Published online Apr 16, 2025. doi: 10.4253/wjge.v17.i4.105238
Revised: March 10, 2025
Accepted: March 27, 2025
Published online: April 16, 2025
Processing time: 89 Days and 0.2 Hours
Esophageal submucosal gland duct adenoma (ESGDA) is very rare, and easily diagnosed as adenocarcinoma.
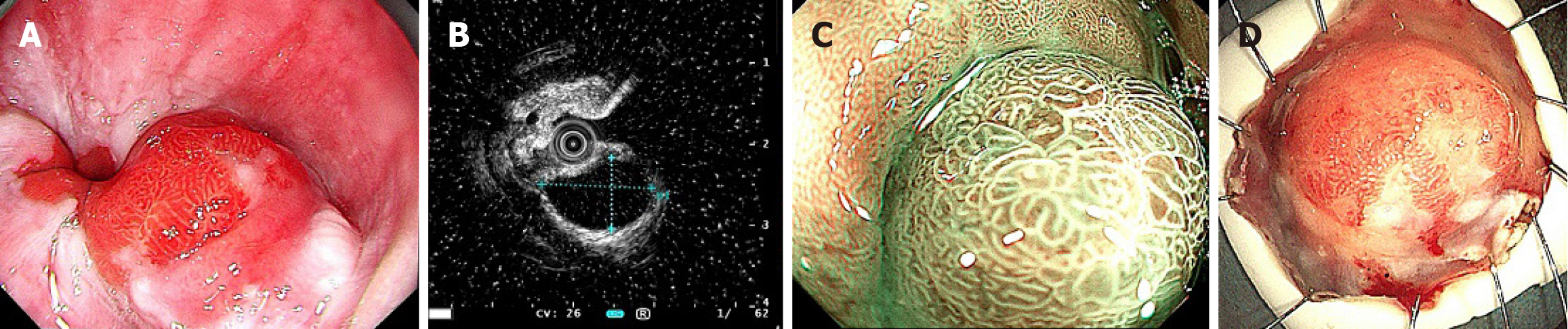
A 70-year-old man presented with abdominal discomfort and intermittent dull pain during swallowing for 10 days. Digestive endoscopy revealed a polypoid bulge at the esophago-gastric junction, which was resected by endoscopic sub
ESGDA is a benign tumor that can be cured by ESD. Accurate diagnosis can prevent unnecessary extensive therapeutic interventions.
Core Tip: We present an exceedingly rare case of esophageal submucosal gland duct adenoma and conduct a comprehensive literature review to elucidate the origin, pathogenesis, clinical features, endoscopic and pathological characteristics, as well as potential genetic alterations of this tumor, thereby enhancing our understanding and preventing misdiagnosis.
- Citation: Lu T, Liu JX, Xia Y, Zhao Y. Clinical, endoscopic and histopathological observation of a rare case of esophageal submucosal gland duct adenoma: A case report. World J Gastrointest Endosc 2025; 17(4): 105238
- URL: https://www.wjgnet.com/1948-5190/full/v17/i4/105238.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i4.105238
It is well known that the submucosal glands of the normal esophagus are composed of lobules and extrolobular ducts. Each submucosal glandular lobule comprises multiple acini and intralobular ducts that converge to form an external lobule duct, which opens into the esophageal cavity. The submucosal glands and ducts are distinctive features of the esophagus and absent in the stomach. The acinus can be mucinous or mucous and serous mixed parotid acinus, which produce mucin and bicarbonate to neutralize gastric acid and growth factors, which are secreted through the extrolobular ducts to the surface of the esophageal mucosa to protect the esophagus[1]. They differ from the gastric cardia-type mucinous glands that are distributed in the lamina propria at the upper and lower end of the esophagus. Intralobular and extralobular ducts have no mucous cells[2]. Esophageal submucosal gland duct adenoma (ESGDA), a benign tumor originating from the esophageal submucosal gland, is extremely rare. Due to the lack of knowledge of clinical patho
A 70-year-old patient presented to our hospital with a 10-day history of upper abdominal discomfort and intermittent dull pain during swallowing. He reported no significant changes in mental status, appetite, or sleep patterns, no food reflux or acid reflux, no nausea, vomiting, abdominal distension, palpitations, chest tightness, chills fever or weight loss.
The patient underwent upper gastrointestinal endoscopy at our hospital. Conventional endoscopy revealed a he
The patient has a 20-year history of hypertension and diabetes. He takes oral Amlodipine daily and receives insulin injections subcutaneously with good control of blood sugar levels. His highest recorded blood pressure was 170/100 mmHg. He underwent a ‘left inguinal hernia repair’ three years ago. No history of other diseases and no food or drug allergies.
The patient denied any family history of hereditary diseases or tumors.
The dimension of the resected tissue measured 3.5 cm × 2.6 cm × 0.2 cm, with a hemispherical polypoid mass measuring 0.7 cm protruding from the mucosal surface (Figure 1D). It was vertically sectioned into strips of tissue, each 2 mm width. Based on the incision profile, the mass was situated beneath the mucous membrane, which appeared largely normal on its surface. The mass exhibited a grayish-white coloration, had a medium consistency, and was distinctly demarcated from adjacent tissue.
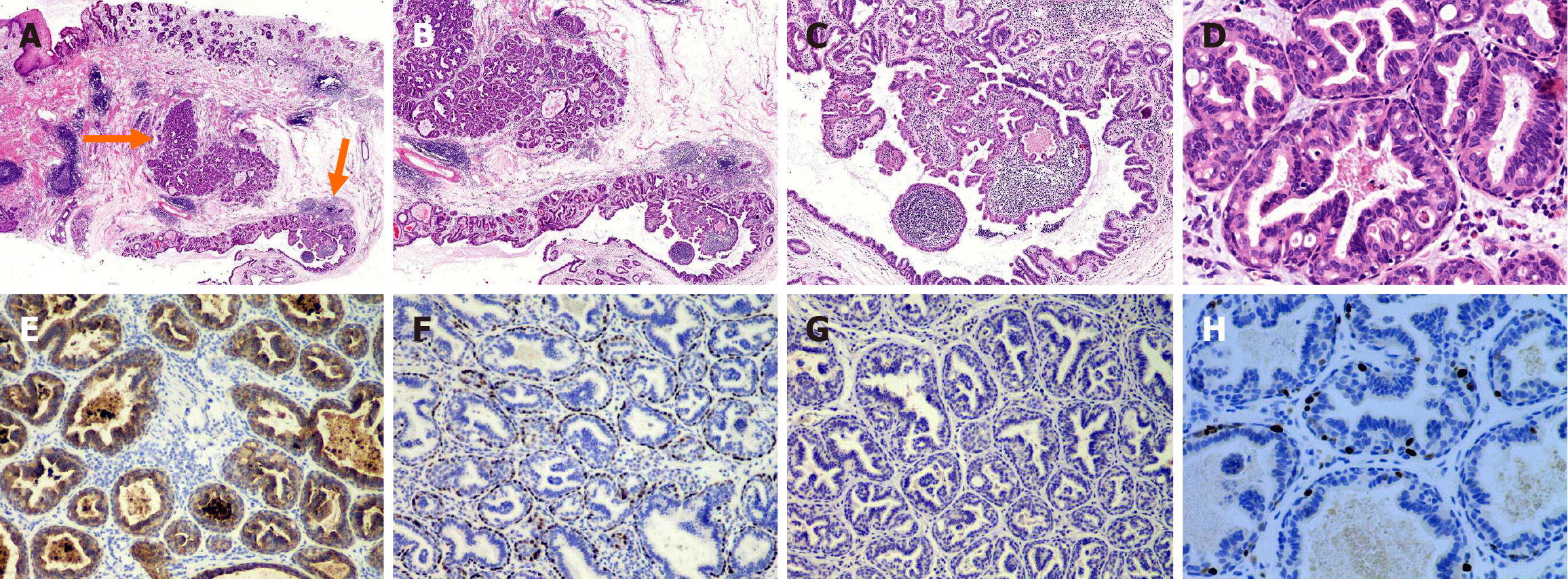
Histologic studies: All tissue strips were cut into slices by the conventional methods, and stained with H&E. The lesion was 0.5 cm × 0.5 cm in size and was located on the submucosal layer without any envelope wrapping (Figure 2A). It was composed of several ducts or cysts with papillary and tubular structures; moreover, lymphocyte clusters were observed in its interstitia along with germinal centers (Figure 2B and C). Furthermore, these ducts or cysts had an inner luminal duct cell layer and an outer myoepithelial cell layer. Both these layers had oncocytic differentiation with granular and eosinophilic cytoplasms. The luminal cells had micropapillary and microglandular hyperplasia in the glandular cavity. There were few nuclear atypicals, lack of mitotic figures, necrosis and mucin production (Figure 2D).
Immunohistochemical studies: Immunohistochemical assay was carried out in strict accordance with the kit instructions. We used EDTA heat antigen repairing methods, with a DAB chromogen. Antibodies used in the immunohistochemistry included: CK7 (OV-TL 12/30; Dako), CK20 (KS20.8; Dako), P63(4A4; Dako), CDX2 (EPR2764Y, Dako), MUC1 (MRQ; Dako), MUC2 (Ccp58; Dako), MUC6 (MRQ-20; Dako), MUC5AC(45M1; Dako), MDM2 (SPM14; Dako) , P53 (BP-53-12; Dako), and Ki-67 (Mib1; Dako).
The luminal duct cells were positive for CK7 (Figure 2E), whereas the myoepithelial cells were positive for P63 (Figure 2F). Both the two-layer cells were negative for CK20, CDX2, MUC1, MUC2, MUC6, and MUC5AC (Figure 2G), and there was no evidence of P53 mutation and MDM2 gene expansion. In fact, Ki-67 showed almost no expression of ductal epithelial cells and expressed in only a few myoepithelial cells (< 5%; Figure 2H).
The patient learned that the tumor was considered benign and successfully removed, he declined our suggestion to conduct relevant gene sequencing for further analysis of the tumor.
After the initial multidisciplinary team discussion, we performed an endoscopic submucosal dissection (ESD) for the patient’s tumor. Following a preliminary pathological examination of the postoperative specimen, we reconvened a multidisciplinary expert consultation which included specialists from both within and outside our institution for the diagnosis of this patient.
After a thorough review of pertinent literature, we arrived at a finally diagnosis of ESGDA.
Following a multidisciplinary discussion and the formulation of a comprehensive treatment plan, we proceeded with ESD on the patient. Tumor was successfully resected en bloc from the submucosa with an estimated blood loss of approximately 10 mL. After the surgery, the patient was placed on fasting status for the first day and received intravenous fluid resuscitation. From the second day, the patient was transitioned to a liquid or semi-liquid diet. Throughout the postoperative period, the patient remained asymptomatic and was subsequently discharged on the fifth postoperative day.
Endoscopic examinations were conducted at 3 months and 1 year post-operation, all of which revealed complete healing of the scar in the surgical area. The patient had no discomfort. Subsequently he underwent biennial endoscopic examina
Rare adenomas of the esophagus have been reported since the middle of the last century. Unfortunately, these reports lacked detailed histological descriptions and photomicrographs[3,4]. ESGDA was first comprehensively introduced by Takubo et al[5] in 1993 and identified as esophageal submucosal tumor. The rarity of this neoplasm has resulted in terminological ambiguity, with various designations including esophageal pleomorphic adenoma, serous cystadenoma, and sialadenoma papilliferum[6,7]. In 1995, Rouse et al[8] formally designated it as ESGDA and posited that it may originate from the ductal components of the submucosal glands. We reviewed the existing literature and found 23 cases in which ESGDA was adequately described in case reports. The clinical characteristics of these cases are summarized in Table 1[5-18].
| Ref. | Age (years)/gender | Symptoms | Location | Gross morphology/size (cm) | Histological morphology | Dysplasia | Necrosis/mitotic figures | Ki-67 index | Follow-up |
| Tsutsumi et al[6], 1990 | 77/male | Nausea | M | Globoid polyp/1.0 | Multiple mycrocysts and papillary proliferation with two layers of cells | Mild and moderate | None/some | NM | Well over 2 years |
| Takubo et al[5], 1993 | 58/male | Abdominal discomfort | M | Dome-like polyp/0.8 | Papillary and tubular structures with two layers of cells | None | None/none | NM | Well over 6 months |
| Rouse et al[8], 1995 | 81/male | Dysphagia | GEJ | Pedunculated polyp/1.5 | Tubules and cystic lumens filled with papillae by two layer of cells | None | None/rare | NM | Well over 12 months |
| Su et al[7], 1998 | 70/male | Abdominal fullness | L | Broad-based polypoid/1.0 | Papillary glandulars with two layers of cells | Benign | None/none | NM | Well over 12 months |
| Agawa et al[9], 2003 | 71/male | NM | L | Sessile polypoid tumor/1.5 | Dilated gland ducts containing papillary and tubular components with two layers of cells | Benign | None/ none | NM | Well over 12 months |
| Hayashi[10] 2004 | 60/female | Abdominal discomfort | M | Dome-like protruding lesion/1.1 | Cysts, papillary and tubular proliferation with two layers of cells | Minimal | None/none | NM | Well over 11 years |
| Chinen et al[11], 2004 | 67/female | None | L | Polypoid lesion/0.6 | Multiple cysts, tubules and papillae with two layers of cells | Mild | None /rare | NM | Well over 6 months |
| Harada et al[12], 2007 | 75/male | NM | L | Well demarcated without a capsule/0.3 | Papillary and cystic structures with two layers of cells | Minimum | None/rare | 1%-2% | NM |
| Nie et al[13], 2016 | 74/male | Retrosternal discomfort | L | Dome-like polypoid tumor/0.5 | Multiple glandular cysts and papillary folds with two layers of cells, | Minimum | None/none | 1% | Well over 4.5 years |
| 54/female | Abdominal discomfort and heartburn | L | Hemispherical protruding lesion/0.3 | Multiple glandular cysts and papillary folds with two layers of cells, | Minimum | None/none | 1% | Well over 4 years | |
| 45/male | NM | L | Hemispherical protruding lesion/0.4 | Multiple glandular cysts and papillary folds with two layers of cells, | Minimum | None/none | 1% | Well | |
| Shibata et al[14], 2017 | 66/female | None | L | Slightly protruding tumor/0.5 | Dilated ducts and papillary proliferations with two layers of cells | Slight | None/none | 2% | NM |
| Genere et al[15], 2019 | 78/female | Dysphagia | U | Submucosal mass with well-defined borders/2.0 | Multiple lobulated cystic proliferations of two layers of cells | Mild | None/rare | NM | Well |
| Yamamoto et al[16], 2020 | 73/female | None | L | Subepithelial tumor with a central depression/0.8 | Tubular and cystic pattern with two-cells layers | None | None/none | NM | Well |
| Chen et al[17], 2023 | 58/male | Gastro-esophageal reflux symptoms | L | Hard mass with well-defined heterogeneous/3.5 | Cystic pattern with two layers of cells | Benign | None/rare | NM | Well over 12 months |
| Hua et al[18], 2025 | 65/male | None | U | SMT/0.5 | Glandular ducts, cysts and papillae, with two-cells layers | Bland | None/none | < 1% | Well over 68 months |
| 75/male | Loss of appetite | U | SMT/1.5 | Well over 46 months | |||||
| 65/male | Belching, acid reflex | M | SMT/1.5 | Well over 36 months | |||||
| 55/female | Discomfort during swallow | L | SMT/0.5 | Well over 25 months | |||||
| 51/female | Acid reflux, vomiting | L | SMT/0.8 | Well over 24 months | |||||
| 73/male | Abdominal pain | L | SMT/0.3 | Well over 50 months | |||||
| 63/male | None | GEJ | SMT/2.0 | Well over 37 months | |||||
| Our case | 70/male | Abdominal discomfort | GEJ | Hemispherical bulge/0.5 | Tubular, cysts and papillary structures with two layers of cells | Few | None/none | < 5% | Well over 69 months |
The average of the 23 patients with ESGDA was 66 years (range: 45-81 years), exhibiting a male predominance (male:female = 15:8). Among them, 2 cases of ESGDA were incidentally discovered due to the presence of esophageal squamous cell carcinoma alongside gastric adenocarcinoma[5,12]; the remaining cases primarily presented with initial symptoms of abdominal discomfort, dysphagia, belching, vomiting, loss of appetite, wasting, acid reflux and abdominal pain, which occasionally posed challenges to their condition and complicated the diagnosis of gastroesophageal reflux disease. During digestive endoscopy, these lesions manifested as small hemispherical or dome-shaped submucosal protrusions. All patients had single ESGDA, which ranged in diameter from 0.3 to 3.5 cm, with an average of 1.0 cm. They were removed by ESD or endoscopic mucosal resection. All ESGDAs exhibited well-defined bondaries but lacked an envelope. Histologically, they were characterized by multiple cystic dilatations of glandular ducts, which contained two layers of epithelial cells exhibiting proliferation and papillary folds. The cytoplasm of the inner luminal ductal epithelial cells was granular and eosinophilic, featuring round to oval nuclei with minimal nuclear atypia. The outer basal cells were spindle with distinct or weak eosinophilic cytoplasms. All the tumors showed a low mitotic activity without necrosis. Diffuse or focal lymphocytic infiltration in the interstitium was commonly observed. In immunohistochemical analysis, both layers expressed epithelial markers like pan cytokeratin and epithelial membrane antigen. The inner ductal epithelial cells demonstrated low molecular weight cytokeratins such as CK7 and CK18, while the outer layer expressed basal cell markers including CK5/6, P63, S-100 among others. Markers such as CK20, CDX2, MUC1, MUC2, MUC5AC, MUC6 and P53 were all negative. The proliferations index of Ki-67 was very low (1%-5%). Few reports on the molecular genetic changes of ESGDA, only Hua et al[18] conducted genomic analysis on 7 cases of ESGDA, and found 5 of them exhibited a BRAF V600E mutation (71.4%). There were no recurrences of ESGDA, and the prognosis was highly favorable.
The glands of the esophagus are categorized into esophageal cardiac glands and submucosal glands, which are distributed in different levels. The esophageal cardiac gland is situated within the mucosal lamina propria and exhibits structural similarities to gastric glands. It comprises MUC6-positive glandular ducts, with a rare presence of parietal cells and chief cells. The epithelium of the glandular pit shows positive expression for MUC5AC, while MUC2 positivity may occur in cases of intestinal metaplasia. Esophageal submucosal glands reside in the submucosa and function as exocrine glands, considered an extension of the small salivary glands of the oropharynx. These glands are dispersed throughout the esophagus along its longitudinal axis but exhibit greater concentration at the junction between the lower esophagus and cardiac, where significant alterations in physical and chemical properties occur. Submucosal gland acinar secretions are collected via ducts that transport them to the esophageal lumen; initially covered by a single cuboidal epithelium, these ducts subsequently transition into a double-layered epithelium before traversing through various layers including the muscularis mucosa, lamina propria mucosa, and the epithelium of the esophagus[17,19].
The occurrence location of ESGDA mirrors that of esophageal submucosal glands. It resided within the submucosa where normal submucosal gland tissues frequently surrounded it. Occasionally observed transitional relationships between both structures suggested an intuitive possibility that ESGDA might originate from these submucosal glands, which was further corroborated by immunohistochemical findings. The immunophenotypic characteristics of the inner ductal epithelial cells of ESGDA were closely align with that of the normal esophageal submucosal duct epithelium. Tumor cells expressed MUC5B, CK7, CK5/6, and CK19 while showing no expression for CK20, CDX2, Villin, MUC5AC, MUC6, MUC2 or GCDFP15. All these indicated a lack of differentiation phenotype characteristic typical to digestive tract-type glandular epithelia or submucosal mucous acinoid epithelia. It is worth mentioning that Harada et al[12] found through immunohistochemistry of MUC5B and electron microscopy that a few ductal epithelial cells in ESGDA displayed limited mucus secretion localized to their subapical regions, implying that tumor cells may have the ability of terminal duct (intercalated duct) differentiation. And They effectively delineated microvilli on both the basement membrane and the apical surface of the luminal duct cells with Alcian blue (PH 2.5) and periodic acid-Schiff (AB-PAS) staining, corroborating the findings from MUC5B immunostaining. The basal layer cells of ESGDA expressed P40, P63, SMA, Calponin, and S-100 protein, suggesting characteristics of myoepithelial differentiation.
Current research indicates that gastroesophageal reflux not only serves as the primary etiological factor in the development of Barrett’s esophagus (BE) but is also intricately linked to the progression of ESGDA[12]. Other environmental factors include long-term smoking, drinking, overheated diet and other irritants. These factors induced injury to the esophageal mucosa and promotes inflammatory cell infiltration within both the mucosal and submucosal layers. This results in a dual impact: On one hand, inflammatory cells impair myoepithelial contractile function; on the other hand, inflammatory exudates or epithelial debris obstruct the ducts of submucosal glands. The cumulative effects of these detrimental factors hinder proper secretion discharge from esophageal submucosal glands, leading to noticeable ex
Adenomas of the esophagus are infrequent and typically associated with intestinal metaplasia, specifically BE, which is a consequence of GERD. These lesions are characterized by raised polypoid mucosal formations composed of intestinal or gastric glandular epithelium exhibiting varying degrees of dysplasia. They may be not represent true adenomas in nature but precursor lesions for BE-related adenocarcinoma[20]. ESGDA discussed in this paper is a true primary submucosal adenoma which is extremely rare and located in the submucosal layer, originating from the submucosal gland duct cells. Its immunophenotypic profile indicates a closer association with tumors of salivary adenoid origin.
In terms of molecular genetics, only Hua et al[18] found BRAF V600E mutations in five of the seven ESGDAs. Given that the BRAF V600E mutation has been previously confirmed in sialadenoma papilliferum, this finding provides additional evidence that ESGDA is an esophageal counterpart of minor salivary gland tumors. The BRAF V600E mutation may promote cell proliferation by activating the MAPK signaling pathway, and its role in ESGDA and whether it may lead to malignant transformation require further investigation.
The pathological diagnostic criteria supporting the diagnosis of ESGDA include: (1) Multiple glands or cysts arranged in a lobular configuration and covered by two layers of cells, the inner luminal epithelial cells and the outer basal or myoepithelial cells; (2) The existence of multilayered epithelium and papillary structures within the glands or cysts, without necrosis and significant cytologic atypia, and nuclear mitotic figures are infrequent; (3) Lymphocytic infiltration accompanied by acinar atrophy or disappearance; and (4) Luminal lining cells exhibiting positivity for MUC5B and various cytokeratins (such as CK5/6, CK7, CK18, CK19), whereas outer cell markers P40, P63, S-100, Calponin and SMA show positive expression alongside a low Ki-67 proliferation index. AB-PAS staining reveals microvilli on the apical surface adjacent to the basement membrane along with tubular epithelial cell[21].
The most important aspect of the diagnosis was identifying the adenocarcinoma, which was always invasive, had an obvious structure and cytological atypia, and was accompanied by multiple mitotic figures and abnormal mitotic figures. The presence of ESGDA bilayer epithelium, lack of cytologic atypia, and lack of mitoses were key criteria for the identification. Similarly, another rare tumor, known as oxyntic gland polyp/adenoma or adenocarcinoma of fundic gland (chief cell-predominant type), was identified[22]. It was mainly composed of proliferation of the chief cells and oxyntic cells along with low-grade cytology and a similar low Ki-67 index as that observed for ESGDA. This tumor showed low-grade malignancy with rare occurrences of lymphatic and venous invasion[23,24].
ESGDA can be cured by ESD regardless of whether it occurs at upper or lower esophagus, and whether it is 0.3 cm or 3.5 cm in size. No recurrence or malignant transformation cases have been found in the literature. We think the incom
To summarize, ESGDA is a benign neoplasm that can be completely resected by ESD. It occurs predominantly in the lower third of the esophagus and is more common in elderly male patients. Symptoms include abdominal discomfort or difficulty swallowing, although it may occasionally be asymptomatic. They are small hemispherical or dome-shaped polypoid submucosal polypoid lesions that can be resected endoscopically, but it is still uncertain whether they can progress from adenoma to adenocarcinoma. Extensive ductal metaplasia, hyperplasia and/or retention cyst formation are considered to be the basis or precursors of ESGDA. The histological, immunohistochemical and molecular evidence of ESGDA support that it is a esophageal counterpart of minor salivary gland tumors.
| 1. | von Furstenberg RJ, Li J, Stolarchuk C, Feder R, Campbell A, Kruger L, Gonzalez LM, Blikslager AT, Cardona DM, McCall SJ, Henning SJ, Garman KS. Porcine Esophageal Submucosal Gland Culture Model Shows Capacity for Proliferation and Differentiation. Cell Mol Gastroenterol Hepatol. 2017;4:385-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Shi L, Der R, Ma Y, Peters J, Demeester T, Chandrasoma P. Gland ducts and multilayered epithelium in mucosal biopsies from gastroesophageal-junction region are useful in characterizing esophageal location. Dis Esophagus. 2005;18:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Schmidt HW, Clagett OT, Harrison EG Jr. Benign tumors and cysts of the esophagus. Ann Otol Rhinol Laryngol. 1961;70:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Plachta A. Benign tumors of the esophagus. Review of literature and report of 99 cases. Am J Gastroenterol. 1962;38:639-652. [PubMed] |
| 5. | Takubo K, Esaki Y, Watanabe A, Umehara M, Sasajima K. Adenoma accompanied by superficial squamous cell carcinoma of the esophagus. Cancer. 1993;71:2435-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Tsutsumi M, Mizumoto K, Tsujiuchi T, Maruyama H, Koizumi M, Inagaki T, Toyokawa M, Konishi Y. Serous cystadenoma of the esophagus. Acta Pathol Jpn. 1990;40:153-155. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Su JM, Hsu HK, Hsu PI, Wang CY, Chang HC. Sialadenoma papilliferum of the esophagus. Am J Gastroenterol. 1998;93:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Rouse RV, Soetikno RM, Baker RJ, Barnard IC, Triadafilopoulos G, Longacre TA. Esophageal submucosal gland duct adenoma. Am J Surg Pathol. 1995;19:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Agawa H, Matsushita M, Kusumi F, Nishio A, Takakuwa H. Esophageal submucosal gland duct adenoma: characteristic EUS and histopathologic features. Gastrointest Endosc. 2003;57:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Hayashi Y, Iwakiri K, Kagawa T, Hirakawa T, Sakamoto C, Arai T, Takubo K. Submucosal adenoma of the esophagus: case report and review of the literature. Esophagus. 2004;1:99-102. [DOI] [Full Text] |
| 11. | Chinen T, Misawa T, Yoshida K, Nasu T, Kubo S, Toyoshima S, Yao T, Harada N. Esophageal submucosal gland duct adenoma. Gastrointest Endosc. 2004;60:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Harada O, Ota H, Katsuyama T, Hidaka E, Ishizaka K, Nakayama J. Esophageal gland duct adenoma: immunohistochemical comparison with the normal esophageal gland and ultrastractural analysis. Am J Surg Pathol. 2007;31:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Nie L, Wu HY, Shen YH, Fan XS, Sun Q, Huang Q, Chen J. Esophageal submucosal gland duct adenoma: a clinicopathological and immunohistochemical study with a review of the literature. Dis Esophagus. 2016;29:1048-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 14. | Shibata M, Kusafuka K, Ono H. A Rare Submucosal Tumor of the Esophagus. Gastroenterology. 2017;152:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Genere JR, Lee HE, Wu TT, Prichard DO, Wang KK. Endoscopic findings of esophageal submucosal gland duct adenoma. VideoGIE. 2019;4:361-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Yamamoto M, Nishida T, Nakamatsu D, Adachi S, Inada M. Endoscopic findings of esophageal gland duct adenoma resected by endoscopic submucosal dissection. Gastrointest Endosc. 2020;92:961-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 17. | Chen SY, Xie ZF, Jiang Y, Lin J, Shi H. Modified endoscopic submucosal tunnel dissection for large esophageal submucosal gland duct adenoma: A case report. World J Gastrointest Surg. 2023;15:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 18. | Hua H, Hu D, Ding Y, Li H. Esophageal submucosal gland duct adenoma: An unrecognised esophageal counterpart of minor salivary gland tumours with frequent BRAF V600E mutations. Histopathology. 2025;86:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lörinc E, Öberg S. Hyperplasia of the submucosal glands of the columnar-lined oesophagus. Histopathology. 2015;66:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Asthana N, Mandich D, Ligato S. Esophageal polypoid dysplasia of gastric foveolar phenotype with focal intramucosal carcinoma associated with Barrett's esophagus. Am J Surg Pathol. 2008;32:1581-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Nie L, Li W, Xue L, Wang L, Shen Y, Fan X. Submucosal gland neoplasms of the esophagus: an update and review. Esophagus. 2020;17:376-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Matsukawa A, Kurano R, Takemoto T, Kagayama M, Ito T. Chief cell hyperplasia with structural and nuclear atypia: a variant of fundic gland polyp. Pathol Res Pract. 2005;200:817-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Chan K, Brown IS, Kyle T, Lauwers GY, Kumarasinghe MP. Chief cell-predominant gastric polyps: a series of 12 cases with literature review. Histopathology. 2016;68:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |