Published online Feb 16, 2012. doi: 10.4253/wjge.v4.i2.33
Revised: January 16, 2012
Accepted: February 6, 2012
Published online: February 16, 2012
AIM: To check the usefulness of blue mode (BM) review in lewis score (LS) calculation, by comparing it with respective LS results obtained by white light (WL) small-bowel capsule endoscopy (SBCE) review and mucosal inflammation as reflected by faecal calprotectin (FC) levels, considered as ‘gold standard’ for this study.
METHODS: Computational analysis of our SBCE database to identify patients who underwent SBCE with PillCam® and had FC measured within a 30-day period from their test. Only patients with prior colonoscopy were included, to exclude any colon pathology-associated FC rise. Each small bowel tertile was reviewed (viewing speed 8 fps) with WL and BM, in a back-to-back mode, by a single experienced reviewer. LS were calculated after each WL and BM reviews. Pearson rank correlation (rho, r) statistic was applied.
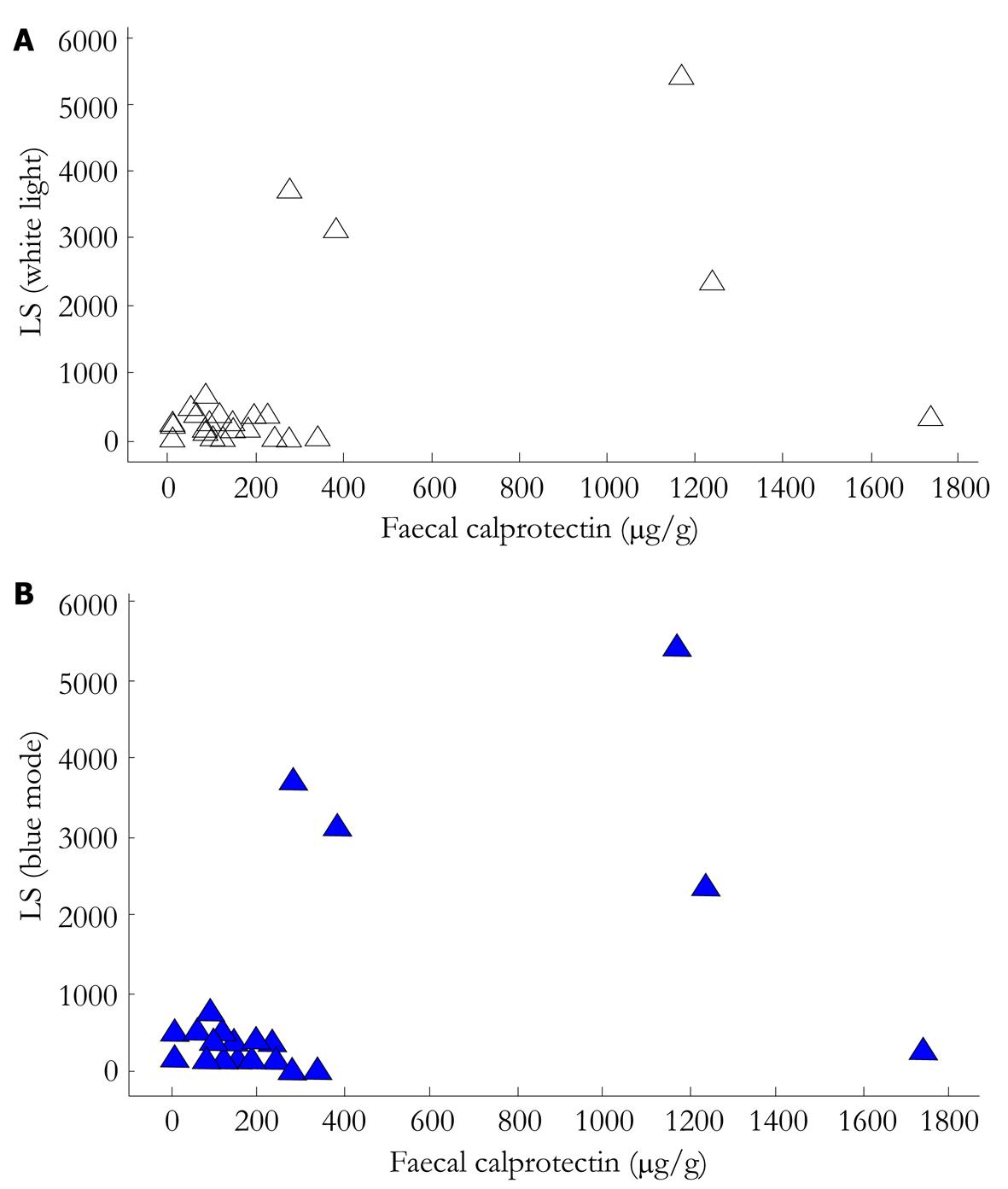
RESULTS: Twenty-seven (n = 27, 20F/7M) patients were included. Thirteen (n = 13) had SBCE with PillCam®SB1, and the remainder (n = 14) with PillCam®SB2. The median level of FC in this cohort was 125 μg/g. LS (calculated in WL SBCE review) correlation with FC levels was r = 0.490 (P = 0.01), while for BM review and LS correlation with FC was r = 0.472 (P = 0.013).
CONCLUSION: Although BM is believed to enhance mucosal details i.e., small mucosal breaks, it did not perform better than WL in the calculation of LS in our cohort.
- Citation: Koulaouzidis A, Douglas S, Plevris JN. Blue mode does not offer any benefit over white light when calculating Lewis score in small-bowel capsule endoscopy. World J Gastrointest Endosc 2012; 4(2): 33-37
- URL: https://www.wjgnet.com/1948-5190/full/v4/i2/33.htm
- DOI: https://dx.doi.org/10.4253/wjge.v4.i2.33
Since its introduction in clinical practice, small-bowel capsule endoscopy (SBCE) has been established as a main, non-invasive imaging modality for the small-bowel. It has already showed to be a superior–to most existing radiological techniques–diagnostic tool in the investigation of obscure gastrointestinal bleeding (OGIB), although its role in Crohn’s (CD) is less clear[1]. Assessment of the full length of the small-intestine is often required not only to evaluate patients with suspected, but also those with established CD[2,3]. However, as the diagnosis of CD remains a clinical one -based on the combination of clinical, radiologic, endoscopic, and histologic findings-, caution is advised in using findings on SBCE as the primary means of making a diagnosis of small-small CD[3]. Furthermore, SBCE is also a useful modality in identifying the impact of non-steroidal anti-inflammatory drugs (NSAIDs), i.e., mucosal breaks, surface denudation and strictures in the small-bowel[4,5].
Until recently, the use of SBCE in monitoring the extent and activity of small-bowel inflammation is limited due to a lack of standardisation in systematically reporting small-bowel mucosal inflammatory change. To this end, Gralnek et al[6] developed a scoring index – known since as the Lewis score (LS) - which examines 3 endoscopic parameters: villous oedema, ulceration and luminal stenosis. The investigators set thresholds where LS < 135 denotes normal or clinically insignificant mucosal inflammatory change, LS > 135 and < 790 denotes mild and LS ≥ 790 severe inflammation.
One of the new features of PillCam® (Given® Imaging Ltd., Yokneam, Israel) reading software (RAPID®) is the integration of the LS and the image enhancement toggle button (in versions 5, 6 and 7). The former provides a screen for LS calculation, while the latter offers both flexible spectral imaging colour enhancement (FICE 1, 2 and 3) as well as blue filtering, all with the simple click of a button. Blue filtering or blue mode (BM) is a colour coefficient shift of light in the short wavelength range (490-430 nm) superimposed onto a white light (WL) (red, blue, green; RGB) image.
Calprotectin, on the other hand, is a protein complex of the S-100 family of calcium binding proteins[7]. It is found in high concentration in the cytosol of neutrophils and is resistant to intestinal degradation for up to a week, thus distributed throughout the stool where it can be readily detected using standard enzyme linked immunosorbent assays (ELISA)[8]. The normal range has been well defined as < 50 µg/g; levels < 20 µg/g are consistent with non-detectable calprotectin in faeces (FC). FC is raised in inflammatory, infective and/or neoplastic enteropathies[9].
Since the initial description by Fagerhol et al[10], several studies have been published showing close correlation between faecal calprotectin (FC) concentration and conventional endoscopy, faecal leukocyte excretion quantified with indium, small bowel MRI and SBCE[11-14]. Therefore, FC is considered as a specific and highly sensitive marker of gut inflammation[11,15].
We are set to examine the usefulness of image enhancement, and in particular BM, in calculating the LS, as compared with relevant scores obtained by WL review of SBCE sequences. FC was used as the gold standard for quantifying small-bowel inflammation.
SBCE video sequences were reviewed with the PillCam® Platform (RAPID®7.0 software), on a 21-inch widescreen monitor using a maximised single view window at a speed of 8 frames per second (fps). The review was performed by a single, experienced reviewer in a room with dimmed lights. Video sequences were not de-identified; however, captured thumbnails were not available to the reader (with the exception of captured anatomical landmarks).
WL review was performed with the Quick Adjust function “on” and with the following predefined settings: sharpness 1, brightness 1 and colour 2.
LS[5] was calculated for each study by inputting the necessary parameters (quantitative and qualitative descriptors relating to villous oedema, ulceration and stenosis) into the RAPID®7.0 workstation algorithm. LS were calculated for each tertile, by switching consecutively between WL and BM review.
In our centre, we have adopted a modified 4-point grading scale (poor, fair, good, and very good; from 0 to 3) to describe small-bowel cleansing. The score depends on the proportion of visualized mucosa and the extent of obscuration by intraluminal food debris, turbid fluids, bubbles or bile as follows: grade 3 (very good visibility): > 75% mucosa visible; grade 2 (good visibility): 50%-75%; grade 1 (average visibility): 25%-50%; and grade 0 (poor visibility): < 25% mucosa seen[16].
Statistical analyses were carried out with a statistical package program for Windows (Minitab® version 16, Minitab Ltd, Coventry, United Kingdom). All P values presented herein are 2-tailed. A P value < 0.05 was considered statistically significant. Numerical values are herein expressed as median with lower (Q1) and upper (Q3) quartile values following in parentheses. Pearsons’ correlation coefficient (r, rho) was used to measure statistical dependence between two variables.
This study was conducted in accordance with United Kingdom research ethics guidelines. After review by the local ethics committee, further specific ethical review and approval were not required, as the study was considered a retrospective audit work using data obtained as part of regular patient care.
Twenty seven (n = 27, 20 females/7 males) patients were included. The median age of the cohort was 40 years (Q1: 24 year, Q3: 55 year). The indications for SBCE were: clinical symptomatology compatible with small-bowel CD (n = 19), abnormal small-bowel radiology (n = 2), reassessement of established CD (n = 3), iron deficiency anaemia ± other clinical symptoms (n = 3).
Thirteen (n = 13) SBCE were performed with PillCam®SB1, the remaider with PillCam®SB2. The capsule endoscope reached the caecum in 25/27 cases, hence 25 cases were used for further analysis. All 25 patients had undergone, for the purpose of their clinical work-up, a colonoscopy prior (and at a reasonable interval) to their SBCE. This was to ensure that the obtained FC results reflected levels of the small-bowel mucosal inflammation and not any colonic pathology[17].
The median small-bowel transit time was 04:11:10, while the median small-bowel cleansing score was assessed at 2.33 (Q1: 1.66, Q3: 2.33). The median FC was 125µg/g (Q1: 87.5 µg/g, Q3: 262.5 µg/g). The median time from obtaining a stool specimen for FC to having SBCE was 0 d (Q1: -6.5 d, Q3: 4.5 d; where the (-) sign denotes that the specimen for FC was obtained after the SBCE was performed).
The correlation between LS (calculated in WL SBCE review) and FC levels was moderate to weak (rho: 0.490, P = 0.010), while the relevant value for BM SBCE review was rho: 0.472, P = 0.013. (Figure 1A and B). There was no statistically significant difference between the LS-WL and LS-BM (P = 0.8976). When only the ulcer-competent of the LS was examined, BM failed to provide any additional information to WL review (P = 0.213).
The cohort (n = 25) was then divided further to 3 sub-groups, based on FC results[15]; group A (n = 8) with FC < 100 µg/g, group B (n = 8) with FC ≥ 100 µg/g and < 200 µg/g, and group C (n = 9) with FC 200 µg/g. In group A, the correlation of LS-WL and LS-BM with FC was rho = 0.479 vs rho = 0.376 (P = 0.842); in group B, rho = 0.123 vs rho = -0.1653 (P = 0.845); and in group C, rho = 0.227 vs rho = 0.215 (P = 0.983), respectively.
Once more, there was no statistical difference between the 2 LS (with WL and BM) calculations in any of the three groups (or groups A, B and C; P = 0.4388, 0.3809 and 0.9935, respectively).
Lewis Score is considered a standardised means of reporting the presence and degree of clinically significant (irrespective of aetiology) mucosal inflammatory changes seen on CE[2]. It was devised, internally validated and presented in 2006/7, by a group of expert gastroenterologists, from the review of a total of 44 de-identified SBCE studies. Its use helps to reduce subjectiveness, as it is based on the variables/parameters associated with mucosal disease, namely mucosal breaks, villous oedema and stenosis. It has since been incorporated into the RAPID® software (Given® Imaging Ltd., Yokneam, Israel) and is easily calculated using the parameter entry algorithm.
Image enhancement techniques, such as FICE and BM, have also been incorporated in the RAPID® software. Virtual chromoendoscopy has been already widely used in conventional endoscopy, aiming to improve diagnostic yield by enhancing the contrast between background and surface mucosal abnormalities, through narrowing the bandwidth of WL to that of blue-green light. To date, the published experience of its use in SBCE is only limited[7,18-21]. Furthermore, the ability of chromoendscopy to improve detection rate of clinically significant lesions during SBCE is still questionable[21]. Imagawa et al[18] have reported that FICE application provided improved image quality of angioectasias, erosion/ulcerations, and various tumours, when FICE wavelength settings 1 and 2 were used. In a more recent pilot study though[19], the same group found that the detection rate of ulceration/erosion did not differ statistically between conventional i.e., WL-SBCE and FICE-SBCE review.
The experience with BM application in SBCE reading is even more limited[7,1,22]. BM is a colour coefficient shift of light in the short wavelength range (490-430 nm) superimposed onto a WL image. Abdelaal et al[22] found that by employing BM in SBCE they detected more superficial erosions and oedema than with WL. They prospectively reviewed a total of 20 SBCE from patients with cirrhosis, at speed of 8 fps, and identified more erosions than with WL. We recently showed that BM provides image improvement for many SBCE lesion categories, but is more useful in enhancing visualisation of surface mucosal changes, e.g., mucosal breaks, ulcerations (in > 90% of cases) and mucosal cobblestoning[7]. This seems to be of particular importance in LS calculation, as one of the three LS parameters is the presence and number of mucosal breaks/ulcers. Although LS has been internally validated, it can be as good as and the current capsule technology level, i.e., lack of directionality, lack of controlled speed of capsule transit, allow to be.
Therefore, in order to compare results from LS calculation with different modes, more objective biochemical markers of small-bowel inflammation i.e. faecal calprotectin or lactoferrin are needed as reference tests. FC is contained in faeces at levels proportional to the amount of neutrophil migration to the intestinal wall and luminal cell shedding. In the absence of colonic pathology, FC levels reflect in an accurate and reliable way, the degree of small-bowel mucosal inflammation[10,12]. As such, it was used as “gold standard” for quantifying small-bowel inflammation, hence mucosal breaks or disruption, for the purposes of this study[23].
With the current study we demonstrated that the use of BM, despite our initial hypothesis[7], offered little aid (in comparison to WL) in LS calculation. In fact, LS calculation with BM showed slighly weaker (as compared to LS-WL) correlation to FC (rho = 0.472 vs rho = 0.490), although this did not reach statistical significance (P = 0.938). Furthermore, it is worth noting that the correlation between LS (irrespective of viewing mode) and FC was weak (rho < 0.5). This could only partially be explained by the fact that the stool specimen collection was obtained on the day of the SBCE test in just one fifth of cases. In the remainder (n = 20), the stool specimen for calprotectin was obtained in period of ± 30 d from the SBCE[12]. Interestingly, Imagawa et al[19] also showed that the difference in erosive/ulcerative lesion detection between conventional SBCE and SBCE-FICE (at the various settings) was not statistically significant. This simply means that although chromoendoscopy works well in improving the image quality of captured lesions, it does not lead to improved lesion detection. Of course, this should not come as a surprise, as chromoendoscopy has nothing to do with the various parameters of image acquisition like the speed of small-bowel transit by the capsule, the lack of directionality or the unpredictable change of field of view.
Our study is retrospective and as such it was not possible to standardise the interval between FC measurement and SBCE/colonoscopy. Furthermore, the use of one reviewer following a strict protocol- may have introduced observational bias, when sequentially comparing images in BM and WL.
The use of small-bowel capsule endoscopy (SBCE) in monitoring the extent and activity of small-bowel inflammation has been limited due to a lack of standardisation in systematically reporting small-bowel mucosal inflammatory change. Lewis score (LS) was developed out of this need and examines 3 endoscopic parameters: villous oedema, ulceration and luminal stenosis. Thresholds are: LS < 135, normal or clinically insignificant mucosal inflammatory change; LS > 135 and < 790, mild inflammation; and LS ≥ 790 severe inflammation. Furthermore, virtual chromoendoscopy (Fujinon® Intelligent Color Enhancement, FICE) has been incorporated in the Rapid software (Given® Imaging Ltd, Yokneam, Israel) with aim to increase the detection of lesions in capsule endoscopy.
There are scanty data on the use of virtual chromoendoscopy (FICE or blue mode filter) in small-bowel capsule endoscopy. The crucial question, should this method becomes a regular adjunct in reviewing SBCE videos, is if it improves the detection rate of clinically relevant lesions. Gupta et al showed that FICE is not better than white light for diagnosing significant lesions on SBCE for obscure GI bleeding, although some vascular lesions could be more accurately characterized with FICE as compared to white light SBCE. Abdelaal et al found that Blue Mode viewing leads to better detection and visualization of vascular and non-vascular lesions. We also extensively checked the use of FICE and Blue Mode in 6 different lesion-categories obtained from 200 capsule endoscopy examinations. We found that comparing with FICE, Blue Mode filter offers better image enhancement in capsule endoscopy.
LS [calculated in white light (WL) SBCE review] correlation with FC levels was r = 0.490 (P = 0.01), while for BM review and LS correlation with FC was r = 0.472 (P = 0.013). There was no statistically significant difference between the LS-WL and LS-BM (P = 0.8976).Although BM is believed to enhance mucosal details i.e., small mucosal breaks, it did not perform better than WL in the calculation of LS in this cohort.
Data on the validity of virtual chromoendoscopy in SBCE are limited and, to a great extent, discordant. Further larger scale, multi-center, randomized controlled trial would be of value to determine if has a role in improving diagnosis in SBCE.
Virtual chromoendoscopy: an imaging technique that is based on narrowing the bandwidth of the conventional endoscopic image arithmetically, using spectral estimation technology. FICE: (Fuji Intelligent Color Enhancement), Fujinon® intelligent chromo endoscopy system. Blue filtering or Blue Mode (BM): a colour coefficient shift of light in the short wavelength range (490-430 nm) superimposed onto a WL (red, blue, green; RGB) image. Lewis score (LS): a SBCE inflammation scoring system which examines 3 endoscopic parameters: villous oedema, ulceration and luminal stenosis.
The present paper is a retrospective cohort study. The article is well written, and topic is interesting and novel.
Peer reviewers: Carlos Robles-Medranda, MD, Head of the Endoscopy Division, Ecuadorian Institute of Digestive Disease, San Antonio Clinic, Av. Reales Tamarindo y Tennis Club, Portoviejo-Manabi-Ecuador, Casilla 13-01-266, Ecuadorian; Sherman M Chamberlain, Associate Professor of Medicine, Section of Gastroenterology, BBR-2538, Medical College of Georgia, Augusta, GA 30912, United States
S- Editor Yang XC L- Editor A E- Editor Yang XC
| 1. | Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy for small-bowel evaluation in Crohn’s disease. Gastrointest Endosc. 2011;74:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Lucendo AJ, Guagnozzi D. Small bowel video capsule endoscopy in Crohn’s disease: What have we learned in the last ten years? World J Gastrointest Endosc. 2011;3:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Aabakken L. Small-bowel side-effects of non-steroidal anti-inflammatory drugs. Eur J Gastroenterol Hepatol. 1999;11:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sidhu R, Brunt LK, Morley SR, Sanders DS, McAlindon ME. Undisclosed use of nonsteroidal anti-inflammatory drugs may underlie small-bowel injury observed by capsule endoscopy. Clin Gastroenterol Hepatol. 2010;8:992-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Krystallis C, Koulaouzidis A, Douglas S, Plevris JN. Chromoendoscopy in small bowel capsule endoscopy: Blue mode or Fuji Intelligent Colour Enhancement? Dig Liver Dis. 2011;43:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Manolakis AC, Kapsoritakis AN, Tiaka EK, Potamianos SP. Calprotectin, calgranulin C, and other members of the s100 protein family in inflammatory bowel disease. Dig Dis Sci. 2011;56:1601-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Logan R. Faecal calprotectin for the diagnosis of inflammatory bowel disease. BMJ. 2010;341:c3636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Aadland E, Fagerhol MK. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:823-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Sander J, Fagerhol MK, Bakken JS, Dale I. Plasma levels of the leucocyte L1 protein in febrile conditions: relation to aetiology, number of leucocytes in blood, blood sedimentation reaction and C-reactive protein. Scand J Clin Lab Invest. 1984;44:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 488] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 13. | Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 308] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Zippi M, Al Ansari N, Siliquini F, Severi C, Kagarmanova A, Maffia C, Parlanti S, Garbarino V, Maccioni F. Correlation between faecal calprotectin and magnetic resonance imaging (MRI) in the evaluation of inflammatory pattern in Crohn’s disease. Clin Ter. 2010;161:e53-e56. [PubMed] |
| 15. | Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Park SC, Keum B, Hyun JJ, Seo YS, Kim YS, Jeen YT, Chun HJ, Um SH, Kim CD, Ryu HS. A novel cleansing score system for capsule endoscopy. World J Gastroenterol. 2010;16:875-880. [PubMed] |
| 17. | Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved visibility of lesions of the small intestine via capsule endoscopy with computed virtual chromoendoscopy. Gastrointest Endosc. 2011;73:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Imagawa H, Oka S, Tanaka S, Noda I, Higashiyama M, Sanomura Y, Shishido T, Yoshida S, Chayama K. Improved detectability of small-bowel lesions via capsule endoscopy with computed virtual chromoendoscopy: a pilot study. Scand J Gastroenterol. 2011;46:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Ibrahim M, Gupta T, Deviere J, Van Gossum A. Is Fujinon Intelligence Computerized Endoscopy (FICE) assisted Capsule Endoscopy (CE) helpful for analyzing Obscure GI Bleeding (OGIB)? ICCD 2010: 23. . |
| 21. | Spada C, Hassan C, Costamagna G. Virtual chromoendoscopy: will it play a role in capsule endoscopy? Dig Liver Dis. 2011;43:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Abdelaal UM, Morita E, Nouda S, Kuramoto T, Miyaji K, Fukui H, Tsuda Y, Fukuda A, Murano M, Tokioka S. W1594:New Method for Better Detection and Visualization of Vascular and Non-Vascular Lesions of Small Bowel by Using Blue Mode Viewing: Capsule Endoscopy Study. Gastrointest Endosc. 2010;71:AB367. [DOI] [Full Text] |
| 23. | Forbes GM. ACP Journal Club. Review: Fecal calprotectin is accurate for screening for suspected IBD in adults but less so in children. Ann Intern Med. 2011;154:JC1-J12. [PubMed] |