Published online Mar 16, 2013. doi: 10.4253/wjge.v5.i3.89
Revised: January 15, 2013
Accepted: February 5, 2013
Published online: March 16, 2013
AIM: To investigate changes in efficiency and resource utilization as a single endoscopist’s experience increased with each subsequent 100 double balloon enteroscopy (DBE) procedures.
METHODS: We reviewed consecutive DBE procedures performed by a single endoscopist at our center over 4 years. DBE was employed when the clinician deemed the procedure was needed for disease management. The approach (oral, anal or both) was chosen based on suspected location of the target lesion. All DBE was performed in a standard endoscopy room with a portable fluoroscopy unit. Fluoroscopy was used to aid in shortening the small intestine and reducing bowel loops. For oral DBE, measurements were taken from the incisors. For anal DBE, measurements were taken from the anal verge. Enteroscopy continued until the target lesion was reached, until the entire small intestine was examined, or until no further progress was deemed possible. The length of small intestine examined (cm), procedure duration (min), and fluoroscopy time (s) were analyzed for sequential groups of 100 DBE. Sub-groups of diagnostic and therapeutic procedures were analyzed using multivariable linear regression.
RESULTS: 802 consecutive DBE procedures were analyzed. For oral DBE, median [interquartile range (IQR)] length of small bowel examined was 230.8 cm (range: 210-248 cm) and for anal DBE was 143.5 cm (range: 100-180 cm). No significant increase in length examined was noted for either the oral or anal approach with advancing position in series. In terms of duration of procedure, the median (IQR) for oral DBE was 86 min (range: 71-105 min) and for anal DBE was 81.3 min (range: 67-105 min). When comparing by the position in series, there was a significant (P value < 0.001) decrease in procedure duration for both upper and lower procedures with increasing experience. Median (IQR) time of exposure to fluoroscopy for oral DBE was 190 s (114-275) compared to anal DBE which was 196.4 s (312-128). This represented a significant (P value < 0.001) decrease in the amount of fluoroscopy used with increasing position in series. For both oral and anal DBE, fluoroscopy time was reduced by greater than 50% over the course of 802 total procedures performed. Sub-group analysis was conducted on therapeutic and diagnostic groups. Out of 802 procedures, a total of 434 were considered therapeutic. Argon plasma coagulation was by far the most common therapeutic intervention performed. There was no evidence of a difference in length examined or fluoroscopy exposure among oral DBE for diagnostic and therapeutic procedures, P = 0.91 and P = 0.32 respectively. The median (IQR) for length was 235 cm (range: 178-280 cm) for diagnostic vs 230 cm (range: 180-275 cm) for therapeutic procedures; additionally, fluoroscopy time median (IQR) was 180 s (range: 110-295 s) and 162 s (range: 102-263 s) for no intervention and intervention. However, there was a significant difference in procedure duration among oral DBE (P < 0.001). The median (IQR) was 80 min (range: 60-97 min) and 94 min (range: 77-110 min) for diagnostic and therapeutic interventions respectively.
CONCLUSION: For a single endoscopist, increased DBE experience with number of performed procedures is associated with increased efficiency and decreased resource utilization.
- Citation: Patel NC, Palmer WC, Gill KR, Cangemi D, Diehl N, Stark ME. Changes in efficiency and resource utilization after increasing experience with double balloon enteroscopy. World J Gastrointest Endosc 2013; 5(3): 89-94
- URL: https://www.wjgnet.com/1948-5190/full/v5/i3/89.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i3.89
Direct, intra-luminal imaging of the entire small bowel through non-surgical means was first made possible with the introduction of capsule endoscopy (CE) to clinical practice in 2001. However, CE is inherently limited by its inability to sample tissue or to perform therapeutic interventions. Double-balloon enteroscopy (DBE), first developed and reported by Yamamoto et al[1,2], subsequently allowed for therapeutic endoscopic interventions of the small bowel, such as hemostasis, tissue biopsy, and polypectomy. The procedure relies on a combination of “push-pull” maneuvers with alternating inflation and deflation of balloons attached at the tip of the endoscope and overtube, allowing for deep intubation of the small intestine. This procedure can be carried out via an oral approach or anal approach. DBE, therefore, allows for non-surgical management of certain small intestine disorders previously requiring intra-operative endoscopy or surgical intervention. It was introduced in the United States in 2004, and is now performed at a limited number of United States centers.
As DBE is still a relatively new procedure performed at a small number of centers, it is not currently implemented as a standard in endoscopic training. The majority of reported experience with DBE has come out of Europe, Japan and the United States[3-6], and has demonstrated effective clinical impact. However, the long length of procedure time and need for specific training have both been well documented, highlighting significant challenges for gastroenterologists who wish to perform DBE procedures.
A past study performed at our institution analyzed the initial experience of a single endoscopist using DBE over the course of 200 procedures[7]. Efficiency was analyzed with respect to procedure duration, estimated length of small intestine examined, and fluoroscopy time, for both oral and anal DBE. The only significant change demonstrated was increase in the estimated length of small bowel visualized after 100 anal DBE procedures[7]. Therefore, we suggested that the development of expertise in performing DBE may require greater than 200 procedures’ worth of experience. In our current study, we examined the increase in efficiency associated with DBE by the same endoscopist with significantly more experience.
We reviewed consecutive DBE procedures performed at our center between September 2005 and February 2009, under IRB review and approval. DBE was employed when the clinician deemed the procedure was needed for disease management. Eight hundred and two consecutive DBE procedures were included in our study. The length of small intestine examined (cm), procedure duration (min), and fluoroscopy time (s) were analyzed for sequential groups of 100 DBE. The length of small intestine examined, procedure duration, and fluoroscopy time were reported as median [interquartile range (IQR)] and were compared among 8 sequential groups of 100 patients each, with the last group containing 102 procedures. Trends were observed regarding different parameters of interest among these groups of patients.
All DBE procedures were performed by a single endoscopist using the Double-Balloon Enteroscopy System (Fujinon Inc, Wayne, NJ), with the Fujinon EN-450T5 enteroscope and TS-13140 overtube (Fujinon), or the EN-450P5 enteroscope and TS-12140 overtube, respectively. The PB-10 Balloon Pump Controller (Fuginon) controlled balloon inflation. The approach (oral, anal or both) was chosen based on suspected location of the target lesion. All DBE was performed in a standard endoscopy room with a portable fluoroscopy unit. The endoscopist was assisted in all cases by a technician and a nurse, all of whom wore lead aprons and cumulative radiation exposure badge monitors. Fluoroscopy was used to aid in shortening the small intestine and reducing bowel loops.
Oral DBE was performed with endotracheal intubation and general anesthesia, in the supine position with right shoulder raised. Measurements were taken from the incisors. Anal DBE was employed under conscious sedation (meperidine and midazolam) with the patient in the left-lateral decubitus or supine position. Measurements were taken from the anal verge. All measurements were documented using the May method[8]. Enteroscopy continued until the target lesion was reached, until the entire small intestine was seen, or until no further progress was deemed possible.
All DBEs were performed by a single endoscopist (Stark ME) with 15 years of endoscopic practice, including over 10 000 colonoscopies, 5000 upper endoscopies, and 200 push enteroscopies. The endoscopist learned the DBE technique through one day of instructions that included hands-on training with animal intestine models and observation of human cases performed by an expert. The endoscopist also reviewed the available literature and discussed the technique with endoscopists already experienced in DBE.
Comparative analyses of oral and anal DBE approaches were conducted by stratifying sequential series of 100 DBE procedures, with P values obtained using the nonparametric Jonckheere-Terpstra test for unidirectional trend. All DBE procedures were categorized into diagnostic or therapeutic procedures and subgroups were analyzed for these same three variables (time of procedure, length of bowel examined, fluoroscopy time used) using multivariable linear regression.
A total of 802 consecutive DBE procedures were analyzed. For oral DBE, median (IQR) length of small bowel examined was 230.8 cm (range: 210-248 cm). Consequently, for anal DBE, median (IQR) length of small bowel was 143.5 cm (range: 100-180 cm) (Table 1). No significant increase in length examined was noted for either the oral or anal approach with advancing position in series. In terms of duration of procedure, the median (IQR) for oral DBE was 86 min (range: 71-105 min) and for anal DBE was 81.3 min (range: 67-105 min). When comparing by the position in series, there was a significant (P value < 0.001) decrease in procedure duration for both upper and lower procedures with increasing experience (Table 1). The final efficiency parameter analyzed was fluoroscopy time. Median (IQR) time of exposure to fluoroscopy for oral DBE was 190 s (range: 114-275 s) compared to anal DBE which was 196.4 s (range: 312-128 s). This represented a significant (P value < 0.001) decrease in the amount of fluoroscopy used with increasing position in series (Table 1). For both oral and anal DBE, fluoroscopy time was reduced by greater than 50% over the course of 802 total procedures performed.
| Position in series (n) | Length, cm, median (IQR) | P value | Duration, min, median (IQR) | P value | Fluoroscopy time, s, median (IQR) | P value |
| Oral DBE | ||||||
| 1-100 (51) | 220 (180-275) | 100 (85-110) | 275 (180-356) | |||
| 101-200 (62) | 210 (150-260) | 83 (80-110) | 278 (211-336) | |||
| 201-300 (58) | 248 (200-285) | 105 (80-12) | 282 (188-339) | |||
| 301-400 (54) | 230 (195-300) | < 0.97 | 92 (75-105) | < 0.001 | 193 (132-295) | < 0.001 |
| 401-500 (55) | 238 (175-295) | 82 (60-100) | 134 (80-159) | |||
| 501-600 (56) | 240 (185-275) | 80 (62-97) | 114 (73-146) | |||
| 601-700 (54) | 235 (180-275) | 75 (62-97) | 117 (85-164) | |||
| 701-802 (54) | 225 (155-270) | 71 (54-91) | 129 (85-192) | |||
| Anal DBE | ||||||
| 1-100 (49) | 100 (70-150) | 105 (75-125) | 312 (191-469) | |||
| 101-200 (38) | 140 (100-170) | 85 (75-105) | 294 (206-360) | |||
| 201-300 (42) | 130 (60-198) | 80 (58-105) | 253 (122-331) | |||
| 301-400 (46) | 180 (100-250) | 0.058 | 75 (56-102) | < 0.001 | 161 (85-223) | < 0.001 |
| 401-500 (45) | 145 (40-170) | 67 (55-88) | 128 (67-174) | |||
| 501-600 (44) | 168 (100-213) | 92 (74-110) | 143 (90-202) | |||
| 601-700 (46) | 140 (80-180) | 76 (52-96) | 138 (80-174) | |||
| 701-802 (48) | 145 (100-190) | 70 (56-87) | 142 (83-204) |
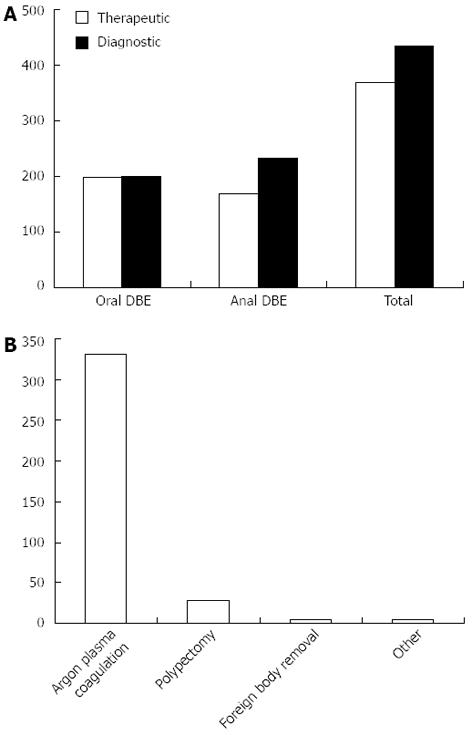
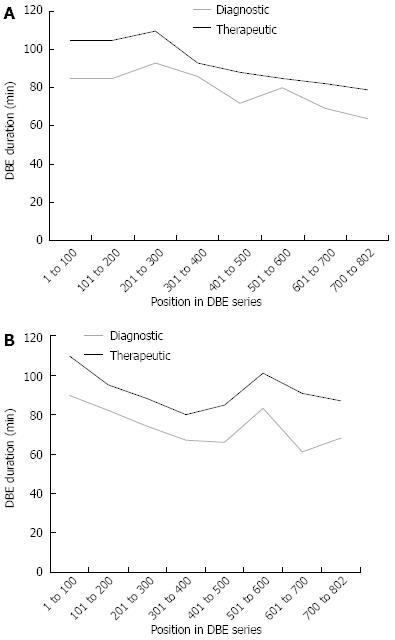
After all 802 DBE procedures were analyzed, sub-group analysis was conducted on therapeutic and diagnostic groups. Out of 802 procedures, a total of 434 were considered therapeutic (Figure 1A). Argon plasma coagulation (APC) was by far the most common therapeutic intervention performed (Figure 1B). There was no evidence of a difference in length examined or fluoroscopy exposure among oral DBE for diagnostic and therapeutic procedures, P = 0.91 and P = 0.32 respectively. The median (IQR) for length was 235 cm (range: 178-280 cm) for diagnostic versus 230 cm (range: 180-275 cm) for therapeutic procedures. Additionally, fluoroscopy time median (IQR) was 180 s (range: 110-295 s) and 162 s (range: 102-263 s) for no intervention and intervention. However, there was a significant difference in time among oral DBE (Figure 2A) compared to anal DBE (Figure 2B). The median (IQR) was 80 min (range: 60-97 min) and 94 min (range: 77-110 min) for diagnostic and therapeutic interventions respectively.
To our knowledge, there are no published reports which quantify how increasing experience with DBE affects efficiency. An early study by Mehdizadeh et al[4], set out to describe the learning curve associated with DBE by analyzing data from DBE procedures performed in six tertiary United States centers over the course of one year. They subsequently found a clinically significant decline in the overall procedure time for oral DBE after the first 10 cases, but no change in anal DBE procedure time. In addition, this study reported a significant decline in mean fluoroscopy usage after 7 cases but no change in the mean depth of insertion via the oral or anal route with experience[4]. Yamamoto et al[2] initially demonstrated that examination of the entire small bowel was possible in up to 86% of patients, with a source of bleeding identified in 76% of patients with gastrointestinal bleeding. Our data corroborate this decrease in procedure time and decrease in fluoroscopy time with further experience by a single endoscopist. Further, our data show no evidence that increasing experience positively affects the length of bowel examined. The authors recognize, however, that in many cases the procedural goal is not to evaluate as much of the bowel as possible, but to reach a specific target lesion.
A recent meta-analysis of short-term data from nineteen different published series worldwide of DBE, including original data from Yamamoto et al[2], revealed mean diagnostic yield of 67% ± 14% (range: 41%-81%), and a mean treatment success rate of 64% ± 13% (range: 42%-84%)[9]. Total enteroscopy was performed on average, in 34% of patients (range: 0%-86%), though the author speculated that the low rate of total enteroscopy could be attributed to pathology being identified on initial approach of the endoscope, or from limiting anatomic factors encountered during the procedure.
By increasing DBE efficiency and thereby reducing fluoroscopy time needed during the procedure, unnecessary radiological exposure and cost of procedure is reduced. While DBE is largely considered a safe procedure for both the staff and the patient, safety monitoring did not show unsafe levels of exposure to our personnel. We did not directly measure radiation exposure to our patients, however, our radiation safety office estimated the exposure to be similar to that of a computed tomography (CT) or an endoscopic retrograde cholangiopancreatography. An increase in DBE efficiency thereby reducing procedural time would theoretically positively impact complication rates as well. This has been shown in a previously published ten year meta-analysis to include a minor complication rate of 9.1%, and major complication rate of 0.72%, and included perforation, pancreatitis, bleeding, and aspiration pneumonia[4]. Previously, the largest-ever study published from the German DBE register demonstrated a major complication rate of 1.2%[10]. By decreasing procedural times through improved technique and efficiency, the endoscopist may limit patient risk for such events.
The overall cost of endoscopy and fluoroscopy are factors in justifying DBE in high volumes. As we show, a more efficient DBE endoscopist will continue to decrease even further the estimated resource utilization of DBE, including total procedure time and amount of fluoroscopy used. Our data demonstrate a significant decrease in procedure duration for both upper and lower procedures with increasing experience, as well as a reduction of fluoroscopy time of greater than 50% over the course of 802 total procedures performed, for both oral and anal DBE.
Our sub-group analysis demonstrates that length of bowel examined and fluoroscopy time were not different in the therapeutic compared to the diagnostic procedures, making therapeutic DBE a practical intervention. At our institution, APC was by far the most common of these DBE interventions as it is often selected as a treatment for obscure gastrointestinal bleeding (OGIB). Generally, the primary indication for DBE is OGIB, in which patients have persistent or recurrent bleeding from the gastrointestinal tract, with no source of bleeding identified after conventional upper endoscopy and colonoscopy. A recent systemic review of studies related to diagnostic DBE published over the past decade revealed that suspected mid-GI bleeding was by far the most common indication for DBE, accounting for 60.2% of the 12 267 procedures reviewed[11].
Cost and resource utilization of DBE have been concerns raised by several recent studies, including Gerson et al[12]. Through cost-effective analysis, the authors determined that initial DBE was more cost effective than push enteroscopy, intraoperative enteroscopy, angiography, small bowel capsule endoscopy (CE), or guided DBE after initial CE in the management of obscure gastrointestinal. The model did not account for indirect costs such as days lost from work, but the combination of diagnostic and intervention provided by initial DBE did prove a statistically significant advantage in bleeding cessation rates compared to other modalities. Also, this study did not account for increasing efficiency as the endoscopist gains experience, which we see in our study as our endoscopist increased his total number of procedures. In a recent study, Benson et al[13] examined predicted and actual cost/profit analysis for an academic tertiary referral center. Although predicted percent margins were lower than expected, the authors found actual margins at 37 cm approaching 5% for double balloon procedures, with even greater margins seen in specific payer subsets. This data further support the financial benefit of double balloon procedures.
This study has many strengths associated with it. It was designed specifically as a follow up to our previous study stating our initial experience with DBE. Being a tertiary referral center, we receive a large volume of patients from which to properly select ideal candidates for the DBE procedure. Most importantly, this study is well powered as there are a large number of consecutive DBE procedures. Additionally, these procedures were all performed by a single endoscopist, controlling for variations in experience and technical ability.
However, this study was not without weaknesses. Primarily, this was a retrospective review of a single endoscopist, and was not randomized or blinded. Our endoscopist had rapid access to advanced imaging such as CT enterography and capsule endoscopy, which allow for better estimation of the target lesion location and approach. This could have introduced selection bias into our statistics. Third, the lack of multiple DBE endoscopists at our center does limit the ability to compare intra-institutional data.
In conclusion, these results show that for a single endoscopist, increased DBE experience is associated with increased efficiency and decreased resource utilization, in terms of total time of procedure and fluoroscopy used. However, there is essentially no significant decrease in resource utilization or increase in efficiency with experience when comparing diagnostic vs therapeutic DBE.
DBE is a useful technique for directly visualizing the small intestine, and is generally accepted as a safe and effective procedure, particularly with regard to obscure gastrointestinal bleed management. In our initial experience of 200 total DBE procedures[7], we did validate that safety and efficacy can be achieved after relatively limited DBE training. As DBE is still a relatively new procedure, and is currently not learned with standard endoscopic training, expectations concerning acquired efficiency over time are not universally established. Our data demonstrate that decreases in both procedural time and resource utilization are made possible by increases in the experience and efficiency of a single DBE endoscopist.
The rising cost of healthcare in the United States require that institutions explore cost-saving measures, and investigate the efficacy of any test or procedure that limits overall expenses of disease treatment. As Gerson et al[12] demonstrated in the case of OGIB management, DBE used in an efficient manner can contribute to reducing medical costs by providing a non-surgical diagnostic and therapeutic intervention.
Double ballon enteroscopy (DBE) relies on a combination of “push-pull” maneuvers with alternating inflation and deflation of balloons attached at the tip of the endoscope and overtube, allowing for deep intubation of the small intestine. As DBE is still a relatively new procedure performed at a small number of centers, it is not currently implemented as a standard in endoscopic training.
DBE’s long length of procedure time and the need for specific training have both been well documented, highlighting significant challenges for gastroenterologists who wish to perform the procedure. A past study performed at the institution analyzed the initial experience of a single endoscopist using DBE over the course of 200 procedures. The authors now present data on subsequent procedures.
For a single endoscopist, increased DBE experience with number of performed procedures is associated with increased efficiency and decreased resource utilization.
The data demonstrate that decreases in both procedure time and resource utilization are made possible by increases in the experience and efficiency of a single DBE endoscopist. In an era of rising health care costs, DBE used in an efficient manner can contribute to reducing medical costs by providing a non-surgical diagnostic and therapeutic intervention.
DBE: Performed with combination of “push-pull” maneuvers with alternating inflation and deflation of balloons attached at the tip of the endoscope and overtube, allowing for deep intubation of the small intestine
The article reports a large, retrospective, single operator series of DBEs over a period of four years. The study strengths and limitations are well highlighted in the discussion section. The article is well written and statistics are well done.
P- Reviewers Karagiannis S, Girelli CM S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [PubMed] |
| 2. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [PubMed] |
| 3. | Ell C, May A, Nachbar L, Cellier C, Landi B, di Caro S, Gasbarrini A. Push-and-pull enteroscopy in the small bowel using the double-balloon technique: results of a prospective European multicenter study. Endoscopy. 2005;37:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Manabe N, Tanaka S, Fukumoto A, Nakao M, Kamino D, Chayama K. Double-balloon enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2006;64:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Di Caro S, May A, Heine DG, Fini L, Landi B, Petruzziello L, Cellier C, Mulder CJ, Costamagna G, Ell C. The European experience with double-balloon enteroscopy: indications, methodology, safety, and clinical impact. Gastrointest Endosc. 2005;62:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Gross SA, Stark ME. Initial experience with double-balloon enteroscopy at a U.S. center. Gastrointest Endosc. 2008;67:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | May A, Nachbar L, Schneider M, Neumann M, Ell C. Push-and-pull enteroscopy using the double-balloon technique: method of assessing depth of insertion and training of the enteroscopy technique using the Erlangen Endo-Trainer. Endoscopy. 2005;37:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Gerson LB. Outcomes associated with deep enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:481-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Möschler O, May AD, Müller MK, Ell C. [Complications in double-balloon-enteroscopy: results of the German DBE register]. Z Gastroenterol. 2008;46:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Xin L, Liao Z, Jiang YP, Li ZS. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Benson ME, Horton W, Gluth J, Pfau PR, Einarsson S, Lucey MR, Soni A, Reichelderfer M, Gopal DV. Fiscal analysis of establishment of a double-balloon enteroscopy program and reimbursement. Clin Gastroenterol Hepatol. 2012;10:371-6.e1-371-6.e2. [PubMed] |