Published online Oct 16, 2013. doi: 10.4253/wjge.v5.i10.476
Revised: July 21, 2013
Accepted: September 4, 2013
Published online: October 16, 2013
Processing time: 154 Days and 20.2 Hours
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract resulting in inflammation, stricturing and fistulae secondary to transmural inflammation. Diagnosis relies on clinical history, abnormal laboratory parameters, characteristic radiologic and endoscopic changes within the gastrointestinal tract and most importantly a supportive histology. The article is intended mainly for the general gastroenterologist and for other interested physicians. Management of small bowel CD has been suboptimal and limited due to the inaccessibility of the small bowel. Enteroscopy has had a significant renaissance recently, thereby extending the reach of the endoscopist, aiding diagnosis and enabling therapeutic interventions in the small bowel. Radiologic imaging is used as the first line modality to visualise the small bowel. If the clinical suspicion is high, wireless capsule endoscopy (WCE) is used to rule out superficial and early disease, despite the above investigations being normal. This is followed by push enteroscopy or device assisted enteroscopy (DAE) as is appropriate. This approach has been found to be the most cost effective and least invasive. DAE includes balloon-assisted enteroscopy, [double balloon enteroscopy (DBE), single balloon enteroscopy (SBE) and more recently spiral enteroscopy (SE)]. This review is not going to cover the various other indications of enteroscopy, radiological small bowel investigations nor WCE and limited only to enteroscopy in small bowel Crohn’s. These excluded topics already have comprehensive reviews. Evidence available from randomized controlled trials comparing the various modalities is limited and at best regarded as Grade C or D (based on expert opinion). The evidence suggests that all three DAE modalities have comparable insertion depths, diagnostic and therapeutic efficacies and complication rates, though most favour DBE due to higher rates of total enteroscopy. SE is quicker than DBE, but lower complete enteroscopy rates. SBE has quicker procedural times and is evolving but the least available DAE today. Larger prospective randomised controlled trial’s in the future could help us understand some unanswered areas including the role of BAE in small bowel screening and comparative studies between the main types of enteroscopy in small bowel CD.
Core tip: Management of small bowel Crohn’s disease has reached new frontiers with the recent renaissance of enteroscopy, that has improved diagnosis and enabled therapeutic interventions. The use of magnetic resonance enteroclysis or wireless capsule endoscopy as the first line modality followed by enteroscopy is the most cost effective. Enteroscopy could be achieved using either a push enteroscope or device-assisted enteroscope (DAE). The latter includes double balloon enteroscopy (DBE), single balloon enteroscopy and more recently spiral enteroscopy. All three DAE modalities are comparable, though most favour DBE due to higher rates of total enteroscopy. The article is intended for the general gastroenterologists, non-gastroenterologists and general practitioners
- Citation: Tharian B, Caddy G, Tham TC. Enteroscopy in small bowel Crohn’s disease: A review. World J Gastrointest Endosc 2013; 5(10): 476-486
- URL: https://www.wjgnet.com/1948-5190/full/v5/i10/476.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i10.476
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract resulting in inflammation, stricturing and fistulae secondary to transmural inflammation[1,2]. Diagnosis relies on clinical history, abnormal laboratory parameters characteristic radiologic and endoscopic changes within the gastrointestinal tract and most importantly histology for confirmation and grading of severity[2]. CD can affect the entire gastrointestinal tract from mouth to anus, in addition to being a multisystem disease. It affects only the small intestine in 30%, ileo-colonic in 50%, colonic disease alone in 30% and upper GI tract in approximately in 5%[3,4]. CD may have characteristic endoscopic features like aphthous ulcers, longitudinal erosions, cobble stone appearance and fissures[4,5] (Figure 1).
The detection of small bowel CD and its management presents its own challenges, especially when the disease is present beyond the reach of the gastroscope and colonoscope. This is mainly due to length of the small bowel but also the tortuous anatomy and the floppy mesentery that leads to looping when a scope is advanced beyond the duodenum[6-13]. The distal 10-20 cm of the ileum can often be accessible with ileo-colonoscopy but more proximal visualisation is often limited by looping. In addition, disease of the ileo-caecal valve can prevent intubation of the ileum. Enteroscopy helps in assessing mucosal disease while cross sectional imaging is better for transmural involvement including fistulae. Small bowel radiological investigations include barium follow through, computed tomography (CT) enteroclysis or enterography, magnetic resonance enteroclysis or enterography and small bowel ultrasound (USS)[7,9-13]. The latter is not widely used since the ultrasound waves have limited penetration through air. However it is useful in assessing thickness of the small bowel and vascularity with Doppler and correlates with active disease. Wireless capsule endoscopy (WCE) is a sensitive test for small bowel disease and is often used to investigate small bowel CD, prior to any invasive deep bowel enteroscopy, once small bowel strictures have been excluded[1,14-19]. Dionissio et al[20] had shown in their meta-analysis comparing 18 prospective studies that WCE was best in evaluation of non-stricturing small bowel CD and magnetic resonance enteroclysis (MRE) had the highest diagnostic yield in known CD. This review is not going to cover the various radiological investigations or WCE[20,21].
Technological advances have extended the reach of the gastroenterologist, enabling access to the entire gut using flexible fibre optic scopes, with a combination of pushing, pulling and torquing to pleat the long and tortuous small bowel. Enteroscopy has improved the field of small bowel CD, in which radiological investigations previously predominated. Despite all these tools to empower the gastroenterologist and radiologist, the assessment of small bowel damage in CD is still far from sufficient. Evidence available from randomised controlled trials comparing the various modalities is limited and at best regarded as Grade C or D (based on expert opinion). Most of the studies performed to date are single centre experiences (retrospective studies) or multicentre trials involving small numbers. Thus a main limitation of this article is lack of comparative data specifically on CD.
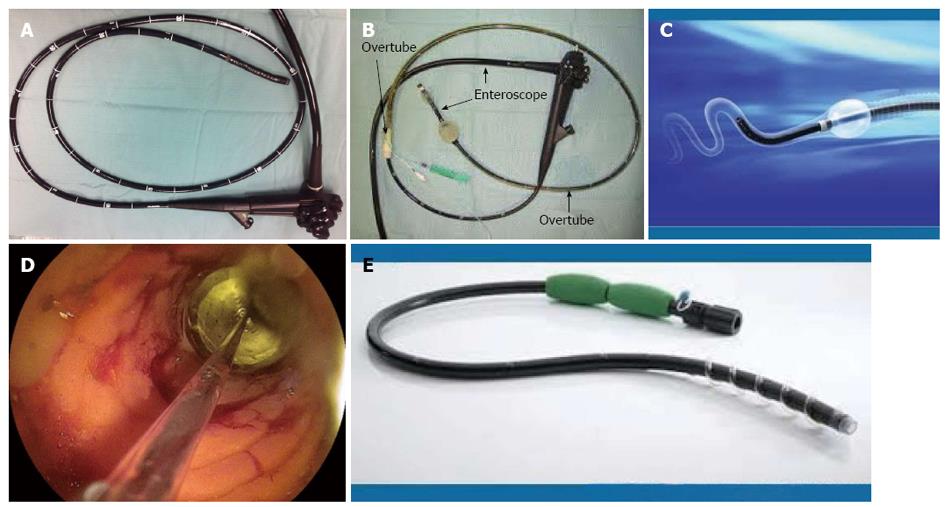
The advantages of enteroscopy include the ability for real-time viewing of the small bowel, to biopsy abnormal mucosa and to undertake therapy such as pneumatic dilatation using the through-the-scope (TTS) balloons, achieving hemostasis, polypectomy, local injection of triamcinolone and immunomodulatory drugs and more recently metallic and biodegradable stent insertion[18,22-25]. Endoscopic dilatation (ED), the commonest therapeutic use of deep enteroscopy in CD, has been used when medical therapy fails to relieve obstruction. These are often done using centre based and regional guidelines, which are often tailored depending on the availability of local expertise, financial constraints and patient preference. The scope of an enteroscope is much wider, including completion colonoscopy and for endoscopic retrograde cholangiopancreatography in surgically altered anatomy[24,25]. The various methods currently available worldwide can be either a push enteroscopy (PE) or device-assisted enteroscopy (DAE) using overtubes (Figure 2). The latter includes balloon-assisted enteroscopes (BAE) [double balloon enteroscopy (DBE) and single balloon enteroscopy (SBE)] and more recently spiral enteroscopy (SE). The complimentary use of cross sectional imaging and endoscopy is invaluable in the diagnosis and management of small bowel CD (Table 1).
Traditional push enteroscope was developed in the 1980’s. PE has a working length between 220 and 250 cm and is inserted per orally directly into the proximal jejunum[26]. The alternative is to use an adult or paediatric colonoscope for the same purpose. It can be used for both diagnostic and therapeutic purposes[26-29]. The push enteroscope may be used with or without an overtube (Figure 3). There have been several studies comparing the use of an overtube in push enteroscopy but not specifically in CD. Taylor and colleagues studied a small group of 38 patients (19 with an overtube and 19 without) and compared the depth of insertion as measured by the distance of insertion with the scope in a shortened position[29]. The median total straightened scope length of insertion just reached significance (125 cm vs 110 cm). From the pylorus the depth of insertion was also significant (70 cm vs 50 cm). However, there was no significant difference in the detection of small bowel pathology[29]. Overall complication rate of this widely available procedure in this study was 1%.
This technique is still commonly used to assess and treat proximal small bowel pathology due to its ease of use. Benz and colleagues studied enteroscopy in a group of 80 patients randomly assigned to enteroscopy with an overtube vs enteroscopy without an overtube[27]. The authors found that depth of insertion as measured by distance in a straightened position from the pylorus and number of counted folds was significantly increased by using an overtube. A further study by the same author compared 2 working lengths of endoscope (250 cm vs 220 cm) to compare the depth of insertion in 28 patients[28]. An overtube was used in all cases. The median insertion from the pylorus was 72.5 cm vs 70.0 cm but no significant difference was demonstrated in depth of small bowel insertion using a longer endoscope[28].
Another method of improving depth of insertion into the small bowel is by using a variable stiffness scope in an attempt to reduce excess looping of the scope within the stomach[30]. Harewood and colleagues prospectively studied enteroscopy in 3 groups of patients (one with standard enteroscope with overtube, one without overtube and a third one with variable stiffness)[31]. Depth of insertion beyond the ligament of Treitz was significantly greater using a variable stiffness enteroscope (89 cm) compared to a standard enteroscope (68 cm) and was over twice that without an overtube (41 cm) (P = 0.03). In this study, patients in the overtube group required significantly more sedation than the other groups, although the overall patient tolerance and procedure duration showed no significant difference. Again, no additional yield of pathological findings was observed with the greater depth of insertion[32]. In a small study by Perez-Cuadrado et al[33], 50% (4 of 8) of this patient group with suspected CD had detectable macroscopic and/or microscopic evidence of small bowel CD not detected by other endoscopic or radiological methods. The same author demonstrated the therapeutic role of PE in small bowel Crohn’s for jejunal stricture dilatation[32]. In a recent study by Darbari et al[34], it was shown that PE was useful and safe in proximal small bowel disease, predominantly CD, leading on to definite change in management. In this study, proximal small bowel CD was detected in 23 out of 44 suspected cases. ED is often considered successful if the scope could be passed through the stricture once dilated. ED should ideally be limited to accessible linear fibrotic strictures under 4 cm length to minimise risk of perforation[35,36].
DBE, originally developed in 2001 by Prof Hironori Yamamoto, is useful in the diagnosis of small bowel diseases including (CD)[9,23,37-39] (Figure 3B). DBE is often used following WCE due to potential miss rate of the latter and to guide the approach of insertion of DBE (antegrade or retrograde). The standard system has an endoscope with an outer diameter of 8.5 mm and a working length of 200 cm[38-40]. It is also provided with a 145 cm soft overtube with 12.2 mm outer diameter and a dedicated pump. One balloon is attached to the tip of the scope, after back loading the overtube (which has an additional balloon attached to the tip of the overtube)[6,25,32,39,41,42]. DBE can be performed with an anterograde (oral) followed by a retrograde (anal) approach or vice versa, with conscious sedation, deep sedation or general anaesthesia. Either air or carbon dioxide can be used, the latter recommended due to better patient tolerance, especially for therapeutic procedures and less post procedural discomfort, when a prolonged procedure is anticipated. Fluoroscopic guidance could be used till competence is achieved, but is not essential[39-41,43].
The overall yield of DBE was better than push enteroscopy and similar to capsule. Oshitani et al[6] showed that, in their study of 30 patients with CD, small bowel ulcers and aphthae were picked up in 9 patients who underwent DBE who had normal small bowel follow through. WCE done in 8 of these patients without symptoms of strictures showed additional finding of small bowel scarring in only one of the patients, though one of the eight developed capsule retention, that was retrieved using DBE. Nine ileal strictures were picked up with barium compared to only 6 with DBE[6].
The scope is inserted as far as possible into the bowel. Then the overtube balloon is inflated to anchor the tip in place and the scope is gently pulled backward to pleat the small bowel behind the balloon. The scope is further advanced into the lumen, followed by inflation of the scope balloon to anchor its tip. Thus by repetitive cycles of balloon inflation/deflation, the scope is advanced. In the early stages of training, this needs two operators, though once experienced one would be sufficient (Figure 4)[39]. A practical tip that is often advocated by Professor Yamamoto to advance an enteroscope is, slight “jiggling” of the scope, with alternating small “in-out” and “right-left” movements, that enables the tip to move forward. The distal most point is tattooed with India ink in the anterograde approach, to be visualised via the retrograde approach for total enteroscopy[24,37,44,45]. The procedure time can vary between 70 to 120 min for the ante-grade procedure and about 15-20 min longer for the retrograde approach, with ileal intubation rates in the latter being over 90% in high volume centre[43]. DBE has a steep learning curve[39,46]. Zhang et al[47] rightly commented that the combined analysis of imaging and gastro endoscopic findings in addition to a diligent clinical history and examination is essential to enhance the diagnostic efficiency of DBE .
In a study of 37 patients with CD who underwent DBE diagnostic yield was 60%. Yield levels increased if direction of insertion (ante-grade or retrograde) was aided by prior investigations[9]. The retrograde approach is useful for lesions noted in the distal 40% of the WCE[48]. In an early retrospective study, the role of DBE in evaluation of 40 patients with CD was found to be superior to radiological studies in detecting mucosal ulcers and strictures[6]. Moreover endoscopic findings often precede radiologic findings that often delay the diagnosis by 1 to 7 years, and hence earlier diagnosis with DBE may lead on to earlier mucosal healing that is the corner stone in management of CD[8,45,49,50]. The ability of therapeutic potential of DBE remains a significant advantage over capsule endoscopy. In a study of 19 patients (10 amenable to endoscopic therapy), Pohl et al[51] demonstrated that dilatation under fluoroscopy yielded a clinical improvement in 80% and avoidance of surgery in 60% albeit over a mean short term follow up period of 10 mo, with no reported complications. The technique is also useful for retrieval of retained capsules[2,38,43,52].
In a similar study, 8 of 9 patients with Crohn’s strictures underwent successful endoscopic dilatation (1 patient had a perforation). Clinical improvement occurred in these 8 patients with no surgical requirement over a follow up of 20 mo. Twenty five percent of patients did require a second dilation[53]. DBE has been shown to alter medical management in patients with established and suspected CD. Mensink and colleagues identified 24 patients with active CD (60% of study population) resulting in a change in management in 75% through a step up approach in these patients medical management. Over 80% of these patients had a clinical improvement with a reduction in CDAI[54,55]. In a further study by the same author a small population of Crohn’s patients with suspected proximal small intestinal Crohn’s underwent DBE. Approximately three quarters of patients had proximal small bowel Crohn’s features, and approximately 50% were beyond reach of standard enteroscopy. There was a change in management in three quarters of those patients with detectable disease by DBE[55]. DBE can also help in assessment of radiologic abnormalities and thus to avoid unnecessary exploratory surgery[8,49,54,56].
The procedure hence is very valuable with a high success rate, but not preferred for those with difficult anatomy due to previous surgery, pathology or acute angle at the stoma due to higher perforation rate (0.4% of procedures and up to 3% when dilated)[43,57-59]. It should also be avoided in those with latex allergy since the balloons are made of latex[8]. The other complications include small risk of pancreatitis (0.3% of procedures), bleeding (0.2% of procedures) and aspiration pneumonia[60-62]. ED should be postponed till the ulcer heals due to higher risk of perforation and is discouraged if over 6 cm long[63].
SBE was introduced in 2007. It uses an enteroscope with 200 cm working length and 2.8 mm channel diameter, an overtube with a silicone balloon that has an outer diameter of 13.2 mm and a balloon controller pump[57,64,65]. The technique is similar to DBE, with the only difference being that the tip of the flexible scope is used to anchor the endoscope, avoiding need of a second balloon[65] (Figure 3C). The depth of insertion ranges from 133 to 270 cm and 73 to 199 cm for the retrograde examination, with a therapeutic yield between 7% to 50%. Total enteroscopy rate is lower than DBE, but is a safe, effective and useful technique for deep small bowel endoscopy[64,66-68]. The main advantage of SBE is the ease of assembling the apparatus taking 5 min compared to 15 min for DBE and overall shorter procedure duration of 55 min compared to 95 for DBE. Secondly it has variable stiffness, thus eliminating the need for a stiffening wire[67,69-73]. Thirdly SBE can be used in patients with latex allergy unlike DBE. Dr. Reddy’s group from Hyderabad, initially described use of “power suction” during straightening of the scope, that can be used instead of inversion of the tip, to minimise the perforation rate that is around 2%[74].
In a small study in children between 8 and 18 years old by de Ridder et al[68], it was shown that SBE is a safe technique and picks up active small bowel Crohn’s that has been missed by magnetic resonance imaging and USS. Similarly, Di Nardo et al[69], showed the safety, yield and therapeutic efficacy of SBE in their study of 16 children with suspected and 14 with known Crohn’s with atypical presentation, who had negative radiologic and conventional upper and lower gastrointestinal endoscopy. In a recent randomised multicenter trial, Domagk et al[66] showed the non-inferiority of SBE over DBE in evaluation of small bowel pathology[71]. Takano et al[70] showed in their randomised controlled trial that, total enteroscopy was much better accomplished with DBE than SBE, though it was a single centre study involving only small numbers. Bortlik et al[75] showed that in their experience of SBE in 35 patients, it provided an evaluation of mucosal healing after treatment and revealed severe inflammatory changes in one third. Therapeutic procedures especially dilation using TTS balloon were done in approximately a third (Figure 3D). SBE is cheaper, easier to perform, has a shorter learning curve than DBE and is a less complex method of balloon assisted enteroscopy[65,66,68,71,73,76]. Current results are conflicting if the SBE and DBE have comparable performance and diagnostic yield. However, more studies favour use of DBE for total small bowel enteroscopy[70].
This is the latest of the armamentarium, available since 2008 to gastroenterologists, to examine the small bowel and is simpler and faster than the predecessors[71,77,78]. The current second generation device uses an FDA approved 118 cm Endo-Ease Discovery™ SB overtube with a soft raised helix, a coupling device to fix the lubricated overtube to the enteroscope 25 cm from its tip, two handles for manual rotation and an injection port for lubrication (C 8)[74,79-82]. The distal end of the device has an external diameter of 16 mm and the internal diameter of the overtube is 9.8 mm. Clockwise rotation pleats the small bowel onto the scope, once engaged and advances the same thus transforming the torquing force into a linear one, the concept developed by Spirus Medical, Inc. and proposed for use in enteroscopy by Dr. Akerman et al[77,81,82] in 2006. Push and rotation technique is used until the scope gets beyond the Ligament of Trietz, followed by only rotation. The small bowel does not get twisted as it is held by the mesentery. It can be performed under conscious sedation or general anaesthetic, preferably the latter. In an intubated patient, the cuff on the endotracheal tube has to be deflated before introducing the spiral enteroscope to prevent oesophageal trauma, until it enters the stomach[77,83,84] (Figure 3E).
The major advantage of SE is the rapid advancement and stable controlled withdrawal enabling therapeutics to be delivered effectively[42,71,77,84]. The overtube can be disengaged from the coupler enabling complete withdrawal of the endoscope and reintroduction (often needed for removal of multiple polyps), without losing the position in the small bowel[42,71,84-86]. The other major advantage is that no dedicated enteroscopy system needs to be purchased and the Endo-Ease spiral overtube could transform an ordinary enteroscope or a paediatric colonoscope to a SE device[40,77,78,81]. Spiral enteroscopy is very useful for proximal small bowel pathology, especially for therapeutic interventions, due to the stability achieved with the overtube.
This procedure requires two operators, one operator handling the scope and the other rotating the overtube. The enteroscope is unlocked from the overtube, advanced and then withdrawn using the hook and suction technique. Anticlockwise rotation of the handle of the overtube is used to withdraw the system allowing visualisation of the mucosa in a controlled fashion. The depth of insertion of SE is usually calculated on the way out. It has not yet been safely demonstrated for retrograde approach, unlike DBE. A promising motorised overtube is in its early stages of development, which could make it single operator dependent. Sore throat and transient difficulty in swallowing are described by around a quarter of the patients, though tiny asymptomatic mucosal disruptions are similar to the balloon assisted devices.
In a study by Buscaglia et al[83] the mean procedure length was around 34 min with a mean insertion depth of 262 cm. One of the early studies by Frieling et al[87] showed that the diagnostic yield of DBE was superior to that of SE. But as the authors commented, one of the main drawbacks was that it involved only small numbers of 17 and 18 subjects respectively. In yet another small cross over study involving 10 patients, May et al[42] showed that SE had a shorter procedure duration by a mean of 22 min, though the depth of insertion was greater by about 60 cm with DBE. Khashab et al[86] in their first comparative study on SE vs SBE, showed greater depth of maximal insertion with the former, although the yield and procedure length were comparable. Akerman et al[77,81] showed an overall severe complication rate less than 0.3% in their review of 2950 patients treated with SE, with perforation occurring in 0.4% of the first 1750 patients and no reported cases of pancreatitis. However Teshima et al[88] showed that asymptomatic hyperamylasemia occurred in up to 20% of patients undergoing SE. Data is limited especially with regards to comparative studies specifically related to use of SE in CD. But overall it is considered to be a safe and quick procedure and compares favourably with other DAE for assessing the small bowel and for delivering therapies in the midgut[71,77,79,80,83-86,89,90].
Intraoperative enteroscopy (IOE) developed over 35 years ago enables the entire gut to be viewed without making an incision on the intestine, by the cooperation of the operating surgeon and the endoscopist[91]. It was done using rigid sigmoidoscopes in the 50’s, until fibre optic scopes became available in the 70’s[92]. Once the surgeon has completed exploring the small bowel laparoscopically and freed the bowel from any adhesions, small bowel loops can be pleated over the orally inserted PE. The current role of IOE is in difficult mid gut pathology, in guiding the surgeon intraoperatively and in marking the lesion with a suture to be dealt with on removing the scope[92-96]. There have not been many studies evaluating role of IOE in CD[94,97]. Complications include standard ones associated with laparoscopy and endoscopy, prolonged post operative ileus, air embolism and multiorgan failure. IOE once regarded as the gold standard for small bowel evaluation has been relegated a “last resort” in the era of less invasive therapeutic total enteroscopy with DAE[91,95-98].
Novel biologic agents and progress in our assessment and management of small bowel CD, which is currently far from sufficient, might help alter the natural history and predict outcomes in Crohn’s disease. However enteroscopy, which is a rapidly evolving field, has had a significant renaissance recently and the small bowel is no longer the black box for the endoscopist or the final frontier. The lack of randomised controlled trial’s (RCT’s) and meta-analysis on enteroscopy in small bowel Crohn’s limits more detailed comparative data between various techniques. PE is still a useful tool in centres that do not have WCE, BAE or SE. An algorithm that we suggest for investigation of small bowel CD would be gastroscopy and colonoscopy (with terminal ileal assessment). This might be followed by either a barium small bowel follow through or CT enteroclysis and increasingly by using MRE, considering the lack of radiation and possibility of repeated studies, considering the fact that the age group affected is often young or middle aged people of child bearing age, to limit radiation exposure. If MRE is normal one could consider WCE, if there is a high index of suspicion of early mucosal disease or malabsorption, which may not show up in MRE. If there is evidence of active small bowel Crohn’s especially strictures or fistulae, then ideally aggressive treatment with anti tumour necrosis factor from the outset. If any complications of CD are seen, such as strictures or bleeding, then DBE/SBE or SE, depending on availability of local expertise, to assess the pathology and consider local treatment-biopsy, diathermy, balloon dilatation or injection of various drugs as might be appropriate to the setting. If initial small bowel imaging at time of first diagnosis is normal, then currently no recommendations are available regarding surveillance intervals or its clinical relevance. There may be multi centre studies in the future can look into appropriate screening intervals and on a more tailored approach for enteroscopy in CD.
A comparison of the various enteroscopy techniques is summarised in the table below. The evidence suggests that all three DAE modalities have comparable insertion depths, diagnostic and therapeutic efficacies and complication rates and can be used as complementary tools. However, most gastroenterologists including the authors, favour DBE due to higher rates of total enteroscopy. Larger prospective RCT’s in the future could help us understand some unanswered areas including the role of BAE in small bowel screening, comparative studies between the main types of BAE in the field of small bowel CD and strengthen the available evidence, especially with regards to their potential roles and clinical impact. Further studies are needed for device refinement and development to make them more cost effective.
P- Reviewers Nielsen OH, Jonaitis L, Yamamoto S, Yamamoto T S- Editor Gou SX L- Editor A E- Editor Liu XM
| 1. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [PubMed] |
| 2. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. [PubMed] |
| 3. | Strobel D, Goertz RS, Bernatik T. Diagnostics in inflammatory bowel disease: ultrasound. World J Gastroenterol. 2011;17:3192-3197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 4. | Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, Barakauskiene A, Villanacci V, Von Herbay A, Warren BF. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55 Suppl 1:i1-15. [PubMed] |
| 5. | Lee SD, Cohen RD. Endoscopy of the small bowel in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:485-493. [PubMed] |
| 6. | Oshitani N, Yukawa T, Yamagami H, Inagawa M, Kamata N, Watanabe K, Jinno Y, Fujiwara Y, Higuchi K, Arakawa T. Evaluation of deep small bowel involvement by double-balloon enteroscopy in Crohn’s disease. Am J Gastroenterol. 2006;101:1484-1489. [PubMed] |
| 7. | Cekiç C, Unsal B. What is the most accurate method for the assessment of small bowel in involvement in Crohn’s disease? Turk J Gastroenterol. 2010;21:80-82. [PubMed] |
| 8. | Semrad CE. Role of double balloon enteroscopy in Crohn’s disease. Gastrointest Endosc. 2007;66:S94-S95. [PubMed] |
| 9. | Manes G, Imbesi V, Ardizzone S, Cassinotti A, Pallotta S, Porro GB. Use of double-balloon enteroscopy in the management of patients with Crohn’s disease: feasibility and diagnostic yield in a high-volume centre for inflammatory bowel disease. Surg Endosc. 2009;23:2790-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Gay G, Delvaux M. Small-bowel endoscopy. Endoscopy. 2008;40:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Wiarda BM, Mensink PB, Heine DG, Stolk M, Dees J, Hazenberg H, Stoker J, van der Woude CJ, Kuipers EJ. Small bowel Crohn’s disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdom Imaging. 2012;37:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Nolan DJ. Radiology of Crohn’s disease of the small intestine: a review. J R Soc Med. 1981;74:294-300. [PubMed] |
| 14. | Caprilli R, Gassull MA, Escher JC, Moser G, Munkholm P, Forbes A, Hommes DW, Lochs H, Angelucci E, Cocco A. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55 Suppl 1:i36-i58. [PubMed] |
| 15. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D'Hoore A, Gassull M, Gomollón F. European Crohn's and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (1)] |
| 16. | Brazilian Study Group of Inflammatory Bowel Diseases. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. 2010;47:313-325. [PubMed] |
| 17. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [PubMed] |
| 18. | Sidhu R, Sanders DS, McAlindon ME, Thomson M. Capsule endoscopy and enteroscopy: modern modalities to investigate the small bowel in paediatrics. Arch Dis Child. 2008;93:154-159. [PubMed] |
| 19. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [PubMed] |
| 20. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (36)] |
| 21. | Sunada K, Yamamoto H. Technology and indications. Gastrointest Endosc Clin N Am. 2009;19:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Boriskin HS, Devito BS, Hines JJ, Scarmato VJ, Friedman B. CT enterography vs. capsule endoscopy. Abdom Imaging. 2009;34:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Murphy SJ, Kornbluth A. Double balloon enteroscopy in Crohn’s disease: where are we now and where should we go? Inflamm Bowel Dis. 2011;17:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Kochhar R, Poornachandra KS. Intralesional steroid injection therapy in the management of resistant gastrointestinal strictures. World J Gastrointest Endosc. 2010;2:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 25. | Pennazio M. Crohn’s disease: diagnostic and therapeutic potential of modern small-bowel endoscopy. Gastrointest Endosc. 2007;66:S91-S93. [PubMed] |
| 26. | Wilmer A, Rutgeerts P. Push enteroscopy. Technique, depth, and yield of insertion. Gastrointest Endosc Clin N Am. 1996;6:759-776. [PubMed] |
| 27. | Benz C, Jakobs R, Riemann JF. Do we need the overtube for push-enteroscopy? Endoscopy. 2001;33:658-661. [PubMed] |
| 28. | Benz C, Jakobs R, Riemann JF. Does the insertion depth in push enteroscopy depend on the working length of the enteroscope? Endoscopy. 2002;34:543-545. [PubMed] |
| 29. | Chong AK, Taylor A, Miller A, Hennessy O, Connell W, Desmond P. Capsule endoscopy vs. push enteroscopy and enteroclysis in suspected small-bowel Crohn’s disease. Gastrointest Endosc. 2005;61:255-261. [PubMed] |
| 30. | Niv Y, Ilani S, Levi Z, Hershkowitz M, Niv E, Fireman Z, O’Donnel S, O’Morain C, Eliakim R, Scapa E. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 31. | Harewood GC, Gostout CJ, Farrell MA, Knipschield MA. Prospective controlled assessment of variable stiffness enteroscopy. Gastrointest Endosc. 2003;58:267-271. [PubMed] |
| 32. | Pérez-Cuadrado E, Molina Pérez E. Multiple strictures in jejunal Crohn’s disease: push enteroscopy dilation. Endoscopy. 2001;33:194. [PubMed] |
| 33. | Perez-Cuadrado E, Macenlle R, Iglesias J, Fabra R, Lamas D. Usefulness of oral video push enteroscopy in Crohn’s disease. Endoscopy. 1997;29:745-747. [PubMed] |
| 34. | Darbari A, Kalloo AN, Cuffari C. Diagnostic yield, safety, and efficacy of push enteroscopy in pediatrics. Gastrointest Endosc. 2006;64:224-228. [PubMed] |
| 35. | Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457-1464. [RCA] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 37. | Gay G, Delvaux M. Double balloon enteroscopy in Crohn’s disease and related disorders: our experience. Gastrointest Endosc. 2007;66:S82-S90. [PubMed] |
| 38. | Mönkemüller K, Weigt J, Treiber G, Kolfenbach S, Kahl S, Röcken C, Ebert M, Fry LC, Malfertheiner P. Diagnostic and therapeutic impact of double-balloon enteroscopy. Endoscopy. 2006;38:67-72. [PubMed] |
| 39. | Tee HP, How SH, Kaffes AJ. Learning curve for double-balloon enteroscopy: Findings from an analysis of 282 procedures. World J Gastrointest Endosc. 2012;4:368-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Moreels TG. Small bowel enteroscopy in Crohn’s disease. Ann Gastroenterol. 2012;25:14-20. |
| 41. | May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62-70. [PubMed] |
| 42. | May A, Manner H, Aschmoneit I, Ell C. Prospective, cross-over, single-center trial comparing oral double-balloon enteroscopy and oral spiral enteroscopy in patients with suspected small-bowel vascular malformations. Endoscopy. 2011;43:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42-48. [PubMed] |
| 44. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. [PubMed] |
| 45. | Pimentel M, Chang M, Chow EJ, Tabibzadeh S, Kirit-Kiriak V, Targan SR, Lin HC. Identification of a prodromal period in Crohn’s disease but not ulcerative colitis. Am J Gastroenterol. 2000;95:3458-3462. [PubMed] |
| 46. | Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 US tertiary care centers. Gastrointest Endosc. 2006;64:740-750. [PubMed] |
| 47. | Zhang SH, Xu J, Qing Q, Zhi FC, Bai Y, Xu ZM, Jiang B, Zhang YL, Chen Y. [Value of deep small-bowel endoscopy in the diagnosis of Crohn’s disease]. Nanfang Yike Daxue Xuebao. 2011;31:637-640. [PubMed] |
| 48. | Li X, Chen H, Dai J, Gao Y, Ge Z. Predictive role of capsule endoscopy on the insertion route of double-balloon enteroscopy. Endoscopy. 2009;41:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Timmer A, Breuer-Katschinski B, Goebell H. Time trends in the incidence and disease location of Crohn’s disease 1980-1995: a prospective analysis in an urban population in Germany. Inflamm Bowel Dis. 1999;5:79-84. [PubMed] |
| 50. | Sunada K, Yamamoto H, Yano T, Sugano K. Advances in the diagnosis and treatment of small bowel lesions with Crohn’s disease using double-balloon endoscopy. Therap Adv Gastroenterol. 2009;2:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Pohl J, May A, Nachbar L, Ell C. Diagnostic and therapeutic yield of push-and-pull enteroscopy for symptomatic small bowel Crohn’s disease strictures. Eur J Gastroenterol Hepatol. 2007;19:529-534. [PubMed] |
| 52. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 53. | Despott EJ, Gupta A, Burling D, Tripoli E, Konieczko K, Hart A, Fraser C. Effective dilation of small-bowel strictures by double-balloon enteroscopy in patients with symptomatic Crohn’s disease (with video). Gastrointest Endosc. 2009;70:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Mensink PB, Groenen MJ, van Buuren HR, Kuipers EJ, van der Woude CJ. Double-balloon enteroscopy in Crohn’s disease patients suspected of small bowel activity: findings and clinical impact. J Gastroenterol. 2009;44:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Mensink PB, Aktas H, Zelinkova Z, West RL, Kuipers EJ, van der Woude CJ. Impact of double-balloon enteroscopy findings on the management of Crohn’s disease. Scand J Gastroenterol. 2010;45:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Kerr JM. Small bowel imaging: CT enteroclysis or barium enteroclysis? Critically appraised topic. Abdom Imaging. 2008;33:31-33. [PubMed] |
| 57. | Gerson LB, Flodin JT, Miyabayashi K. Balloon-assisted enteroscopy: technology and troubleshooting. Gastrointest Endosc. 2008;68:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Barreto-Zuñiga R, Tellez-Avila FI, Chavez-Tapia NC, Ramirez-Luna MA, Sanchez-Cortes E, Valdovinos-Andraca F, Zepeda-Gomez S. Diagnostic yield, therapeutic impact, and complications of double-balloon enteroscopy in patients with small-bowel pathology. Surg Endosc. 2008;22:1223-1226. [PubMed] |
| 59. | Lo SK. Techniques, tricks, and complications of enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Yano T, Yamamoto H. Current state of double balloon endoscopy: the latest approach to small intestinal diseases. J Gastroenterol Hepatol. 2009;24:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Gustavsson A, Magnuson A, Blomberg B, Andersson M, Halfvarson J, Tysk C. Endoscopic dilation is an efficacious and safe treatment of intestinal strictures in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 63. | Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177-182, 1182.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Bordas JM, Llach J, Mata A. [Utility of single- and double-balloon enteroscopy]. Gastroenterol Hepatol. 2009;32:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Manno M, Barbera C, Bertani H, Manta R, Mirante VG, Dabizzi E, Caruso A, Pigo F, Olivetti G, Conigliaro R. Single balloon enteroscopy: Technical aspects and clinical applications. World J Gastrointest Endosc. 2012;4:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Domagk D, Mensink P, Aktas H, Lenz P, Meister T, Luegering A, Ullerich H, Aabakken L, Heinecke A, Domschke W. Single- vs. double-balloon enteroscopy in small-bowel diagnostics: a randomized multicenter trial. Endoscopy. 2011;43:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Riccioni ME, Urgesi R, Cianci R, Spada C, Nista EC, Costamagna G. Single-balloon push-and-pull enteroscopy system: does it work? A single-center, 3-year experience. Surg Endosc. 2011;25:3050-3056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | de Ridder L, Mensink PB, Lequin MH, Aktas H, de Krijger RR, van der Woude CJ, Escher JC. Single-balloon enteroscopy, magnetic resonance enterography, and abdominal US useful for evaluation of small-bowel disease in children with (suspected) Crohn’s disease. Gastrointest Endosc. 2012;75:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Di Nardo G, Oliva S, Aloi M, Rossi P, Casciani E, Masselli G, Ferrari F, Mallardo S, Stronati L, Cucchiara S. Usefulness of single-balloon enteroscopy in pediatric Crohn’s disease. Gastrointest Endosc. 2012;75:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Takano N, Yamada A, Watabe H, Togo G, Yamaji Y, Yoshida H, Kawabe T, Omata M, Koike K. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: a randomized, controlled trial. Gastrointest Endosc. 2011;73:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Lenz P, Domagk D. Double- vs. single-balloon vs. spiral enteroscopy. Best Pract Res Clin Gastroenterol. 2012;26:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Mönkemüller K, Fry LC, Bellutti M, Malfertheiner P. Balloon-assisted enteroscopy: unifying double-balloon and single-balloon enteroscopy. Endoscopy. 2008;40:537; author reply 539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Sidhu R, McAlindon ME, Drew K, Hardcastle S, Cameron IC, Sanders DS. Evaluating the role of small-bowel endoscopy in clinical practice: the largest single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Rao GV. Spiral enteroscopy: a preliminary experience in Asian population. J Gastroenterol Hepatol. 2010;25:1754-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Bortlik M, Bouzkova E, Duricova D, Komarek V, Machkova N, Lukas M. Endoscopic balloon dilatation of anastomotic strictures in patients with Crohn's disease: Effect of immediate endoscopic success and biological therapy. Gastroenterology. 2011;140:S-281. |
| 76. | Upchurch BR, Vargo JJ. Single-balloon enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Akerman PA, Haniff M. Spiral enteroscopy: prime time or for the happy few? Best Pract Res Clin Gastroenterol. 2012;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Mensink PB. Spiral enteroscopy: from “new kid on the block” to established deep small-bowel enteroscopy tool. Endoscopy. 2010;42:955-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Judah JR, Draganov PV, Lam Y, Hou W, Buscaglia JM. Spiral enteroscopy is safe and effective for an elderly United States population of patients with numerous comorbidities. Clin Gastroenterol Hepatol. 2010;8:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Morgan D, Upchurch B, Draganov P, Binmoeller KF, Haluszka O, Jonnalagadda S, Okolo P, Grimm I, Judah J, Tokar J. Spiral enteroscopy: prospective U.S. multicenter study in patients with small-bowel disorders. Gastrointest Endosc. 2010;72:992-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Akerman PA, Agrawal D, Chen W, Cantero D, Avila J, Pangtay J. Spiral enteroscopy: a novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest Endosc. 2009;69:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Akerman PA, Agrawal D, Cantero D, Pangtay J. Spiral enteroscopy with the new DSB overtube: a novel technique for deep peroral small-bowel intubation. Endoscopy. 2008;40:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 83. | Buscaglia JM, Dunbar KB, Okolo PI, Judah J, Akerman PA, Cantero D, Draganov PV. The spiral enteroscopy training initiative: results of a prospective study evaluating the Discovery SB overtube device during small bowel enteroscopy (with video). Endoscopy. 2009;41:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Akerman PA, Cantero D. Spiral enteroscopy and push enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Ross AS. Diving deeper into the small bowel: a comparison of spiral and single-balloon enteroscopy. Gastrointest Endosc. 2010;72:773-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Khashab MA, Lennon AM, Dunbar KB, Singh VK, Chandrasekhara V, Giday S, Canto MI, Buscaglia JM, Kapoor S, Shin EJ. A comparative evaluation of single-balloon enteroscopy and spiral enteroscopy for patients with mid-gut disorders. Gastrointest Endosc. 2010;72:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Frieling T, Heise J, Sassenrath W, Hülsdonk A, Kreysel C. Prospective comparison between double-balloon enteroscopy and spiral enteroscopy. Endoscopy. 2010;42:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Teshima CW, Aktas H, Kuipers EJ, Mensink PB. Hyperamylasemia and pancreatitis following spiral enteroscopy. Can J Gastroenterol. 2012;26:603-606. [PubMed] |
| 89. | Albert JG. Small bowel imaging in managing Crohn’s disease patients. Gastroenterol Res Pract. 2012;2012:502198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Buscaglia JM, Richards R, Wilkinson MN, Judah JR, Lam Y, Nagula S, Draganov PV. Diagnostic yield of spiral enteroscopy when performed for the evaluation of abnormal capsule endoscopy findings. J Clin Gastroenterol. 2011;45:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Bombeck CT. Intraoperative esophagoscopy, gastroscopy, colonoscopy, and endoscopy of the small bowel. Surg Clin North Am. 1975;55:135-142. [PubMed] |
| 92. | Cave DR, Cooley JS. Intraoperative enteroscopy. Indications and techniques. Gastrointest Endosc Clin N Am. 1996;6:793-802. [PubMed] |
| 93. | Douard R, Wind P, Panis Y, Marteau P, Bouhnik Y, Cellier C, Cugnenc P, Valleur P. Intraoperative enteroscopy for diagnosis and management of unexplained gastrointestinal bleeding. Am J Surg. 2000;180:181-184. [PubMed] |
| 94. | Smedh K, Olaison G, Nyström PO, Sjödahl R. Intraoperative enteroscopy in Crohn’s disease. Br J Surg. 1993;80:897-900. [PubMed] |
| 95. | Lau WY. Intraoperative enteroscopy--indications and limitations. Gastrointest Endosc. 1990;36:268-271. [PubMed] |
| 96. | Kopácová M, Bures J, Vykouril L, Hladík P, Simkovic D, Jon B, Ferko A, Tachecí I, Rejchrt S. Intraoperative enteroscopy: ten years’ experience at a single tertiary center. Surg Endosc. 2007;21:1111-1116. [PubMed] |
| 97. | Monsanto P, Almeida N, Lérias C, Figueiredo P, Gouveia H, Sofia C. Is there still a role for intraoperative enteroscopy in patients with obscure gastrointestinal bleeding? Rev Esp Enferm Dig. 2012;104:190-196. [PubMed] |
| 98. | Bonnet S, Douard R, Malamut G, Cellier C, Wind P. Intraoperative enteroscopy in the management of obscure gastrointestinal bleeding. Dig Liver Dis. 2013;45:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |