Published online Apr 16, 2013. doi: 10.4253/wjge.v5.i4.174
Revised: March 11, 2013
Accepted: March 15, 2013
Published online: April 16, 2013
AIM: To study the endocytoscopic visualization of squamous cell islands within Barrett’s epithelium.
METHODS: Endocytoscopy (ECS) has been studied in the surveillance of Barrett’s esophagus, with controversial results. In initial studies, however, a soft catheter type endocytoscope was used, while only methylene blue dye was used for the staining of Barrett’s mucosa. Integrated type endocytoscopes (GIF-Q260 EC, Olympus Corp, Tokyo, Japan) have been recently developed, with the incorporation of a high-power magnifying endocytoscope into a standard endoscope together with narrow-band imaging (NBI). Moreover, double staining with a mixture of 0.05% crystal violet and 0.1% of methylene blue (CM) during ECS enables higher quality images comparable to conventional hematoxylin eosin histopathological images.
RESULTS: In vivo endocytoscopic visualization of papillary squamous cell islands within glandular Barrett’s epithelium in a patient with long-segment Barrett’s esophagus is reported. Conventional white light endoscopy showed typical long-segment Barrett’s esophagus, with small squamous cell islands within normal Barrett’s mucosa, which were better visualized by NBI endoscopy. ECS after double CM staining showed regular Barrett’s esophagus, while higher magnification (× 480) revealed the orifices of glandular structures better. Furthermore, typical squamous cell papillary protrusion, classified as endocytoscopic atypia classification (ECA) 2 according to ECA, was identified within regular glandular Barrett’s mucosa. Histological examination of biopsies taken from the same area showed squamous epithelium within glandular Barrett’s mucosa, corresponding well to endocytoscopic findings.
CONCLUSION: To our knowledge, this is the first report of in vivo visualization of esophageal papillary squamous cell islands surrounded by glandular Barrett’s epithelium.
Core tip: Endocytoscopy has been also studied in surveillance of Barrett’s esophagus, with controversial results. In initial studies, however, a soft catheter type endocytoscope was used, while only methylene blue dye was used for staining of Barrett’s mucosa. In the present study, in vivo endocytoscopic visualization of papillary squamous cell islands within glandular Barrett’s epithelium in a patient with long-segment Barrett’s esophagus is reported.
- Citation: Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, Hosoya T, Maselli R, Kudo SE. Endocytoscopic visualization of squamous cell islands within Barrett’s epithelium. World J Gastrointest Endosc 2013; 5(4): 174-179
- URL: https://www.wjgnet.com/1948-5190/full/v5/i4/174.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i4.174
Endocytoscopy (ECS) with ultra-high magnification (× 400-1100) represents the most recent innovation in endoscopic imaging, permitting in vivo cellular imaging of gastrointestinal (GI) mucosa and visualization of nuclear atypia in neoplastic lesions during routine endoscopic examination[1-5]. Not only structural atypia, but also cellular atypia, with observation of lumens and nuclei, is achieved by recent advances in ECS[5-9].
Two different integrated type endocytoscopes (GIF-Q260, Olympus Medical Systems Corp, Tokyo, Japan) have been recently developed[2,6]. The first is a dual charged couple device (CCD) integrated type (CIF-Y0001, EC1 Olympus, Tokyo, Japan) and the other is a single CCD integrated type (CIF-Y0002, EC2 Olympus).
The dual CCD prototype carries both conventional magnification (× 80) and ultra-high magnification (× 480) abilities, which can be easily interchanged by pushing a button on the endocytoscope[2,6].
The single CCD prototype endocytoscope (CIF-Y0002, EC2 Olympus) has only one lens that can consecutively increase the magnification power from the conventional magnification power to × 380 using a hand lever. The video processor (prototype, Olympus CV-260X) with a light source (Olympus CLV-260) allows narrow-band imaging (NBI)[2].
Methylene blue or toluidine blue single staining was initially used for endocytoscopic evaluation of esophageal lesions[6,10,11]. Recently, however double staining with a mixture of 0.05% crystal violet and 0.1% methylene blue (CM) has been also proposed during ECS[2,3]. Crystal violet alone effectively stains the cytoplasm, while methylene blue single staining dyes both nuclei and cytoplasm, revealing details of cell structure[11,12]. Double CM staining enables well balanced staining of both cytoplasm and nuclei, resulting in improved endocytoscopic visualization of GI lesions, comparable to conventional hematoxylin eosin histopathological images[1].
Minami et al[2] has recently described a five type endocytoscopic atypia classification (ECA) of esophageal squamous cell lesions based on size and uniformity of nuclei, number of cells and regularity of cellular arrangement. ECA-1 to ECA-3 lesions correspond to histological categories 1 to 3, according to the revised Vienna[13,14] histological classification of gastrointestinal epithelial neoplasia, while ECA-4 to ECA-5 lesions correspond to Vienna categories 4 to 5 (Table 1). According to the results of Minami et al[2], overall accuracy of ECS in evaluation of esophageal squamous cell lesions was 91.3%, providing images similar to conventional hematoxylin and eosin staining[2]. Other endocytoscopic atypia classification systems of esophageal lesions based on “nuclear density” and “nuclear abnormality” have also been studied, with promising results[15].
| Category | Diagnosis |
| Group 1 | Negative for neoplasia |
| Group 2 | Indefinite for neoplasia |
| Group 3 | Mucosal low grade neoplasia |
| Low grade adenoma | |
| Low grade dysplasia | |
| Group 4 | Mucosal high grade neoplasia |
| Subgroup 4.1 | High grade adenoma/dysplasia |
| Subgroup 4.2 | Non-invasive carcinoma (carcinoma in situ) |
| Subgroup 4.3 | Suspicious for invasive carcinoma |
| Subgroup 4.4 | Intramucosal carcinoma |
| Group 5 | Submucosal invasion by carcinoma |
Endocytoscopy has also been studied in surveillance of Barrett’s esophagus, with controversial results[16,17]. In initial studies, however, a soft catheter type endocytoscope was used, while only methylene blue dye was used for staining of Barrett’s mucosa[16,17]. Although a standardized endocytoscopic atypia classification system for Barrett’s esophageal glandular lesions has not been yet described, endocytoscopically, dysplasia was diagnosed on the basis of polarity of cells and nuclei (spacing, orientation); size, shape and uniformity of nuclei; chromatin; nucleoli; and nucleus to cytoplasm ratio[17].
In the present study, in vivo endocytoscopic visualization of papillary squamous cell islands within glandular Barrett’s epithelium in a patient with long-segment Barrett’s esophagus is reported.
The dual CCD integrated prototype endocytoscope (CIF-Y0001, EC1 Olympus, Tokyo, Japan) was used for evaluation of long-segment Barrett’s esophagus in the present study. In order to compare endocytoscopic images to histological images, biopsies were taken from the same area of ECS by an experienced endoscopist.
Conventional magnifying endoscopy and ECS was performed under conscious sedation with intravenous pethidine hydrochloride (35 mg; Opystan, Mitsubishi Tanabe Pharma Corporation, Osaka, Japan), supplemented with diazepam (5-10 mg, Takeda Pharmaceutical Co., Osaka, Japan). In order to suppress esophageal peristalsis, scopolamine butylbromide (20 mg; Buscopan, Boeringer Ingelhei, GmbH, Ingelheim, Germany) was also administered intravenously. Conventional and ultra-high magnification examination was performed simultaneously. Flushing with water containing a small amount of simethicone was carried out to eliminate gas and foamy mucus from the esophagus before the procedure.
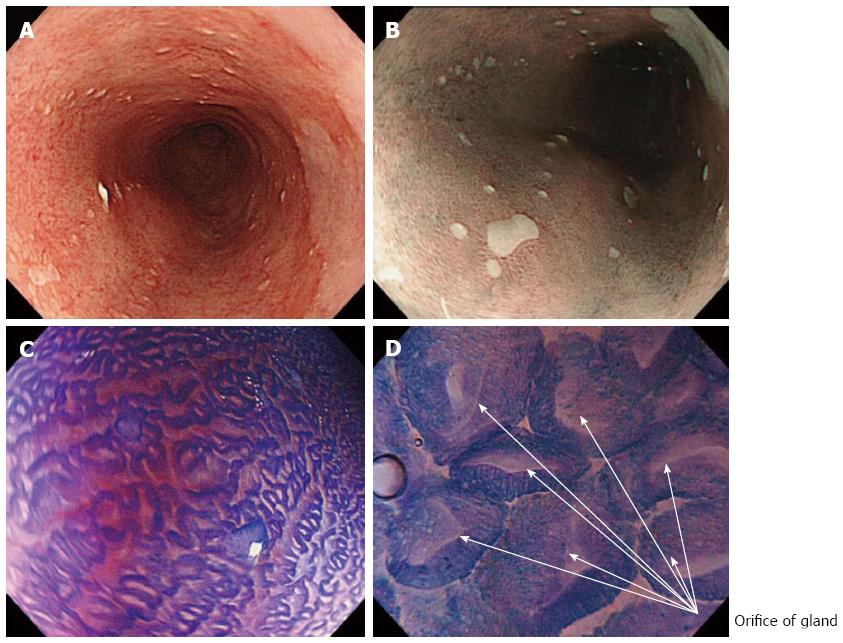
Conventional white light endoscopy (WLE) showed typical long-segment Barrett’s esophagus, without visible lesions (Figure 1A). NBI clearly visualized small squamous cell islands within normal Barrett’s mucosa, which were also identified by WLE with difficulty (Figure 1B).
After double CM staining, ECS with gradual magnification followed. A total amount of 10 mL CM mixture was directly injected through the working channel with a 5 mL syringe to esophageal Barrett’s mucosa. No catheter spray was necessary. The CM mixture is routinely prepared for ECS use, from 0.05% crystal violet and 0.1% methylene blue due solutions. After waiting 60 s to stain nuclei and cytoplasm, ECS followed.
Initially, detailed endocytoscopic observation on the background mucosa showed regular Barrett’s esophagus, without endocytoscopic signs of dysplasia (Figure 1C), while with higher magnification the adenomatous Barrett’s glandular orifices were better visualized (Figure 1D). Particularly, high quality endocytoscopic images revealed normal cellular structures, with cells similar in size and shape, without crowding or overlapping and an equal uptake of methylene blue, uniformly oriented in a glandular structure. Furthermore, nuclei were uniform, regular in shape, small in size with normal nucleus/cytoplasm ratio.
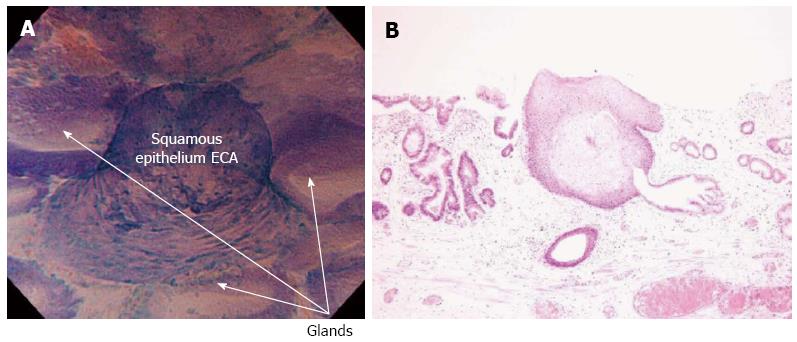
Subsequently, ECS focused on the largest squamous cell island surrounded by regular Barrett’s epithelium, which was previous identified by NBI. A typical squamous papillary protrusion was clearly identified within regular glandular Barrett’s mucosa (Figure 2A). Endocytoscopic findings revealed combined round-shaped cytoplasm-rich cells in an almost regular arrangement, while different sized small nuclei were observed, corresponding to ECA2 according to endocytoscopic atypia classification[2] (Figure 2A). These findings were suggestive of mild inflammatory changes of esophageal squamous epithelium (DVD).
After detailed observation, biopsies were taken from the same area in order to obtain a pathological diagnosis. The location of endocytoscopic images were matched to histological images and complete correspondence of endocytoscopic images with histopathological images was obtained (Figure 2) based on the records of endocytoscopic examination (DVD).
Histological examination showed squamous epithelium within non-dysplastic columnar Barrett’s epithelium (Figure 2B). No dysplasia or atypia was found in histopathology of both squamous cell islands and adenomatous Barrett’s epithelium, which was in accordance with endocytoscopic images.
Barrett’s esophagus is the transformation of the normal squamous esophageal mucosa into columnar epithelium and is considered a premalignant condition with high risk of esophageal adenocarcinoma[18-21]. Traditionally, the diagnosis of Barrett’s esophagus is based on histology of biopsy specimens and hematoxylin eosin stain, revealing glandular structures combined with goblet cells[22,23]. The presence of goblet cells is the sine qua non of Barrett’s esophagus[24,25].
Long-term endoscopic surveillance with multiple and repeated sets of biopsies are the standard recommended practice in Barrett’s esophagus in an attempt to detect dysplasia or carcinoma at an early and potentially curable stage[26-29]. The Seattle multiple biopsy protocol (4 quadrant jumbo biopsies every 1 cm with additional biopsies of mucosal abnormalities), is considered to be the optimal method for surveillance of Barrett’s esophagus, although it has never been validated[27,30]. However, even the most intensive biopsy protocols are associated with significant sampling errors[31,32].
By convention, there are four broad categories used by pathologists to describe the dysplastic process in Barrett’s: (1) no dysplasia; (2) indefinite for dysplasia; (3) low-grade dysplasia; and (4) high-grade dysplasia; which corresponds to groups 1 to 4 according to the revised Vienna[14] classification for gastrointestinal epithelial neoplasia. The most significant category, high-grade dysplasia, is characterized by carcinoma in situ with malignant cells that do not invade the lamina propria. Category (5) corresponds to submucosal invasion by carcinoma[14,18].
However, the ability to grade dysplasia remains a subjective endeavor, particularly outside specialized centers with expert gastrointestinal pathologists[33]. Even among focused gastrointestinal pathologists there is discordance, particularly with regard to the presence of low-grade dysplasia[34]. This lack of precision inherent in histopathological grading has stimulated efforts to identify alternative methods of surveillance in patients with Barrett’s esophagus, including more objective molecular and biochemical indicators of an increased risk for progression[18].
ECS is a revolutionized endoscopic imaging technique aiming to replace the histological examination of biopsy specimens, making “optical biopsy” possible while facilitating real time decision-making[8].
ECS after double CM staining using modern integrated type endocytoscopes enables in vivo visualization of living cells and evaluation of tissue atypia by approximating the tip of the endoscope onto the mucosal surface[10]. No serious complications of ECS have been reported yet[6].
At present, a standardized endocytoscopic atypia classification system has been described for esophageal squamous cell lesions[2] and colorectal[5] adenomatous lesions. ECS has been also applied for Barrett’s esophagus[16,17,35,36], with controversial results, however, and without a standardized endocytoscopic classification system.
In contrast to previous endocytoscopic studies in Barrett’s esophagus[16,17] where a soft catheter type endocytoscope was used, endocytoscopic evaluation of long-segment Barrett’s esophagus in the present study was performed by a dual CCD integrated endocytoscope[2]. This scope has the advantage of gradual magnification at the center of the monitor, ensuring biopsies from the same area of ECS. This is important to compare endocytoscopic images to histological images. Standard endoscopy, supplemented by NBI and conventional magnification endoscopy was also performed by the same endoscope[2].
Another interesting finding of the present study is the use of the double CM staining technique, which provided higher quality endocytoscopic images of both Barrett’s metaplastic epithelium and esophageal squamous cell islands. Although double CM staining has been used in ECS of esophageal squamous cell lesions, to our knowledge, it has not been previously reported in endocytoscopic evaluation of Barrett’s esophagus.
ECS may further allow target biopsy, as in the presented case, which is extremely important in surveillance of Barrett’s esophagus where random biopsy protocols are currently in use. In the present case, ECS permitted in vivo high quality images of squamous cell islands within long-segment Barrett’s epithelium comparable to histology. To our knowledge, this is the first report of in vivo visualization of typical esophageal squamous cell islands surrounded by glandular Barrett’s epithelium. According to the positive results of the present study, although from only one case, endocytoscopic evaluation of Barrett’s mucosa is promising. However, further studies and expertise are necessary.
Barrett’s esophagus is the transformation of the normal squamous esophageal mucosa into columnar epithelium and is considered a premalignant condition with high risk of esophageal adenocarcinoma. Multiple biopsy protocols are currently the optimal practice in surveillance of Barrett’s esophagus, with significant sampling errors, however. Moreover, there is discordance regarding the ability to grade dysplasia in Barrett’s esophagus even among focused gastrointestinal pathologists. This lack of precision inherent in histopathological grading has stimulated efforts to identify alternative methods of surveillance in patients with Barrett’s esophagus.
Endocytoscopy (ECS) has emerged as a novel method of in vivo diagnosis of gastrointestinal mucosal lesions aimed at replacing the histological examination of biopsy specimens while facilitating real time decision-making.
ECS has been studied in surveillance of Barrett’s esophagus, with controversial results. In contrast to previous studies in which a soft catheter type endocytoscope was used after single methylene blue dye for staining of Barrett’s mucosa, in the present study, a novel integrated type endocytoscope after double crystal violet and methylene blue (CM) staining resulted in higher quality endocytoscopic images, corresponding to hematoxylin eosin histopathological images. To the knowledge, this is the first report of in vivo endocytoscopic visualization of typical esophageal squamous cell islands within regular glandular Barrett’s epithelium.
Based on the encouraging results of the present study, ECS, according to the technique described in this article, would be reliably used for real time, in vivo diagnosis of Barrett’s esophagus as an alternative to histological examination of biopsy specimens. ECS may allow target biopsy, as in the presented case, which is extremely important in surveillance of Barrett’s esophagus where random biopsy protocols are currently in use. However, further studies and expertise are necessary, while a standardized endocytoscopic atypia classification system, similar to that described for esophageal squamous cell lesions and colorectal adenomatous lesions, is necessary and awaited.
CCD: charged couple device; ECS is a novel endoscopic imaging of gastrointestinal mucosa, with ultra-high magnification (× 400-1100), permitting in vivo cellular imaging and observation of lumens and nuclei during routine endoscopic examination; The dual CCD integrated prototype (CIF-Y0001, EC1, Olympus, Tokyo, Japan) endocytoscope (× 480) carries both conventional magnification (× 80) and ultra-high magnification (× 480) abilities, which can be easily interchanged by pushing a button on the endocytoscope; The single CCD prototype (CIF-Y0002, EC2 Olympus) endocytoscope (× 380) has only one lens that can consecutively increase the magnification power from the conventional magnification power to × 380 using a hand lever; The revised Vienna classification of gastrointestinal epithelial neoplasia, which is based on the severity of cytological and architectural changes and on invasion status, has to some extent, resolved the differences between Western and Japanese pathologists in the diagnostic classification of gastrointestinal epithelial neoplastic lesions, especially in the use of the terminology of dysplasia, adenoma, early cancer and advanced cancer.
It is very interesting brief report. Superb images and careful description of the technique are the strong points of the paper.
P- Reviewer Maluf-Filho F S- Editor Song XX L- Editor Roemmele A E- Editor Zhang DN
| 1. | Inoue H, Yokoyama A, Kudo SE. [Ultrahigh magnifying endoscopy: development of CM double staining for endocytoscopy and its safety]. Nihon Rinsho. 2010;68:1247-1252. [PubMed] |
| 2. | Minami H, Inoue H, Yokoyama A, Ikeda H, Satodate H, Hamatani S, Haji A, Kudo S. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus. 2012;25:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Ochiai T, Momma K, Odajima H, Kawachi H, Nemoto T, Kawano T. Endocytoscopic observation of esophageal squamous cell carcinoma. Dig Endosc. 2010;22:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tomizawa Y, Abdulla HM, Prasad GA, Wong Kee Song LM, Lutzke LS, Borkenhagen LS, Wang KK. Endocytoscopy in esophageal cancer. Gastrointest Endosc Clin N Am. 2009;19:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Odajima H, Ochiai T, Kawano T, Takubo K. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis. 2012;13:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Singh R, Chen Yi Mei SL, Tam W, Raju D, Ruszkiewicz A. Real-time histology with the endocytoscope. World J Gastroenterol. 2010;16:5016-5019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R, Neurath MF. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther. 2011;33:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Galloro G. High technology imaging in digestive endoscopy. World J Gastrointest Endosc. 2012;4:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Inoue H, Sasajima K, Kaga M, Sugaya S, Sato Y, Wada Y, Inui M, Satodate H, Kudo SE, Kimura S. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: a pilot trial. Endoscopy. 2006;38:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Dutt MK. Basic dyes in the staining of DNA-phosphate groups and DNA-aldehyde molecules in cell nuclei. Microsc Acta. 1982;85:361-368. [PubMed] |
| 13. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 499] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kumagai Y, Kawada K, Yamazaki S, Iida M, Momma K, Odajima H, Kawachi H, Nemoto T, Kawano T, Takubo K. Endocytoscopic observation for esophageal squamous cell carcinoma: can biopsy histology be omitted? Dis Esophagus. 2009;22:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Eberl T, Jechart G, Probst A, Golczyk M, Bittinger M, Scheubel R, Arnholdt H, Knuechel R, Messmann H. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007;39:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Pohl H, Koch M, Khalifa A, Papanikolaou IS, Scheiner K, Wiedenmann B, Rösch T. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus. Endoscopy. 2007;39:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Oh DS, Demeester SR. Pathophysiology and treatment of Barrett’s esophagus. World J Gastroenterol. 2010;16:3762-3772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256. [PubMed] |
| 20. | Katona BW, Falk GW. Barrett’s esophagus surveillance: When, how often, does it work? Gastrointest Endosc Clin N Am. 2011;21:9-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Fléjou JF. Barrett’s oesophagus: from metaplasia to dysplasia and cancer. Gut. 2005;54 Suppl 1:i6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Wood NJ. Barrett esophagus: Need for ongoing surveillance called into question for patients with non-dysplastic Barrett esophagus. Nat Rev Gastroenterol Hepatol. 2011;8:657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Bergman JJ, Tytgat GN. New developments in the endoscopic surveillance of Barrett’s oesophagus. Gut. 2005;54 Suppl 1:i38-i42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Odze RD. What the gastroenterologist needs to know about the histology of Barrett’s esophagus. Curr Opin Gastroenterol. 2011;27:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Oberg S, DeMeester TR, Peters JH, Hagen JA, Nigro JJ, DeMeester SR, Theisen J, Campos GM, Crookes PF. The extent of Barrett’s esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg. 1999;117:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Gupta N, Mathur SC, Dumot JA, Singh V, Gaddam S, Wani SB, Bansal A, Rastogi A, Goldblum JR, Sharma P. Adequacy of esophageal squamous mucosa specimens obtained during endoscopy: are standard biopsies sufficient for postablation surveillance in Barrett’s esophagus? Gastrointest Endosc. 2012;75:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, Lightdale CJ. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736-742; quiz 710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Seewald S, Ang TL, Groth S, Zhong Y, Bertschinger P, Altorfer J, Thonke F, Soehendra N. Detection and endoscopic therapy of early esophageal adenocarcinoma. Curr Opin Gastroenterol. 2008;24:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Ramus JR, Gatenby PA, Caygill CP, Winslet MC, Watson A. Surveillance of Barrett’s columnar-lined oesophagus in the UK: endoscopic intervals and frequency of detection of dysplasia. Eur J Gastroenterol Hepatol. 2009;21:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Peters FP, Curvers WL, Rosmolen WD, de Vries CE, Ten Kate FJ, Krishnadath KK, Fockens P, Bergman JJ. Surveillance history of endoscopically treated patients with early Barrett’s neoplasia: nonadherence to the Seattle biopsy protocol leads to sampling error. Dis Esophagus. 2008;21:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 31. | Kariv R, Plesec TP, Goldblum JR, Bronner M, Oldenburgh M, Rice TW, Falk GW. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin Gastroenterol Hepatol. 2009;7:653-668; quiz 606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Mannath J, Ragunath K. Era of Barrett’s surveillance: does equipment matter? World J Gastroenterol. 2010;16:4640-4645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Alikhan M, Rex D, Khan A, Rahmani E, Cummings O, Ulbright TM. Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest Endosc. 1999;50:23-26. [PubMed] |
| 34. | Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Thekkek N, Anandasabapathy S, Richards-Kortum R. Optical molecular imaging for detection of Barrett’s-associated neoplasia. World J Gastroenterol. 2011;17:53-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Shukla R, Abidi WM, Richards-Kortum R, Anandasabapathy S. Endoscopic imaging: How far are we from real-time histology? World J Gastrointest Endosc. 2011;3:183-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |