Published online Mar 16, 2013. doi: 10.4253/wjge.v5.i3.122
Revised: August 22, 2012
Accepted: January 23, 2013
Published online: March 16, 2013
AIM: To investigate the yield, etiologies and impact of capsule endoscopy (CE) in Thai patients with obscure gastrointestinal bleeding (OGIB).
METHODS: The present study is a retrospective cohort study. All patients with OGIB who underwent CE in Siriraj Hospital, Bangkok, Thailand during 2005-2009 were included in the study. All the patients’ medical records and results of the CE videos were reviewed. CE findings were classified as significant, suspicious/equivocal and negative. Sites of the lesions were located to duodenum, jejunum, jejunoileum, ileum and diffuse lesions by the localization device of the CE. Impact of CE on the patients’ management was defined by any investigation or treatment given to the patients that was more than an iron supplement or blood transfusion. Patients’ outcomes (rebleeding, persistent bleeding, anemia or requirement of blood transfusion) were collected from chart reviews and direct phone interviews with the patients.
RESULTS: Overall, there were 103 patients with OGIB included in the study. Mean age of the patients was 64 ± 16 years (range 9-88 years) and 57 patients (55%) were male. Types of OGIB were overt in 80 (78%) and occult in 23 patients (22%). The median time interval of CE after onset of OGIB was 10 d (range 1-180 d). The median time of follow-up was 19 mo (range 1-54 mo). Capsules reached caecum in 77 patients (74%) and capsule retention was found in 1 patient (1%). The diagnostic yield of CE revealed significant lesions in 37 patients (36%), suspicious/equivocal lesions in 15 patients (15%) and 51 patients (49%) had negative CE result. Among the significant lesions, the bleeding etiologies were small bowel ulcers in 44%, angiodysplasia in 27%, small bowel tumor in 13%, miscellaneous in 8% and active bleeding without identifiable causes in 8%. Patients with small bowel ulcers were significantly associated with the use of non-steroidal anti-inflammatory drugs (48%, P = 0.034), while patients with small bowel tumors were more commonly female (86%, P = 0.043) compared to the other etiologies. The rate of rebleeding, persistent bleeding or anemia in patients with positive, equivocal and negative CE results were 5%, 0% and 18%, respectively (P = 0.078). All the 9 patients with rebleeding after negative CE were subsequently found to be from hematologic disorders (4), colonic diverticulosis (2), colonic Dieulafoy’s (1), hemorrhoid (1) and hemosuccus pancreaticus (1). Results of CE had a positive impact on the patients’ management in 35% of the patients whose results were positive, but none on the patients whose results were equivocal or negative CE (P < 0.001).
CONCLUSION: In Thai OGIB patients, CE had low yield and small bowel ulcer was most common. Positive CE impacted managements and outcomes. Negative CE caused low rebleeding.
- Citation: Pongprasobchai S, Chitsaeng S, Tanwandee T, Manatsathit S, Kachintorn U. Yield, etiologies and outcomes of capsule endoscopy in Thai patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc 2013; 5(3): 122-127
- URL: https://www.wjgnet.com/1948-5190/full/v5/i3/122.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i3.122
Obscure gastrointestinal bleeding (OGIB) is defined as a persistent or recurrent gastrointestinal bleeding without a source being identified by standard evaluations, including esophagogastroduodenoscopy and colonoscopy. It is further classified into overt and occult gastrointestinal bleeding[1]. OGIB accounts for an approximately 5% of all gastrointestinal bleeding and the most common site of bleeding is in the small intestine[1]. The difficulty in examining the entire small intestine has made an assessment of small intestinal sources of bleeding problematic.
Capsule endoscopy (CE) has recently been developed as a non invasive method for examining the small bowel. CE provides opportunity to obtain images from the entire length of the small intestine in most patients, thus has been accepted as a first-line investigation of patients with OGIB[1,2]. Recent meta-analysis showed that CE provided the highest diagnostic yield for OGIB (61%-63%)[3,4], comparable to double-balloon enteroscopy[5], but much higher than push enteroscopy (28%) and small bowel radiography (8%)[3]. The most common cause of OGIB identified by CE in most studies was angiodysplasia[6,7] and results of CE were shown to impact management in 50%-70% of cases[8].
However, there are some debates on the long-term outcome of patients with negative CE, whether negative CE predicts low or substantial rebleeding remains controversial. Furthermore, data of CE in OGIB in Asia is limited and the etiology of OGIB among Asians may be different from Westerners. This study aims to evaluate the etiologies of OGIB among Thai patients, and to determine the diagnostic yield and the impacts on patient management and outcomes in association with the results of CE.
All patients with OGIB who had CE done at the Division of Gastroenterology, Siriraj Hospital during 2005-2009 were identified and included in the study. The study was approved by Siriraj Institute Review Board. OGIB was defined as an evidence of melena, hematochezia, drop of hemoglobin (Hb) level for at least 2 g/dL or a positive fecal occult blood test, accompanied by negative EGD and colonoscopy.
CE studies (PillCam®, Given Imaging, Israel) were performed according to standard protocol, which included an overnight fast, the use of bowel preparation (2 sachets of polyethylene glycol in 2 L of fluid), a prokinetic agent (metoclopramide 10 mg intravenous injection) for immobilized patients, patients with longstanding diabetes or patients with known slow transit. A second recorder technique was done periodically to check the position of the capsule[9]. All videos were reviewed by 2 readers, one of which is an experienced reader who had officially reviewed more than 500 cases of CE (SP).
CE findings were classified as highly significant lesions (P2) which was likely to explain source of bleeding, suspicious or equivocal lesions (P1) and negative for any lesions (P0) according to the report by Saurin et al[10]. Examinations that demonstrated one or more P2 lesions were recorded as positive studies, whereas those with P1 and P0 lesions were considered equivocal and negative, respectively.
Location of the lesions was classified using the localization device on the screen of the CE platform. Duodenum was located right after the capsule entered duodenum and quickly moved along the path of C-loop from right- to left-side of the of the localization device. Jejunum was located when capsule was in the left side of the localization device or the proximal third of the small bowel transit. Ileum was located when capsule was in the right side of the localization device or the distal third of the small bowel transit. Capsule located in the mid abdomen or in the middle third of the small bowel was classified as jejunoileal lesion.
Change of patient management was defined by any treatment other than iron supplement and blood transfusion. They included any of the followings, i.e., surgery, further endoscopy and specific medications.
Long-term outcomes were evaluated by the presence of persistent or rebleeding episodes. Rebleeding was defined as an evidence of melena, hematochezia, a documented fall of Hb 2 g/dL from baseline or more and the need for blood transfusion. Follow-up data were obtained from the medical records and direct phone interview with the patients.
All the data collected were subjected to a descriptive analysis. For numerical variables, the results were expressed as a mean ± SD. For quantitative variables, percentages are shown with 95%CI. The comparison of numerical variables between groups was accomplished by using the Student’s t-test or the Mann-Whitney test, as appropriate. For comparison of percentages, the χ2 test or the fisher’s exact test were used together with the calculation of the odds ratio and its 95%CI. Long-term outcome was analyzed by using Kaplan-Meier analysis. The value of P < 0.01 was considered significant. The SPSS 13.0 for windows was used for the statistical analysis.
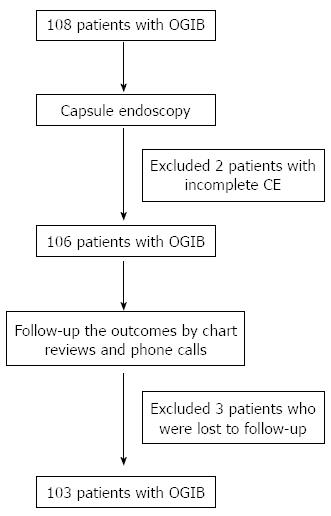
Overall, there were 108 patients, who underwent CE for OGIB at Siriraj Hospital during the 5-year periods from 2005-2009. Five patients were excluded because 2 of them failed to complete the procedures, and 3 patients lost contact and were impossible to follow their clinical outcomes. Finally, 103 patients were included in the analysis (Figure 1).
The baseline characteristics of the studied population are shown in Table 1. Mean age was 64 ± 16 years (range 9-88 years) and 57 patients (55%) were male. Types of OGIB were found to be overt in 80 (78%) and occult in 23 patients (22%). Median time interval of CE after onset of OGIB was 10 d (range 1-180 d). The proportions of patients who had CE done within 2 d, 3-7 d, 8-14 d and more than 14 d were 7%, 30%, 31% and 32%, respectively. The median time of follow-up was 19 mo (range 1-54 mo).
| Parameters | Type of OGIB | P value | ||
| Total(n = 103) | Overt(n = 80) | Occult(n = 23) | ||
| Age (yr), mean ± SD | 64 ± 16 | 62 ± 16 | 69 ± 14 | 0.059 |
| Male, n | 57 (55) | 51 (64) | 6 (26) | 0.001 |
| Episode, n | ||||
| First | 79 (77) | 56 (70) | 23 (100) | 0.003 |
| Recurrent | 24 (23) | 24 (30) | 0 | |
| Hb (g/dL), mean ± SD | 8.5 ± 2.4 | 8.7 ± 2.5 | 8.2 ± 1.9 | 0.371 |
| Drugs, n | ||||
| NSAIDs | 30/100 (30) | 22/77 (29) | 8/23 (35) | 0.568 |
| Anticoagulants | 4/100 (4) | 2/77 (3) | 2/23 (9) | 0.226 |
Capsules reached caecum in 77 patients (74%) and capsule retention was found in 1 patient (1%) due to undiagnosed stricture in Crohn’s disease. However, the capsule passed beyond the stricture spontaneously 3 d after initiating corticosteroid treatment. Diagnostic yield of CE revealed significant lesions which could explain GI bleeding in 37 patients (36%), suspicious or equivocal lesions in 15 patients (15%) while 51 patients (49%) had negative CE result. Among the significant lesions, the bleeding etiologies were found to be small bowel ulcers in 44%, angiodysplasia in 27%, small bowel tumor in 13%, miscellaneous in 8% and active bleeding without identifiable causes in 8% (Table 2). Natures of the small bowel ulcers were caused by nonsteroidal anti-inflammatory drugs (NSAIDs)-induced in 8 (35%), CD in 3, radiation in 1, chemotherapy-induced in 1 and undetermined cause in 10 patients.
| Parameters | Type of OGIB | P value | ||
| Total(n = 103) | Overt(n = 80) | Occult(n = 23) | ||
| Reaching caecum, n | 77 (74) | 60 (75) | 17 (74) | 0.916 |
| Capsule endoscopy findings, n | ||||
| Positive (P2) | 37 (36) | 28 (35) | 9 (39) | |
| Equivocal (P1) | 15 (15) | 11 (14) | 4 (17) | 0.792 |
| Negative (P0) | 51 (49) | 41 (51) | 10 (43) | |
| Etiologies, n1 | ||||
| Ulcers | 23 (44) | 15 (38) | 8 (61) | |
| Angiodysplasia | 14 (27) | 12 (31) | 2 (15) | |
| Tumors | 7 (13) | 6 (15) | 1 (8) | 0.462 |
| Miscellaneous | 4 (8) | 3 (8) | 1 (8) | |
| Active bleeding | 4 (8) | 3 (8) | 1 (8) | |
| Location of lesions, n2 | ||||
| Duodenum | 6 (13) | 4 (11) | 2 (17) | |
| Jejunum | 15 (31) | 11 (31) | 4 (33) | |
| Jejuno-ileal | 12 (25) | 9 (25) | 3 (25) | 0.969 |
| Ileum | 14 (29) | 11 (30) | 3 (25) | |
| Diffuse | 1 (2) | 1 (3 ) | 0 | |
Patients’ characteristics according to their etiology were demonstrated in Table 3. Patients’ age and type of OGIB were similar in all etiologies, however, patients with small bowel tumors were more commonly female (86%, P = 0.043) as compared to other etiologies. History of NSAIDs use was more common in patients with small bowel ulcers (48%, P = 0.034) but their sensitivity, specificity, positive predictive value and negative predictive value were 48%, 75%, 37% and 83%, respectively.
The results of CE finally determined patient management in 13 (35%) of the 37 patients with positive CE result. Surgery was performed in 8 patients, immunosuppressive therapy for inflammatory bowel disease was initiated in 4 patients and anti-parasitic agent was administered in 1 patient. On the other hand, none of the patients with equivocal or negative CE resulted in changing of management plan (P < 0.001) and all were treated with iron supplement and blood transfusion.
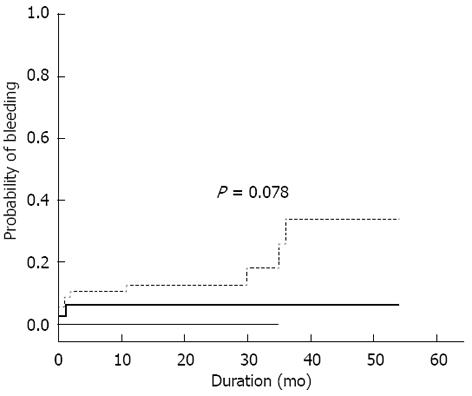
Rebleeding, persistent bleeding or persistent anemia occurred in 5%, 0% and 18% of patients with positive, equivocal and negative CE results, respectively (P = 0.078, Figure 2). Positive CE resulted in fewer rebleeding as compared to patients with negative CE (hazards ratio 0.31, 95%CI 0.07-1.43). Causes of rebleeding in all CE-negative patients (n = 9) were subsequently found to be associated with hematologic disorders, i.e., myelodysplastic syndrome in 4 patients, colonic diverticulosis in 2 patients, colonic Dieulafoy’s in 1 patient, hemorrhoid in 1 patient and hemosuccus pancreaticus in 1 patient.
In the present study, the diagnostic yield, etiology and outcomes of 103 patients with OGIB who underwent CE in a single institution in Thailand were reported. This is the largest study on capsule endoscopy in OGIB in Thai patients. The main information obtained from this study was that the diagnostic yield for significant lesions of OGIB was substantially low (36%). The most common etiology was small bowel ulcers (44%). The long-term rebleeding rate in patients with negative CE results was low (18%) and most rebleedings were from non-small bowel etiologies.
The diagnostic yield of CE in OGIB in the present study (36%) was lower than those reported in western literatures (42%-71%)[6,7,10-16] and those reported in meta-analyses (61%-63%)[3,4]. There are many possible reasons. Firstly, the timing of CE after onset of OGIB varied, particularly the overt OGIB group of patients might be different from other studies. The study by Pennazio et al[6] showed that the yield of CE was highest in patients with ongoing overt bleeding, while the yield could be as low as 13% in patients without ongoing bleeding, particularly, if CE was performed after 2 wk of bleeding. The median time interval of CE after onset of OGIB in the present study was 10 d but they ranged from 1-180 d. This wide range of interval was because most patients were referred from other institutes where significant delay in CE was problematic. The late performance of CE in this study might underdiagnose small bowel ulcers caused by NSAIDs as suggested by a recent study[17] which were found to be the most common cause of OGIB in this study. Most small bowel ulcers were able to heal spontaneously within days to weeks. Secondly, the low diagnostic yield might be due to the low prevalence of angiodysplasia in Thai patients (27%) which is the major cause of OGIB in other studies (33%-79%)[6,7,10-16].
The finding that small bowel ulcer, instead of angiodysplasia was the most common cause of OGIB in this study needs attention. Angiodysplasia is well-known to be the most common cause of OGIB among Western patients but no convincing data to show that this holds true among Asians[18-21]. Some[18,20] reported angiodysplasia as a main cause of OGIB, while others[19,21] report ulcers to be the main etiology. However, when considered large studies that include more than 100 patients, one study from India[21] and two double-balloon enteroscopy studies from Japan[22,23], all showed that small bowel ulcers were the most common cause of OGIB (41%-53%), more common than angiodysplasia (23%-24%). These yielding rates are much closer to the results of our study (44% and 27% for ulcers and angiodysplasia, respectively). Therefore, small bowel ulcer is likely the most common cause of OGIB among Asians.
In the present study, the demographic data of patients with OGIB from various etiologies were mostly indistinguishable. Although we found significantly more small bowel tumors in female, many recent larger studies of small bowel tumors did not confirm this finding and most demonstrated male predomination in 60%-65%[24-26]. Thus, the finding in our study might be from type I error. We found that history of NSAID use correlated with the finding of small bowel ulcers, which is straightforward. However, it was also common in other causes of OGIB and the predictive values were too low to have significant clinical implication.
Information regarding to the site of the lesions obtained from this study can be helpful to guide the route of balloon-assisted enteroscopy (BAE) if it needs to be done. Assuming from the location of the lesions by CE and findings from recent studies on the predictive role of CE for the route of enteroscopy[27,28], antegrade BAE would reach the lesions in 79%, whereas retrograde BAE would do so in 56%. Thus, antegrade route may have higher yield than retrograde route for patients with OGIB in Thailand.
Positive CE results in this study also lead to change in patient management plans in 35% of patients. They were given either specific medication, BAE with or without specific interventions or surgery. Patients with equivocal or negative CE results obviously had no change in their management plan. The change in patient management plan in this study is slightly lower than the 50%-70% rate of management changes reported in the recent review[8]. The explanation might be because the criteria of the change of management in these studies were different from ours and the findings in our study that large proportion of OGIB was NSAID-induced small bowel ulcers. Such small bowel ulcer can often heal spontaneously without any specific treatment and thus the rate of change in patient management would be low.
Patient outcome after CE was another important significant finding in the present study. The very low rebleeding rate in patients with positive CE results (together with appropriate management according to the CE findings) is found to be lower than those reported from the West[6,7,10-16]. The low rebleeding in our study could be due to the spontaneously improved nature of the disease (i.e., NSAID-induced ulcers) and the ability to provide specific treatments, i.e., surgery for small bowel tumors, which were also prevalent in this study. In contrast, the main cause of OGIB found in the West is angiodysplasia which is often difficult or impossible to completely eradicate and the natural course is often not well-understood[1]. These similar reasons may also explain the substantially low rebleeding rate in the CE-negative group in the present study. Another important finding from this study that all rebleeding in the CE-negative patients were finally found to be non-small bowel lesions, strongly supports CE as an important tool to rule out small bowel source of OGIB. It also emphasizes the importance of thorough evaluation of non-small bowel causes before heading to investigate with capsule endoscopy.
The strong aspect of present study is its large number of sample size and that all CE videos were reviewed by 2 reviewers. However, the main drawback of this study is its retrospective design and some clinical outcomes might not be completely recorded as retrospective phone interview might not be completely accurate.
In conclusion, the diagnostic yield of CE for OGIB in Thai patients seems to be lower than those in Westerners. Small bowel ulcers are the most common causes of OGIB, while angiodysplasia is less common. Positive CE strongly impacted on management plan and outcomes of OGIB. Negative CE was associated with a substantially low rebleeding rate and all etiologies were from non-small bowel origins. However, further studies, i.e., randomized controlled trial, are necessary in order to confirm these results.
The authors would like to thank Ms. Jaruwan Kongkaew for her strong efforts in carrying all the CE procedures and her assistance in data collection.
Capsule endoscopy (CE) is now accepted as the first line investigation of obscure gastrointestinal bleeding (OGIB). The yield is high, angiodysplasia is the most common cause in Westerners but impact on patients’ outcome is controversial. Data in Asians is limited.
Knowledge on the yield, etiologies and impact of CE on outcomes of OGIB in Asians are lacking and may be different from those in the Westerners.
Yield of CE in Thai OGIB is lower than the Westerners. Small bowel ulcers are more common than angiodysplasia and tumors are quite common. CE impacts management and outcomes. Rebleeding is low and most are from non-small bowel causes.
CE should be used as first-line investigation of OGIB in Asians because of the noninvasiveness, the high yield, the positive impacts on management and the ability to rule-out small bowel causes of OGIB when negative.
OGIB is gastrointestinal bleeding that the etiology is not detected by both upper endoscopy and colonoscopy.
This is the first manuscript on Thai patients with OGIB managed by CE. Figures and results are well made and easy to be understood for readers.
P- Reviewer Yoshida N S- Editor Song XX L- Editor A E- Editor Zhang DN
| 1. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 2. | Pennazio M, Eisen G, Goldfarb N. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy. 2005;37:1046-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 5. | Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, Fleischer DE, Sharma VK. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 604] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Estévez E, González-Conde B, Vázquez-Iglesias JL, de Los Angeles Vázquez-Millán M, Pértega S, Alonso PA, Clofent J, Santos E, Ulla JL, Sánchez E. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006;18:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, Seidman EG, Cellier C, Murray J, de Franchis R. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Seitz U, Bohnacker S, Soehendra N. A simple method to determine the location of the capsule and thus whether prokinetic drugs are needed during video capsule endoscopy. Endoscopy. 2002;34:1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Saurin JC, Delvaux M, Vahedi K, Gaudin JL, Villarejo J, Florent C, Gay G, Ponchon T. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy. 2005;37:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Viazis N, Papaxoinis K, Theodoropoulos I, Sgouros S, Vlachogiannakos J, Pipis P, Markoglou C, Avgerinos A. Impact of capsule endoscopy in obscure small-bowel bleeding: defining strict diagnostic criteria for a favorable outcome. Gastrointest Endosc. 2005;62:717-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Neu B, Ell C, May A, Schmid E, Riemann JF, Hagenmüller F, Keuchel M, Soehendra N, Seitz U, Meining A. Capsule endoscopy versus standard tests in influencing management of obscure digestive bleeding: results from a German multicenter trial. Am J Gastroenterol. 2005;100:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Leighton JA, Sharma VK, Hentz JG, Musil D, Malikowski MJ, McWane TL, Fleischer DE. Capsule endoscopy versus push enteroscopy for evaluation of obscure gastrointestinal bleeding with 1-year outcomes. Dig Dis Sci. 2006;51:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hindryckx P, Botelberge T, De Vos M, De Looze D. Clinical impact of capsule endoscopy on further strategy and long-term clinical outcome in patients with obscure bleeding. Gastrointest Endosc. 2008;68:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Matsumura T, Arai M, Sazuka S, Saito M, Takahashi Y, Maruoka D, Suzuki T, Nakagawa T, Sato T, Katsuno T. Negative capsule endoscopy for obscure gastrointestinal bleeding is closely associated with the use of low-dose aspirin. Scand J Gastroenterol. 2011;46:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Park JJ, Cheon JH, Kim HM, Park HS, Moon CM, Lee JH, Hong SP, Kim TI, Kim WH. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. 2010;71:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Ghoshal UC, Lakshmi CP, Kumar S, Das K, Misra A, Rai P, Mohindra S, Saraswat VA, Kumar A, Choudhuri G. Capsule endoscopy for obscure gastrointestinal bleeding in the tropics: report from India. Dig Endosc. 2011;23:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Goenka MK, Majumder S, Kumar S, Sethy PK, Goenka U. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2011;17:774-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, Itoh A, Hirooka Y, Niwa Y, Maeda O. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66:S72-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Shinozaki S, Yamamoto H, Yano T, Sunada K, Miyata T, Hayashi Y, Arashiro M, Sugano K. Long-term outcome of patients with obscure gastrointestinal bleeding investigated by double-balloon endoscopy. Clin Gastroenterol Hepatol. 2010;8:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Cheung DY, Lee IS, Chang DK, Kim JO, Cheon JH, Jang BI, Kim YS, Park CH, Lee KJ, Shim KN. Capsule endoscopy in small bowel tumors: a multicenter Korean study. J Gastroenterol Hepatol. 2010;25:1079-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, Murakami T, Murakami H, Ikeda Y, Yokota T. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56:2366-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Lee BI, Choi H, Choi KY, Byeon JS, Jang HJ, Eun CS, Cheon JH, Shin SJ, Kim JO, Lee MS. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56:2920-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Li X, Chen H, Dai J, Gao Y, Ge Z. Predictive role of capsule endoscopy on the insertion route of double-balloon enteroscopy. Endoscopy. 2009;41:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |