Published online Mar 16, 2013. doi: 10.4253/wjge.v5.i3.128
Revised: September 17, 2012
Accepted: December 22, 2012
Published online: March 16, 2013
We report a case of multiple flat adenomas and cancer of the rectum that occurred 15 years after pelvic irradiation following surgery for uterine cancer. Adenoma borders were diagnosed accurately by magnifying chromoendoscopy, leading to their adequate excision using endoscopic submucosal dissection. This enabled minimal dissection of the irradiated pelvis that would have otherwise been difficult. Furthermore, our approach probably helped minimize loss of bowel function, thereby preserving the patient’s quality of life as much as possible. Pathology of the resected specimens revealed thickened walls of the submucosal layer vessels, indicating chronic radiation proctitis. Pelvic irradiation of the bowel carries a high risk of causing flat adenomas and cancer. Close and long-term surveillance may be useful in such cases, using not only conventional colonoscopy but also chromoendoscopy with indigo carmine dye spray and magnifying endoscopy.
- Citation: Asayama N, Ikehara H, Yano H, Saito Y. Endoscopic submucosal dissection of multiple flat adenomas in the radiated rectum. World J Gastrointest Endosc 2013; 5(3): 128-131
- URL: https://www.wjgnet.com/1948-5190/full/v5/i3/128.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i3.128
Pelvic irradiation is frequently used as a definitive or adjunctive treatment for pelvic malignancy, and development of cancer in this region is considered a late complication[1-4]. We recently experienced a case of multiple flat adenomas and cancer in the rectum that occurred 15 years after pelvic irradiation following surgery for uterine cancer. The borders of these adenomas could be accurately diagnosed by magnifying chromoendoscopy, leading to their adequate excision using endoscopic submucosal dissection (ESD). This enabled us to minimize the extent of surgical dissection in the irradiated pelvis when removing the remaining neoplasm.
To our knowledge, there have been no similar reports of endoscopic resection of multiple flat adenomas in the irradiated rectum. The superficial neoplastic lesions were described according to the Paris endoscopic classification of superficial neoplastic lesions[5].
A 46-year-old woman presented with bloody stools in November 2010. She had undergone radical hysterectomy followed by pelvic irradiation (total 54 Gy) for uterine cancer 15 years earlier. There was no family history of colorectal cancer. On physical examination, her lower abdomen was slightly hard because of the surgical scar, bowel sound was normal, and superficial lymph nodes were not palpable. All other physical examinations were unremarkable. Laboratory studies, including complete blood cell counts, serum electrolytes, blood biochemistry, carcinoembryonic antigen, and CA19-9 were within normal limits.
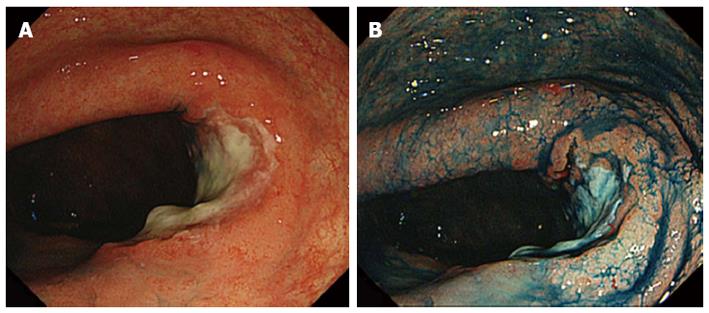
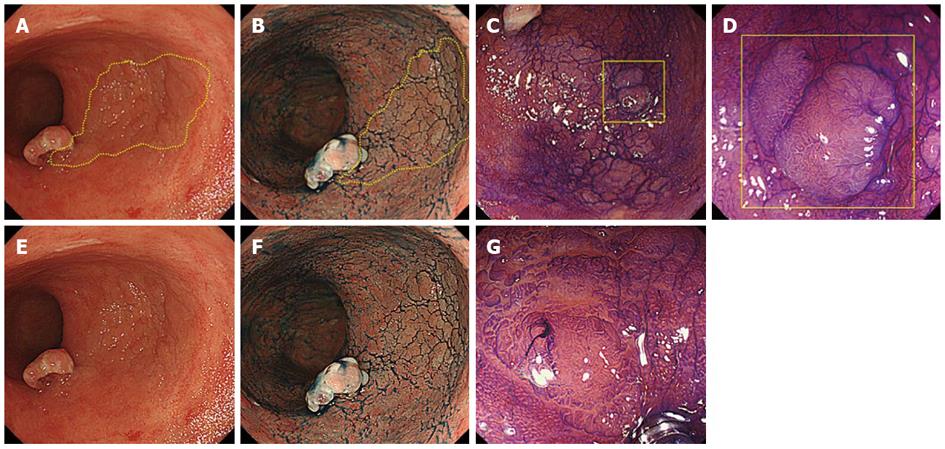
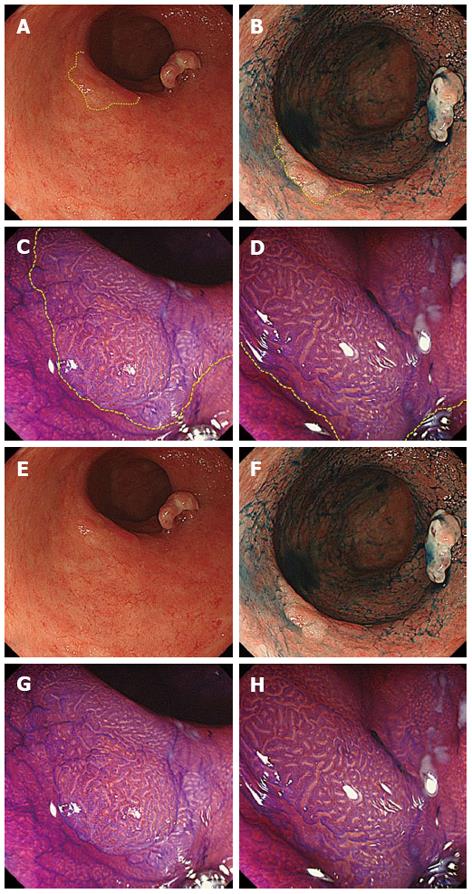
Colonoscopy using a magnifying videoscope (CF H260AZI high vision scope, Olympus, Tokyo, Japan) revealed five lesions: a 35-mm flat adenoma in the sigmoid colon, an advanced cancer, a 30-mm flat adenoma in the rectum (Figure 1), and two flat adenomas (35 mm and 10 mm) in the lower rectum. Although we could highlight the irregular surface by spraying indigo carmine solution, it was difficult to trace the lesion margin. On magnifying endoscopy with crystal violet staining, the surface structure of the four lesions was found to be composed mainly of IIIL and IV pits, which suggested adenomas. Magnifying chromoendoscopy helped to delineate the borders of these lesions distinctly (Figures 2, 3). ESD was performed for two rectal flat lesions, which minimized the extent of dissection in the subsequent anterior resection of the rectal cancer (pT3 N0 M0) in January 2011.
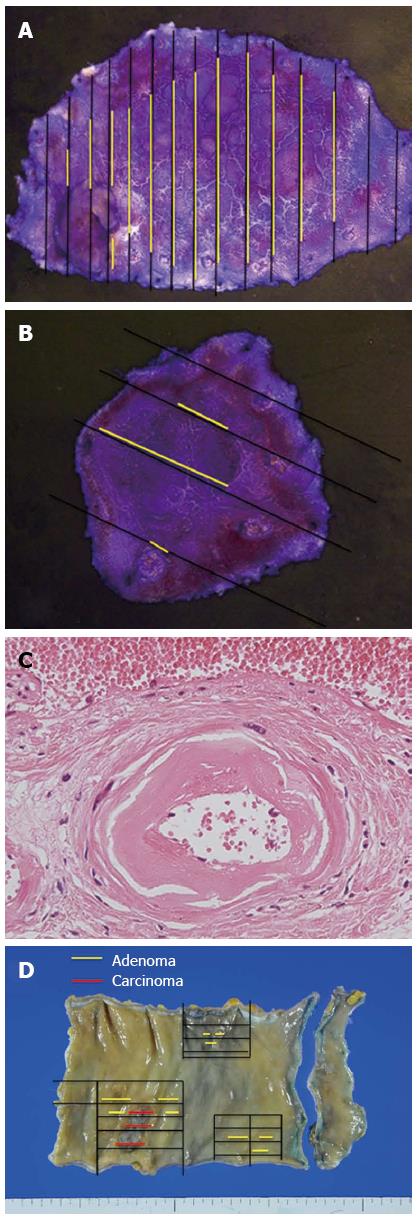
Pathological examination revealed the two flat lesions in the lower rectum to be tubular adenomas. The resected specimens measured 52 mm × 30 mm and 18 mm × 18 mm with sufficient margins (Figure 4A, B). In the resected rectum specimen, wall-thickening of the vessels in the submucosal and subserosal layers was evident (Figure 4C), indicating chronic radiation proctitis[6]. No recurrence was observed during follow-up.
To our knowledge, no cases of endoscopic removal of flat adenomas arising in the radiated rectum have been reported. The current case is particularly interesting not only because the patient developed advanced rectal cancer 15 years after pelvic irradiation therapy, but also because she simultaneously developed multiple sizeable flat adenomas that would have been extremely difficult to diagnose on routine colonoscopy alone. In fact, magnifying chromoendoscopy proved useful for accurate diagnosis, and led to adequate resection using ESD. Chromoendoscopy with crystal violet is indeed time-consuming. However, magnifying chromoendoscopy enabled accurate diagnosis of the surface structure of the lesions. Narrow-band imaging (NBI) is a recent development designed to enhance standard endoscopy with superior delineation of mucosal surface capillaries. In the present case, we did not estimate these lesions by using NBI. However, the features of NBI suggest that it is more useful than conventional colonoscopy for evaluating flat adenomas. Pathology of the resected specimens revealed thickened vessel walls in the submucosal layer, indicating chronic radiation proctitis[6].
We predicted that using endoscopic mucosal resection it would be difficult to achieve complete one-piece resection with tumor-free margins because adequate lifting of the lesions after submucosal injection was not obtained due to severe submucosal fibrosis after the previous radiation therapy. Therefore, we performed ESD for two flat adenomas in the lower rectum. By adequately removing the adenomas in the low rectum by ESD, we were able to minimize the extent of dissection of the irradiated pelvis which would have otherwise been difficult. Furthermore, it is likely that this approach helped minimize the loss of bowel function, thereby minimizing the impact on quality of life.
Flat adenomas are generally hard to detect on routine colonoscopy. Moreover, background radiation proctocolitis, resulting in flat lesions, makes accurate diagnosis more difficult. In fact, the pathology of the surgically resected specimen revealed a cancer-associated adenomatous component which endoscopic observation had failed to detect (Figure 4D). It is important to bear in mind that patients who have undergone pelvic irradiation may be at high risk of developing flat adenomas or neoplasms in the irradiated bowel[1-4]. Close and long-term surveillance using not only conventional colonoscopy but also chromoendoscopy with indigo carmine dye spray and magnifying endoscopy may therefore prove useful in such patients.
P- Reviewer Yoshida N S- Editor Song XX L- Editor Hughes D E- Editor Zhang DN
| 1. | Jao SW, Beart RW, Reiman HM, Gunderson LL, Ilstrup DM. Colon and anorectal cancer after pelvic irradiation. Dis Colon Rectum. 1987;30:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Castro EB, Rosen PP, Quan SH. Carcinoma of large intestine in patients irradiated for carcinoma of cervix and uterus. Cancer. 1973;31:45-52. [PubMed] |
| 3. | Morita H, Koyama N, Tamura Y. Development of flat adenoma and superficial rectal cancer after pelvic radiation. J Clin Gastroenterol. 1998;26:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Kimura T, Iwagaki H, Hizuta A, Nonaka Y, Tanaka N, Orita K. Colorectal cancer after irradiation for cervical cancer--case reports. Anticancer Res. 1995;15:557-558. [PubMed] |
| 5. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [PubMed] |
| 6. | Qizilbash AH. Radiation-induced carcinoma of the rectum. A late complication of pelvic irradiation. Arch Pathol. 1974;98:118-121. [PubMed] |