to next duodenoscopy | Conservative therapy | Surgical treatment |
| 0 (0 points) | 4 (maximum 5 yr) | No | No |
| I (1-4 points) | 3 (maximum 5 yr) | No | No |
| II (5-6 points) | 2-3 | Chemoprevention with or without endoscopic therapy | No |
| III (7-8 points) | 6-12 (maximum 1-2 yr) | Chemoprevention with or without endoscopic therapy1 | Acceptable |
| IV (9-12 ponits) | 6-12 (maximum 1-2 yr) | Endoscopic therapy and endoscopic ultrasonography | Duodenectomy with pancreas/pylorus preservation |
Ideally, treatment should include complete removal or destruction of adenomas and minimal morbid risk. Endoscopic management may be performed with standard polipectomy and local ablation techniques (thermal ablation, argon plasma coagulation or photodynamic therapy)[12,35]. Endoscopic therapy with argon plasma coagulation and Nd-YAG lasers has been attempted with varying results.
The plaque-like morphology of the majority of duodenal adenomas may pose some technical difficulties in performing endoscopic polipectomy, and new techniques of mucosal elevation/resection and hemostasis using different tools may reduce the risks of bleeding, pancreatitis and perforation. Another possible advantage of endoscopic treatment is the postponement of major operations such as duodenopancreatectomy. Although polipectomy or polyp destruction in stage II and stage III patients may be useful, long-term results have demonstrated adenoma recurrence rates of 50%-100%, and complications are not rare[26,49,65]. Thus, endoscopy generally does not affect disease course and follow-up remains necessary.
In this context, low-risk lesions (small, tubular, low-grade adenomas) should be biopsied and observed. Conversely, high-risk lesions (adenomas greater than 1 cm and those with villous patterns or high-grade dysplasia) may be treated via transduodenal resection[61]. Endoscopic or surgical ampullectomy should be used on lesions that have developed in the ampulla of Vater (mainly those with severe dysplasia, Tis or T1), despite the associated morbidity[66].
Patients with large stage III polyps (or stage IV, for which surgical treatment is not appropriate) may be candidates for endoscopic polipectomy. The use of general anesthesia may optimize therapeutic maneuvers by allowing the introduction of front and lateral endoscopes to evaluate the papilla, and third and fourth portions of duodenum. Such a strategy aims to avoid progression to stage IV disease, as this results in a greater risk (1 in 3 patients) of duodenal cancer[33]. The management of stage IV patients with desmoids disease, unfavorable clinical conditions or diffuse involvement of duodenal mucosa remains a significant problem.
Surgical management includes local procedures (duodenotomy with polypectomy and/or ampullectomy), pancreas- and pylorus-sparing duodenectomies, and pancreatico-duodenectomy (Whipple’s operation). The specific choice of which procedure to use appears to be related to technical expertise, local features (size and site of polyp) and disease severity. In the final analysis, the morbidity and mortality of these procedures must be weighed against the risk of developing duodenal adenocarcinoma.
Whereas radical resection is the obvious option for patients with carcinomas, a prophylactic operation (pancreas and pylorus sparing duodenectomy) to avoid cancer is also justified in cases of severe adenomatosis (Spigelman IV) or after a failed attempt at local resection (endoscopic or surgical)[33,67]. Even patients with stage III polyposis have been considered for surgery[49,68,69]. However, no randomized studies to help guide surgical selection have been published thus far.
Duodenotomy with local resection may be indicated in selected patients who present with one or two dominant duodenal lesions and in whom endoscopic resection would be considered dangerous. In a recent review on this subject, Brosens et al[12] indicated that this approach might be useful for delaying major procedures in young patients. Otherwise, high recurrence rates have been reported after local surgical resection, similarly to what occurs after endoscopic resection. Moreover, patients who have previously undergone prophylactic colectomy and present with desmoids tumors have a significant risk of developing complications from duodenectomy[37,57].
Pancreatico-duodenectomy remains a last resort for advanced duodenal and ampullary adenomatosis, despite the risks of this complex procedure and the possibility of inducing desmoid tumor formation[58].
Chemoprevention is defined as the use of pharmaceutical drugs, natural agents or dietary supplements to reduce the incidence or delay the onset of diseases, including cancer[70]. In FAP patients, the colorectum, ileal pouch and duodenum represent the most clinically relevant sites of carcinogenesis[71]. Consequently, FAP patients constitute an ideal group for assessing the efficacy of various chemopreventive strategies at delaying polyp progression, postponing prophylactic colectomy and preventing the recurrence of adenoma following colectomy with IRA. These effects have also been evaluated in the upper gastrointestinal tract, particularly in the duodenum[72].
As prophylactic surgical resection of an ampulla and/or duodenum may be accompanied by significant morbidity, duodenal resection is currently reserved for only severe cases of duodenal polyposis or duodenal carcinoma. In this context, chemoprevention should be the strategy employed to control premalignant lesions[72]. Secondary chemoprevention has been attempted with the use of agents such as non-steroidal anti-inflammatory drugs (NSAIDs)[73-75]. The use of the cyclooxygenase (COX) non-selective inhibitor sulindac and of the selective COX-2 inhibitors celecoxib and rofecoxib may be beneficial when duodenal polyposis develops by inducing polyposis regression or stabilization.
Studies using sulindac have revealed the drug to have a statistically significant effect on small (2 mm) duodenal polyps, whereas larger (> 3 mm) polyps were unaffected[76,77]. In a different study, the administration of 300 mg/d of sulindac for 10 mo resulted in a 30% discontinuation rate due to side effects and no regression of polyps; furthermore, three patients developed large polyps and one developed an infiltrating carcinoma while on this drug[78].
In a large randomized trial, the use of celecoxib resulted in a 14%-31% reduction in the regions of the duodenum that were affected by adenomatosis and therefore this drug may be recommended as a therapeutic alternative to patients with moderate adenomatosis[33,79]. However, the promising use of coxibs in chemoprevention must be weighed against their potential cardiovascular and renal side effects[80,81]. In addition to the fact that celecoxib may delay worsening of polyposis, there have not been sufficient long-term results or evidence from controlled studies on cancer protection to routinely recommend these agents during follow-up[14,51,58].
In conclusion, although they may reduce the progression and even lead to regression of small adenomas, the role of NSAIDs and other compounds in duodenal polyposis regression remains unclear, and thus far the results have primarily been ambiguous[12]. The evidence must prove to be reproducible, and potential cardiovascular and renal side effects, in addition to the risk of gastrointestinal bleeding, must be taken into account[24]. Moreover, duodenal adenomas are less likely to degenerate compared to colonic polyps, and they also appear to be less responsive to chemoprevention with NSAIDs[12,82].
To date, no medical therapy has demonstrated long-term effectiveness and safety in the management of duodenal adenomatosis. There has been a single report indicating an apparent disappearance of duodenal polyposis in a patient who was treated with FOLFOX chemotherapy for an ileal pouch adenocarcinoma[83].
As dietary chemoprevention has shown no effective results, a new line of interventions focus on the role of the estrogen receptor (ER) in reducing polyp numbers and sizes, based on the supposed preventive effects of CRC. In an interesting study, Calabrese et al[84] evaluated whether dietary supplementation with phytoestrogens, which are selective agonists of the estrogen receptor, was able to prevent the progression of duodenal polyps. They demonstrated that short-term (90 d) supplementation with Eviendep® in FAP patients with recurrent adenomas in the duodenal mucosa resulted in a 32% reduction of polyp numbers and 51% reduction in polyp size.
This study clearly demonstrates that researches with FAP patients will always have a lead role in the testing of new agents, favoring their own interests and those of non-familial adenomas, a problem with even greater social impact. For the next future, the role of NSAIDs in chemoprevention has gained renewed interest in sporadic adenoma prevention, although the long-term risks associated with its use have always been a source of concern[85,86].
As chemoprevention may eventually avoid surgical resection of at-risk duodenal adenomas, it would desirable to identify patients important to select advanced adenomas that would be candidates. In an interesting research, it was reported that mRNA levels of glutathione S-transferase A1 (28.16% vs 38.24%, P = 0.008) and caspase-3 (3.30% vs 5.31%, P = 0.001) were significantly lower in patients with FAP vs non-FAP patient controls, respectively[87]. This finding points at a lower capacity to detoxify toxins and carcinogens, with subsequent increased susceptibility for malignant degeneration[88]. Previous studies have already found lower GDT enzyme activity in colonic mucosa but no differences in duodenal mucosa when compared to patient controls[89,90]. Other eventual risk factors include the development of small intestinal adenomas and location of APC mutation[91-93].
All of these findings indicate that routine gastroduodenal endoscopy in FAP patients is necessary[94-96]. In this setting of surveillance, both endoscopy and EUS are extremely important to select advanced adenomas that are candidates for endoscopic intervention instead of surgical resection[97,98]. Moreover, although these lesions progress in severity (size and degree of dysplasia), their progression rate to carcinoma is slow[96,99].
The authors would like to thank the Drs. Olympio Meirelles, Michel Gardere and Cristiane Cruz (Gastrocentro-UNICAMP) by endoscopic images illustrating this manuscript and doctor Umberto Morelli by elaboration of the audio core tip.
P- Reviewer: Almeida N, Ando T S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL
| 1. | Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153-161. [PubMed] |
| 2. | Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet. 2015;8:95-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661-665. [PubMed] |
| 4. | Bertario L, Russo A, Sala P, Varesco L, Giarola M, Mondini P, Pierotti M, Spinelli P, Radice P. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol. 2003;21:1698-1707. [PubMed] |
| 5. | Campos FG. Surgical treatment of familial adenomatous polyposis: dilemmas and current recommendations. World J Gastroenterol. 2014;20:16620-16629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | van Heumen BW, Nieuwenhuis MH, van Goor H, Mathus-Vliegen LE, Dekker E, Gouma DJ, Dees J, van Eijck CH, Vasen HF, Nagengast FM. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery. 2012;151:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Bülow S, Bülow C, Nielsen TF, Karlsen L, Moesgaard F. Centralized registration, prophylactic examination, and treatment results in improved prognosis in familial adenomatous polyposis. Results from the Danish Polyposis Register. Scand J Gastroenterol. 1995;30:989-993. [PubMed] |
| 8. | Björk J, Akerbrant H, Iselius L, Alm T, Hultcrantz R. Epidemiology of familial adenomatous polyposis in Sweden: changes over time and differences in phenotype between males and females. Scand J Gastroenterol. 1999;34:1230-1235. [PubMed] |
| 9. | Ficari F, Cama A, Valanzano R, Curia MC, Palmirotta R, Aceto G, Esposito DL, Crognale S, Lombardi A, Messerini L. APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis. Br J Cancer. 2000;82:348-353. [PubMed] |
| 10. | de Campos FG, Perez RO, Imperiale AR, Seid VE, Nahas SC, Cecconello I. Evaluating causes of death in familial adenomatous polyposis. J Gastrointest Surg. 2010;14:1943-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Campos FG, Martinez CA, Novaes M, Nahas SC, Cecconello I. Desmoid tumors: clinical features and outcome of an unpredictable and challenging manifestation of familial adenomatous polyposis. Fam Cancer. 2015;14:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 12. | Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034-1043. [PubMed] |
| 13. | Hashimoto T, Ogawa R, Matsubara A, Taniguchi H, Sugano K, Ushiama M, Yoshida T, Kanai Y, Sekine S. Familial adenomatous polyposis-associated and sporadic pyloric gland adenomas of the upper gastrointestinal tract share common genetic features. Histopathology. 2015;Mar 31; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, Vasen HF. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 258] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 15. | Hauser G. Ueber Polyposis intestinalis adenomatosa und deren Beziehungen zur Krebsentwicklung. Deutsche Archive Klinische Medizinische. 1895;55:429-448. |
| 16. | Funkenstein O. Über Polyposis intestinalis. Z Med Lab Diagn. 1904;55:236-248. |
| 17. | Cabot RC, Harris NL, Shepard JA, Rosenberg ES, Cort AM, Ebeling SH, Peters CC, Misra M, Parangi S, Ross DS. Case records of the Massachusetts General Hospital: Case 38-2010: a 13-year-old girl with an enlarging neck mass. N Engl J Med. 2010;363:2445-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Duncan BR, Dohner VA, Priest JH. The Gardner syndrome: need for early diagnosis. J Pediatr. 1968;72:497-505. [PubMed] |
| 19. | Macdonald JM, Davis WC, Crago HR, Berk AD. Gardner’s syndrome and periampullary malignancy. Am J Surg. 1967;113:425-430. [PubMed] |
| 20. | Jones TR, Nance FC. Periampullary malignancy in Gardner’s syndrome. Ann Surg. 1977;185:565-573. [PubMed] |
| 21. | Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg. 1998;85:742-750. [PubMed] |
| 22. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [PubMed] |
| 23. | Church JM, McGannon E, Hull-Boiner S, Sivak MV, Van Stolk R, Jagelman DG, Fazio VW, Oakley JR, Lavery IC, Milsom JW. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35:1170-1173. [PubMed] |
| 24. | Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306-314. [PubMed] |
| 25. | Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc. 1999;49:358-364. [PubMed] |
| 26. | Bertoni G, Sassatelli R, Nigrisoli E, Pennazio M, Tansini P, Arrigoni A, Ponz de Leon M, Rossini FP, Bedogni G. High prevalence of adenomas and microadenomas of the duodenal papilla and periampullary region in patients with familial adenomatous polyposis. Eur J Gastroenterol Hepatol. 1996;8:1201-1206. [PubMed] |
| 27. | Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis. 2001;16:63-75. [PubMed] |
| 28. | Iida M, Aoyagi K, Fujimura Y, Matsumoto T, Hizawa K, Nakamura S. Nonpolypoid adenomas of the duodenum in patients with familial adenomatous polyposis (Gardner’s syndrome). Gastrointest Endosc. 1996;44:305-308. [PubMed] |
| 29. | Koritala T, Zolotarevsky E, Bartley AN, Ellis CD, Krolikowski JA, Burton J, Gunaratnam NT. Efficacy and safety of the band and slough technique for endoscopic therapy of nonampullary duodenal adenomas: a case series. Gastrointest Endosc. 2015;81:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Fatemi SR, Safaee A, Pasha S, Pourhoseingholi MA, Bahrainei R, Molaei M. Evaluation of endoscopic characteristics of upper gastrointestinal polyps in patients with familial adenomatous polyposis. Asian Pac J Cancer Prev. 2014;15:6945-6948. [PubMed] |
| 31. | Moussata D, Napoleon B, Lepilliez V, Klich A, Ecochard R, Lapalus MG, Nancey S, Cenni JC, Ponchon T, Chayvialle JA. Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc. 2014;80:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Debinski HS, Spigelman AD, Hatfield A, Williams CB, Phillips RK. Upper intestinal surveillance in familial adenomatous polyposis. Eur J Cancer. 1995;31A:1149-1153. [PubMed] |
| 33. | Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636-641. [PubMed] |
| 34. | Björk J, Akerbrant H, Iselius L, Bergman A, Engwall Y, Wahlström J, Martinsson T, Nordling M, Hultcrantz R. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology. 2001;121:1127-1135. [PubMed] |
| 35. | Latchford AR, Neale KF, Spigelman AD, Phillips RK, Clark SK. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2009;7:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980-1982. [PubMed] |
| 37. | Basford PJ, Bhandari P. Endoscopic management of nonampullary duodenal polyps. Therap Adv Gastroenterol. 2012;5:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Eswaran SL, Sanders M, Bernadino KP, Ansari A, Lawrence C, Stefan A, Mattia A, Howell DA. Success and complications of endoscopic removal of giant duodenal and ampullary polyps: a comparative series. Gastrointest Endosc. 2006;64:925-932. [PubMed] |
| 39. | Lepistö A, Kiviluoto T, Halttunen J, Järvinen HJ. Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy. 2009;41:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Vasen HF, Bülow S, Myrhøj T, Mathus-Vliegen L, Griffioen G, Buskens E, Taal BG, Nagengast F, Slors JF, de Ruiter P. Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut. 1997;40:716-719. [PubMed] |
| 41. | Lal G, Gallinger S. Familial adenomatous polyposis. Semin Surg Oncol. 2000;18:314-323. [PubMed] |
| 42. | Bertario L, Presciuttini S, Sala P, Rossetti C, Pietroiusti M. Causes of death and postsurgical survival in familial adenomatous polyposis: results from the Italian Registry. Italian Registry of Familial Polyposis Writing Committee. Semin Surg Oncol. 1994;10:225-234. [PubMed] |
| 43. | Belchetz LA, Berk T, Bapat BV, Cohen Z, Gallinger S. Changing causes of mortality in patients with familial adenomatous polyposis. Dis Colon Rectum. 1996;39:384-387. [PubMed] |
| 44. | Church J, Xhaja X, LaGuardia L, O’Malley M, Burke C, Kalady M. Desmoids and genotype in familial adenomatous polyposis. Dis Colon Rectum. 2015;58:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Schiessling S, Kihm M, Ganschow P, Kadmon G, Büchler MW, Kadmon M. Desmoid tumour biology in patients with familial adenomatous polyposis coli. Br J Surg. 2013;100:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | de Leon MP, Urso ED, Pucciarelli S, Agostini M, Nitti D, Roncucci L, Benatti P, Pedroni M, Kaleci S, Balsamo A. Clinical and molecular features of attenuated adenomatous polyposis in northern Italy. Tech Coloproctol. 2013;17:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Groves C, Lamlum H, Crabtree M, Williamson J, Taylor C, Bass S, Cuthbert-Heavens D, Hodgson S, Phillips R, Tomlinson I. Mutation cluster region, association between germline and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol. 2002;160:2055-2061. [PubMed] |
| 48. | Friedl W, Caspari R, Sengteller M, Uhlhaas S, Lamberti C, Jungck M, Kadmon M, Wolf M, Fahnenstich J, Gebert J. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut. 2001;48:515-521. [PubMed] |
| 49. | Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, Cohen Z, Bapat B. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 1998;62:1290-1301. [PubMed] |
| 50. | Matsumoto T, Lida M, Kobori Y, Mizuno M, Nakamura S, Hizawa K, Yao T. Genetic predisposition to clinical manifestations in familial adenomatous polyposis with special reference to duodenal lesions. Am J Gastroenterol. 2002;97:180-185. [PubMed] |
| 51. | Skipworth JR, Morkane C, Raptis DA, Vyas S, Olde Damink SW, Imber CJ, Pereira SP, Malago M, West N, Phillips RK. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB (Oxford). 2011;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 53. | King JE, Dozois RR, Lindor NM, Ahlquist DA. Care of patients and their families with familial adenomatous polyposis. Mayo Clin Proc. 2000;75:57-67. [PubMed] |
| 54. | Mathus-Vliegen EM, Boparai KS, Dekker E, van Geloven N. Progression of duodenal adenomatosis in familial adenomatous polyposis: due to ageing of subjects and advances in technology. Fam Cancer. 2011;10:491-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Bülow S, Christensen IJ, Højen H, Björk J, Elmberg M, Järvinen H, Lepistö A, Nieuwenhuis M, Vasen H. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis. 2012;14:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 807] [Article Influence: 53.8] [Reference Citation Analysis (2)] |
| 57. | Gallagher MC, Phillips RK, Bulow S. Surveillance and management of upper gastrointestinal disease in Familial Adenomatous Polyposis. Fam Cancer. 2006;5:263-273. [PubMed] |
| 58. | Johnson JC, DiSario JA, Grady WM. Surveillance and Treatment of Periampullary and Duodenal Adenomas in Familial Adenomatous Polyposis. Curr Treat Options Gastroenterol. 2004;7:79-89. [PubMed] |
| 59. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 60. | Al-Kawas FH. The significance and management of nonampullary duodenal polyps. Gastroenterol Hepatol (N Y). 2011;7:329-332. [PubMed] |
| 61. | Shibata C, Ogawa H, Miura K, Naitoh T, Yamauchi J, Unno M. Clinical characteristics of gastric cancer in patients with familial adenomatous polyposis. Tohoku J Exp Med. 2013;229:143-146. [PubMed] |
| 62. | Gallagher MC, Shankar A, Groves CJ, Russell RC, Phillips RK. Pylorus-preserving pancreaticoduodenectomy for advanced duodenal disease in familial adenomatous polyposis. Br J Surg. 2004;91:1157-1164. [PubMed] |
| 63. | Saurin JC, Ligneau B, Ponchon T, Leprêtre J, Chavaillon A, Napoléon B, Chayvialle JA. The influence of mutation site and age on the severity of duodenal polyposis in patients with familial adenomatous polyposis. Gastrointest Endosc. 2002;55:342-347. [PubMed] |
| 64. | Heiskanen I, Kellokumpu I, Järvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;31:412-416. [PubMed] |
| 65. | Morpurgo E, Vitale GC, Galandiuk S, Kimberling J, Ziegler C, Polk HC. Clinical characteristics of familial adenomatous polyposis and management of duodenal adenomas. J Gastrointest Surg. 2004;8:559-564. [PubMed] |
| 66. | Mantas D, Charalampoudis P, Nikiteas N. FAP related periampullary adenocarcinoma. Int J Surg Case Rep. 2013;4:684-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Mackey R, Walsh RM, Chung R, Brown N, Smith A, Church J, Burke C. Pancreas-sparing duodenectomy is effective management for familial adenomatous polyposis. J Gastrointest Surg. 2005;9:1088-1093; discussion 1093. [PubMed] |
| 68. | de Vos tot Nederveen Cappel WH, Järvinen HJ, Björk J, Berk T, Griffioen G, Vasen HF. Worldwide survey among polyposis registries of surgical management of severe duodenal adenomatosis in familial adenomatous polyposis. Br J Surg. 2003;90:705-710. [PubMed] |
| 69. | Kalady MF, Clary BM, Tyler DS, Pappas TN. Pancreas-preserving duodenectomy in the management of duodenal familial adenomatous polyposis. J Gastrointest Surg. 2002;6:82-87. [PubMed] |
| 70. | Schönthal AH, Chen TC, Hofman FM, Louie SG, Petasis NA. Celecoxib analogs that lack COX-2 inhibitory function: preclinical development of novel anticancer drugs. Expert Opin Investig Drugs. 2008;17:197-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Laukaitis CM, Erdman SH, Gerner EW. Chemoprevention in patients with genetic risk of colorectal cancers. Colorectal Cancer. 2012;1:225-240. [PubMed] |
| 72. | Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Chan AT. Aspirin and familial adenomatous polyposis: coming full circle. Cancer Prev Res (Phila). 2011;4:623-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Matsumoto T, Nakamura S, Esaki M, Yao T, Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. J Gastroenterol Hepatol. 2006;21:251-257. [PubMed] |
| 75. | Koornstra JJ, Rijcken FE, Oldenhuis CN, Zwart N, van der Sluis T, Hollema H, deVries EG, Keller JJ, Offerhaus JA, Giardiello FM. Sulindac inhibits beta-catenin expression in normal-appearing colon of hereditary nonpolyposis colorectal cancer and familial adenomatous polyposis patients. Cancer Epidemiol Biomarkers Prev. 2005;14:1608-1612. [PubMed] |
| 76. | Debinski HS, Trojan J, Nugent KP, Spigelman AD, Phillips RK. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet. 1995;345:855-856. [PubMed] |
| 77. | Kim KY, Jeon SW, Park JG, Yu CH, Jang SY, Lee JK, Hwang HY. Regression of Colonic Adenomas After Treatment With Sulindac in Familial Adenomatous Polyposis: A Case With a 2-Year Follow-up Without a Prophylactic Colectomy. Ann Coloproctol. 2014;30:201-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Richard CS, Berk T, Bapat BV, Haber G, Cohen Z, Gallinger S. Sulindac for periampullary polyps in FAP patients. Int J Colorectal Dis. 1997;12:14-18. [PubMed] |
| 79. | Phillips RK, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, Wakabayashi N, Shen Y, Zimmerman S, Godio L. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857-860. [PubMed] |
| 80. | Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, Booker SV, Robinson CR, Offerhaus GJ. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313-1316. [PubMed] |
| 81. | Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946-1952. [PubMed] |
| 82. | Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and Aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2004;CD004079. [PubMed] |
| 83. | Pioche M, Aguero Garcete G, Forestier J, Lépilliez V, Nozières C, Lombard-Bohas C, Saurin JC. Macroscopic and histologic regression of duodenal polyposis with FOLFOX4 chemotherapy for an ileal pouch adenocarcinoma in a patient with familial adenomatous polyposis. Endoscopy. 2012;44:1165-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Calabrese C, Praticò C, Calafiore A, Coscia M, Gentilini L, Poggioli G, Gionchetti P, Campieri M, Rizzello F. Eviendep® reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch-anal anastomosis. World J Gastroenterol. 2013;19:5671-5677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Lynch PM. Chemoprevention with special reference to inherited colorectal cancer. Fam Cancer. 2008;7:59-64. [PubMed] |
| 86. | Garcia Rodriguez LA, Cea-Soriano L, Tacconelli S, Patrignani P. Coxibs: pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res. 2013;191:67-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | van Heumen BW, Roelofs HM, te Morsche RH, Nagengast FM, Peters WH. Duodenal mucosal risk markers in patients with familial adenomatous polyposis: effects of celecoxib/ursodeoxycholic acid co-treatment and comparison with patient controls. Orphanet J Rare Dis. 2013;8:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2571] [Cited by in RCA: 2689] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 89. | Grubben MJ, van den Braak CC, Nagengast FM, Peters WH. Low colonic glutathione detoxification capacity in patients at risk for colon cancer. Eur J Clin Invest. 2006;36:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Berkhout M, Roelofs HM, Friederich P, van Krieken JH, Nagengast FM, Peters WH. Detoxification enzymes in the duodenal mucosa of patients with familial adenomatous polyposis. Br J Surg. 2005;92:754-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Jaganmohan S, Lynch PM, Raju RP, Ross WA, Lee JE, Raju GS, Bhutani MS, Fleming JB, Lee JH. Endoscopic management of duodenal adenomas in familial adenomatous polyposis--a single-center experience. Dig Dis Sci. 2012;57:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Matsumoto T, Esaki M, Yanaru-Fujisawa R, Moriyama T, Yada S, Nakamura S, Yao T, Iida M. Small-intestinal involvement in familial adenomatous polyposis: evaluation by double-balloon endoscopy and intraoperative enteroscopy. Gastrointest Endosc. 2008;68:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Park SY, Ryu JK, Park JH, Yoon H, Kim JY, Yoon YB, Park JG, Lee SH, Kang SB, Park JW. Prevalence of gastric and duodenal polyps and risk factors for duodenal neoplasm in korean patients with familial adenomatous polyposis. Gut Liver. 2011;5:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Freeman HJ. Appearance of attenuated intestinal polyposis during chronic non-steroidal anti-inflammatory drugs use. World J Gastrointest Pharmacol Ther. 2012;3:100-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Wood LD, Salaria SN, Cruise MW, Giardiello FM, Montgomery EA. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 96. | Drini M, Speer A, Dow C, Collier N, Bhathal P, Macrae FA. Management of duodenal adenomatosis in FAP: single centre experience. Fam Cancer. 2012;11:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Gluck N, Strul H, Rozner G, Leshno M, Santo E. Endoscopy and EUS are key for effective surveillance and management of duodenal adenomas in familial adenomatous polyposis. Gastrointest Endosc. 2015;81:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Samadder NJ, Jasperson K, Burt RW. Hereditary and common familial colorectal cancer: evidence for colorectal screening. Dig Dis Sci. 2015;60:734-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |