Published online Feb 16, 2025. doi: 10.4253/wjge.v17.i2.102075
Revised: December 6, 2024
Accepted: January 18, 2025
Published online: February 16, 2025
Processing time: 124 Days and 21.9 Hours
Rectal schwannoma (RS) is a rare subtype of schwannoma that presents diag
A 71-year-old Chinese woman presented to our outpatient clinic with a 4-year history of a rectal mucosal mass for a follow-up surveillance colonoscopy. A neu
EFTR is safe and effective for resecting gastrointestinal stromal tumors, especially those with extraluminal growth and no lymph node involvement.
Core Tip: This report highlights a rare case of rectal schwannoma that was not identified before resection but was successfully treated with endoscopic full-thickness resection alone, without the need for additional therapy. Our experience suggests that this technique may be a simple and effective treatment for colorectal submucosal tumors that are difficult to diagnose preoperatively, especially when there is no lymph node involvement.
- Citation: Zhang YJ, Yuan MX, Wen W, Jian Y, Zhang CM, Yuan J, He L. Endoscopic full-thickness resection of rectal schwannoma: A case report. World J Gastrointest Endosc 2025; 17(2): 102075
- URL: https://www.wjgnet.com/1948-5190/full/v17/i2/102075.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i2.102075
Schwannomas are common neurogenic tumors originating from Schwann cells of the peripheral nervous system. They often occur in the skin and somatic soft tissue, but rarely involve the gastrointestinal tract[1]. The most common submucosal tumor of the gastrointestinal tract is gastrointestinal stromal tumor, while schwannomas account for only 2%-6% of cases[2]. Gastrointestinal schwannomas most commonly occur in the stomach, followed by the duodenum, jejunum, and ileum, with rare involvement of the colon and rectum[3].
Colorectal schwannoma (RS) is a rare subtype of schwannoma[4]. These tumors grow in a similar layer and pattern as gastrointestinal stromal tumors. Consequently, endoscopic mucosal biopsy often provides limited information to differentiate them from other mesenchymal tumors of the gastrointestinal tract before surgery, including gastrointestinal stromal tumors, neuroendocrine tumors, and leiomyomas. Therefore, preoperative diagnosis of RS remains challenging. Currently, the preferred treatment strategy is complete surgical excision of the tumor to ensure negative margins and minimize the risk of tumor recurrence[5]. Pathological information and immunohistochemistry are crucial for the diagnosis of tumors after surgery.
Herein, we report a case of RS successfully treated exclusively through endoscopic full-thickness resection (EFTR), with postoperative pathology confirming complete tumor resection. The patient exhibited no recurrence or metastasis of the tumor one year after the operation. Notably, EFTR achieved treatment results comparable to those of surgical resection.
Rectal mucosal mass.
A 71-year-old Chinese woman presented to our outpatient clinic with a 4-year history of a rectal mucosal mass.
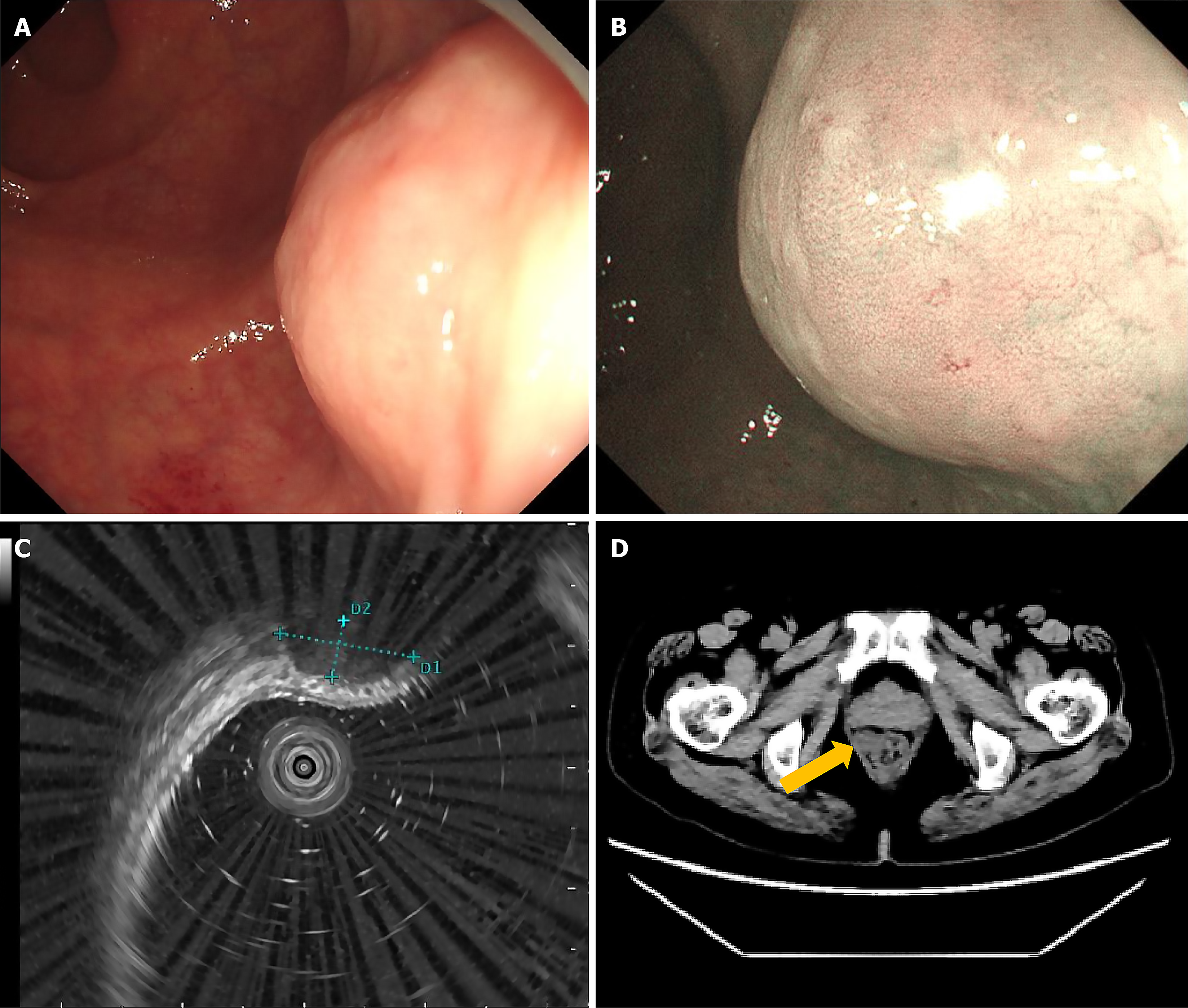
A mucosal mass-like “fortress”, which protruded into the rectum lumen, was identified 5 cm from the anus on endos
Abdominopelvic computed tomography (CT) revealed a 2.0 cm homogeneous, regular, round mass without lobulation at the rectum, with low-density shadowing. No larger peripheral lymph nodes were identified, and the main growth pattern was extraluminal (Figure 1D). Chest CT showed no significant abnormalities.
Given these clinical and imaging findings, the diagnosis of a neurogenic tumor with extraluminal growth as the primary growth pattern was strongly considered, necessitating resection through either EFTR or surgery.
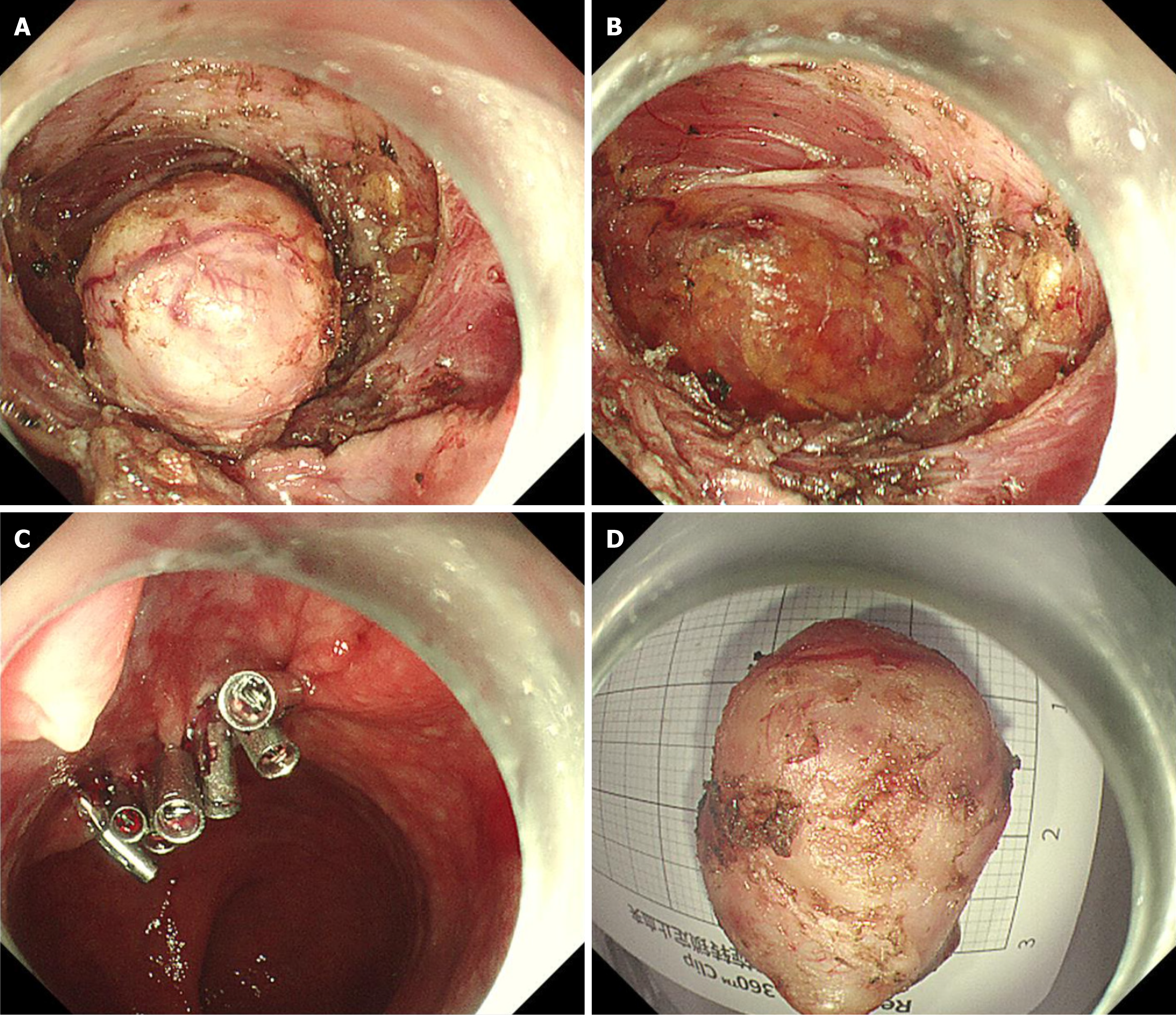
After discussing the options with the patient and her family, EFTR was selected and performed with their informed consent (Figure 2A-C). On endoscopic exploration, a smooth surface extruding mass was identified at the rectum. The patient was discharged 48 hours after the operation without the occurrence of infection or bleeding.
During examination after resection, a white-grey round mass approximately 2.0 cm × 1.5 cm × 1.5 cm in size originating from the muscularis propria was identified; it was solid with an intact capsule (Figure 2D). Histological analysis revealed well-arranged spindle cells with light eosinophilic cytoplasm, no mitotic activity, and nuclear hyperpigmentation. Two distinct histological growth types were observed: Antoni A, characterized by tightly arranged areas of densely packed spindle cells in a palisade formation, including Verocay bodies; and Antoni B, which exhibited a looser distribution of spindle cells with round or elongated nuclei, accompanied by abundant myxoid stroma and xanthoma cells.
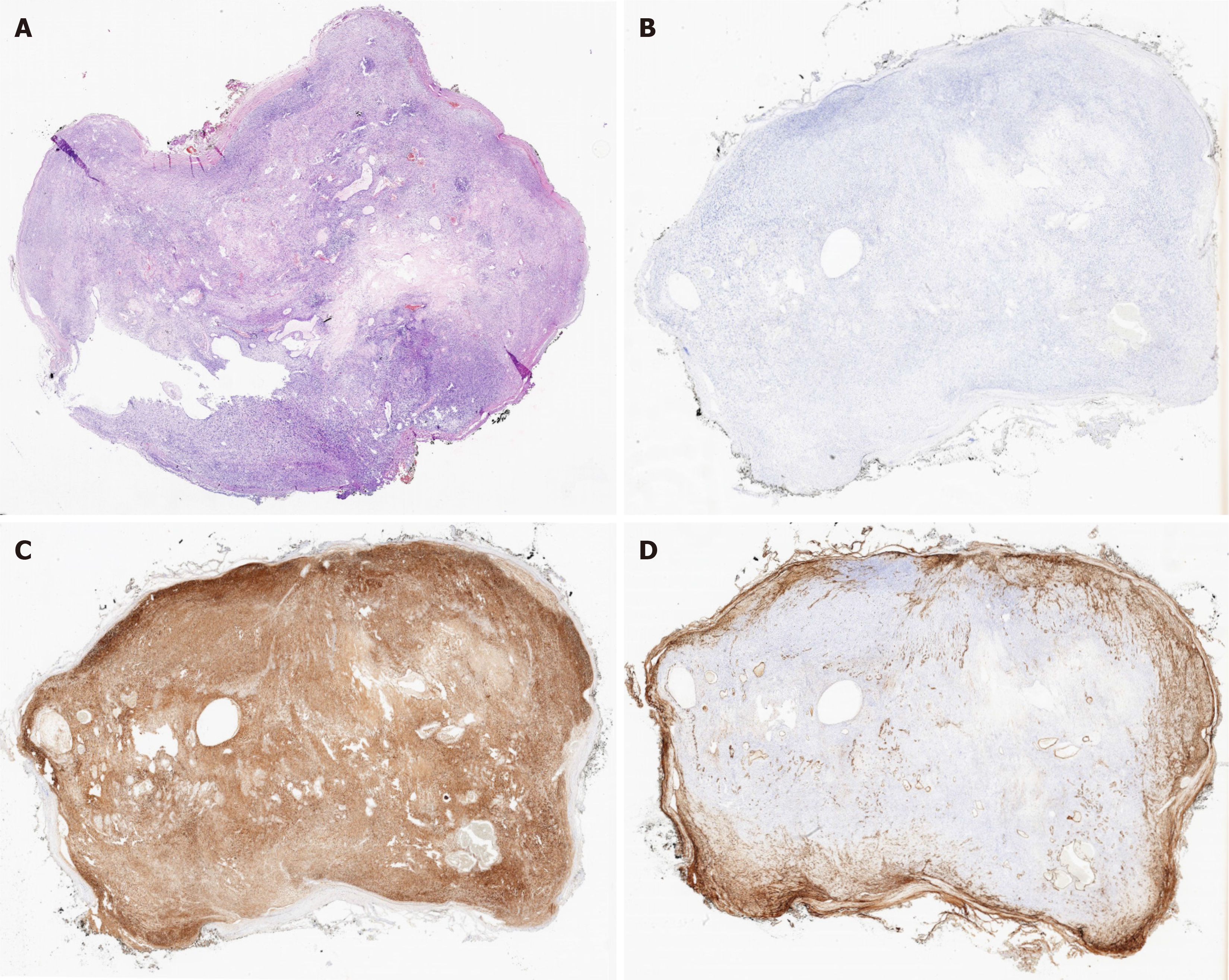
Immunohistochemistry indicated that the resected mass was negative for CD-34, CD-117, and pan-cytokeratin, while positive for S100 and SOX-10, with a Ki-67 proliferative index of approximately 3% (Figure 3).
The patient did not undergo any adjuvant therapy.
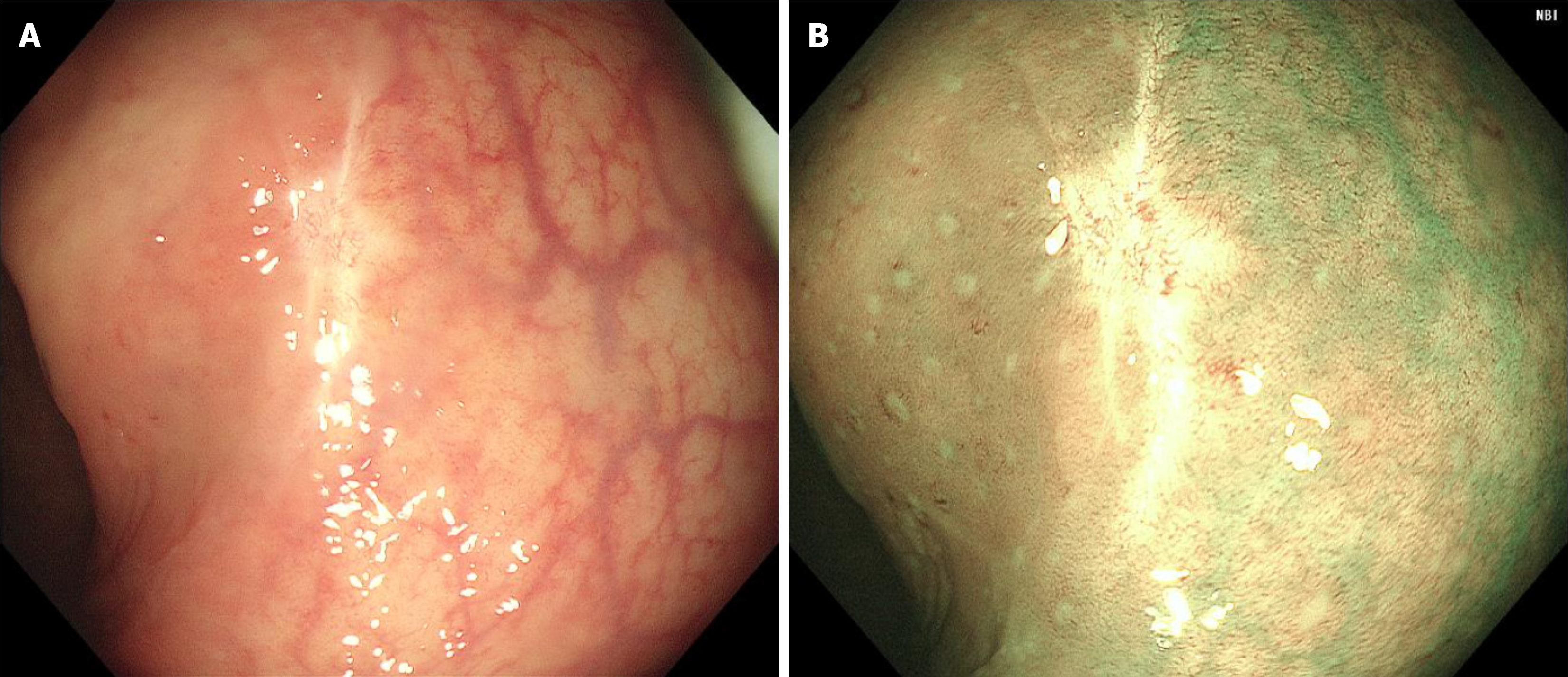
Nearly one year later, a follow-up surveillance colonoscopy and an abdominal and pelvic plain plus enhancement CT scan were performed at a local hospital (Figure 4), and no tumor recurrence or metastasis was found.
Primary schwannoma in the gastrointestinal tract is extremely rare, and most occur in the stomach, followed by the duodenum, jejunum, and ileum, with minimal colorectal involvement. These tumors are believed to originate from Auerbach’s myenteric plexus rather than Meissner’s submucosal plexus[3], although the exact pathogenesis remains unclear.
Although intestinal schwannoma can present at any age, individuals over 60 years of age are more likely to be diagnosed. The most common sites of RSs are the descending colon and sigmoid colon, followed by the cecum, ascending colon, and rectum[5]. A higher proportion of schwannomas of the colon and rectum occur in women than in men[6]. Moreover, most cases of colonic and RSs are reported in PubMed, Scopus, and Cochrane databases as case reports and case series. Diagnosing RSs before surgery is highly challenging, as they usually appear as submucosal tumors with normal endoscopic mucosal biopsy findings[7]. Diagnosis is typically made after surgery, although a small proportion of cases have been confirmed through endoscopic biopsy and successfully treated via wedge resection[7]. Cases of successful treatment through endoscopy alone are rare. However, endoscopic removal offers several distinct advantages. First, it leaves the rectum intact after RS removal; second, it significantly reduces the length of hospital stay; and third, it is relatively low-cost. Overall, EFTR for RS is a cost-effective treatment option that requires fewer resources compared to traditional surgery.
The final diagnosis depends on postoperative pathology and immunostaining results. Macroscopically, gastrointestinal schwannomas are generally located within the muscularis propria, presenting as oval-shaped nodules, ranging between 2 to 10 cm in diameter. They have a solid, grayish-yellow cut surface and a texture similar to that of classic schwannomas. These tumors have a capsule but lack secondary changes such as hemorrhage, necrosis, or cystic degeneration. Tumor cells typically show positive immunostaining for vimentin, S100 protein, or CD56, but negative staining for desmin, CD34, or CD68[8].
RSs are often considered benign neoplasms; nonetheless, distant metastasis has been observed in approximately 2% of cases[9]. A Ki-67 value exceeding 5% is associated with increased tumor invasiveness, while a value exceeding 10% indicates malignancy. A higher risk of recurrence and/or metastasis is associated with a mitotic rate of over five mitoses per field at high magnification or a tumor diameter greater than 5 cm[10].
EFTR is emerging as a viable option for the resection of gastrointestinal stromal tumors, especially in cases where the main growth pattern is extraluminal or originates from the muscularis propria, owing to its advantages in safety, success, and efficiency[11]. Our experience further indicates that EFTR is an effective approach, particularly when lymph node involvement is absent.
We would like to thank Professor Bing Hu of West China Hospital Sichuan University gave scientific guidance.
| 1. | de Mesquita Neto JW, Lima Verde Leal RM, de Brito EV, Cordeiro DF, Costa ML. Solitary schwannoma of the cecum: case report and review of the literature. Case Rep Oncol. 2013;6:62-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Baig MMAS, Patel R, Kazem MA, Khan A. Schwannoma in the ascending colon, a rare finding on surveillance colonoscopy. J Surg Case Rep. 2019;2019:rjz046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Tsunoda C, Kato H, Sakamoto T, Yamada R, Mitsumaru A, Yokomizo H, Yoshimatsu K, Ogawa K, Aiba M, Haga S. A Case of Benign Schwannoma of the Transverse Colon with Granulation Tissue. Case Rep Gastroenterol. 2009;3:116-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Bohlok A, El Khoury M, Bormans A, Galdon MG, Vouche M, El Nakadi I, Donckier V, Liberale G. Schwannoma of the colon and rectum: a systematic literature review. World J Surg Oncol. 2018;16:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Dickson-Lowe RA, James CL, Bailey CM, Abdulaal Y. Caecal schwannoma: a rare cause of per rectal bleeding in a 72-year-old man. BMJ Case Rep. 2014;2014:bcr2014205643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Zhou GYJ, Hu JL, Wang S, Ge N, Guo JT, Liu X, Wang GX, Sun SY. [Endoscopic diagnosis and treatment of colorectal schwannomas]. Xiandai Zhongliu Yixue. 2021;11:1907-1911. [DOI] [Full Text] |
| 7. | Kojima Y, Yamaguchi T, Taguchi S, Kondo E, Yokoyama M, Shirayama S, Nikaido T, Yanagida O. Ascending Colon Schwannoma Surgically Treated after Accurate Preoperative Diagnosis. Case Rep Gastroenterol. 2020;14:483-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Sun XL, Wen K, Xu ZZ, Wang XP. Magnetic resonance imaging findings for differential diagnosis of perianal plexiform schwannoma: Case report and review of the literature. World J Clin Cases. 2018;6:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Akgul E, Inal M, Soyupak SK, Binokay F, Bicakci K. Benign rectal schwannoma. Eur J Radiol Extra. 2003;45:67-70. [DOI] [Full Text] |
| 10. | Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Tian X, Shi B, Chen WQ. Modified endoscopic full-thickness resection of gastric stromal tumor originating from the muscularis propria layer. J Gastrointest Oncol. 2020;11:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |