Published online Feb 16, 2025. doi: 10.4253/wjge.v17.i2.100665
Revised: December 12, 2024
Accepted: January 11, 2025
Published online: February 16, 2025
Processing time: 174 Days and 16.1 Hours
At present, there is a lack of non-invasive indicators to evaluate the changes in endoscopic activity between two visits for patients with Crohn's disease (CD).
To develop a model for predicting whether endoscopic activity will improve in CD patients.
This is a single-center retrospective study that included patients diagnosed with CD from January 2014 to December 2022. The patients were randomly divided into a modeling group (70%) and an internal validation group (30%), with an external validation group from January 2023 to March 2024. Univariate and binary logistic regression analyses were conducted to identify independent risk factors, which were used to construct a nomogram model. The model's performance was evaluated using receiver operating characteristic curves, calibration curves, and decision curve analysis (DCA). Additionally, further sensitivity analyses were performed.
One hundred seventy patients were included in the training group, while 64 were included in the external validation group. A binary logistic stepwise regression analysis revealed that the changes in the amplitudes of albumin (ALB) and fibrinogen (FIB) were independent risk factors for endoscopic improvement. A nomogram model was developed based on these risk factors. The area under the curve of the model for the training group, internal validation group, and external validation group were 0.802, 0.788, and 0.787, respectively. The average absolute errors of the calibration curves were 0.011, 0.016, and 0.018, respectively. DCA indicated that the model performs well in clinical practice. Additionally, sensitivity analysis demonstrated that the model has strong robustness and applicability.
Our study shows that changes in the amplitudes of ALB and FIB are effective predictors of endoscopic improvement in patients with CD during follow-up visits compared to their previous ones.
Core Tip: Endoscopic activity assessment is crucial for Crohn's disease (CD) patients. However, most current studies use serological indicators at the time of their visit to predict their current endoscopic activity, while doctors focus more on whether the patient's condition has improved compared to the previous visit. This study aimed to establish a clinical prediction model for evaluating the changes in endoscopic activity during follow-up visits. The final results indicate that the combined changes in albumin and fibrinogen between two visits can effectively predict whether the endoscopic activity of patients with CD will improve during follow-up visits.
- Citation: Wang HG, Nima CL, Zhou Q. Development and validation of a predictive model for endoscopic improvement of Crohn's disease. World J Gastrointest Endosc 2025; 17(2): 100665
- URL: https://www.wjgnet.com/1948-5190/full/v17/i2/100665.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i2.100665
Crohn's disease (CD) is a chronic inflammatory disease that can affect any part of the digestive tract, from the mouth to the anus. It is known for being resistant to treatment, having a tendency to relapse, and presenting a prolonged course, which makes it a significant challenge in medical practice[1,2]. In recent decades, there has been a steady increase in the incidence of CD[3]. Frequent endoscopic evaluations are essential throughout the disease due to its relentless nature. However, this invasive procedure can be financially burdensome and emotionally distressing for patients. As a result, many patients experience apprehension and reluctance about undergoing the examination[4]. Additionally, endoscopies carry risks associated with anesthesia and may not be suitable for certain individuals.
Many studies have utilized non-invasive indicators, such as serum, urine, and fecal markers, as well as imaging techniques like ultrasound, computed tomography, magnetic resonance imaging. However, most of these studies focused on evaluating patients' endoscopic activity at specific time points rather than accurately tracking disease progression or regression over time. While imaging methods such as ultrasound can indicate whether a patient's condition is in remission or has worsened by comparing imaging features before and after treatment, they are often cumbersome, expensive, and associated with radiation exposure or hepatorenal toxicity. These factors limit their widespread use as primary monitoring tools.
Current research primarily relies on serological markers to assess disease activity in patients with CD. However, these markers are often inadequate for capturing the dynamic changes in a patient's condition. Most studies currently use serological indicators from the time of a patient's visit to predict their current endoscopic activity. However, clinicians focus on whether a patient's condition has improved since the previous visit to evaluate treatment effectiveness and make timely adjustments to the medication regimen. Despite this, there is a lack of studies investigating whether changes in relevant serological indicators correlate with improvements in patients' endoscopic activity. Therefore, further research in this area is urgently needed. Based on the published literature, we found that body mass index (BMI)[5-10], erythrocyte sedimentation rate (ESR)[11,12], hemoglobin (Hb)[13,14], C-reactive protein (CRP)[15,16], albumin (ALB)[17-22], ALB and globulin ratio (A/G)[23], and fibrinogen (FIB)[24,25] are not only closely related to the inflammation and nutritional status in CD, but also significantly related to its prognosis. Numerous studies have demonstrated that fluctuations in disease activity among patients with CD are closely linked to variations in serological indicators. For instance, the research by Reinisch et al[26] found that achieving early clinical remission and normalizing CRP levels are the strongest predictors of treatment efficacy, mucosal healing, and the need for increased doses of adalimumab within the first year of treatment for CD. In a study examining the treatment of CD with ustekinumab (UST), researchers discovered a negative correlation between endoscopic activity and ALB levels, with a correlation coefficient of -0.60 (P = 0.0007). This suggests that serum ALB levels may increase during endoscopic remission. Additionally, serum UST trough concentrations and ALB levels were significantly higher in the endoscopic response group compared to the non-endoscopic response group, measuring 3.3 μg/mL vs 1.8 μg/mL, which further supports this association[27]. In this study, we incorporated several factors (BMI, ESR, Hb, CRP, ALB, A/G, and FIB) that are associated with inflammation, nutritional status, and prognosis in patients with CD into a prediction model. The goal was to noninvasively assess changes in disease activity in these patients. This approach aims to assist clinicians in the treatment and management of CD, ultimately reducing the need for endoscopic examinations.
This prospective study enrolled patients who initially visited Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between January 2014 and December 2022. All patients were diagnosed with CD. A total of 170 patients were included in the training group through our hospital's electronic case system. These patients were then randomly allocated into a modeling group and an internal validation group in a ratio of 7:3. The modeling group was used to construct a nomogram model, while the internal validation group was used to further verify the model's accuracy. Additionally, patients hospitalized between January 2023 and March 2024 were selected as an external validation group. The study was reviewed and approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No. TJ-IRB202409069).
The inclusion criteria were: (1) Patients must be admitted to the hospital; (2) Age must be between 18 and 70 years; and (3) Patients must have undergone a complete endoscopic examination (colonoscopy, ileocolonoscopy, or capsule endoscopy) during both the initial and follow-up visits, and endoscopic evaluation only assessed the condition of the terminal ileum, colon, and rectum. The exclusion criteria included: (1) Patients with severe infections, rheumatic diseases, immune system disorders (such as rheumatoid arthritis, systemic lupus erythematosus, or ankylosing spondylitis), vascular embolism, coagulation disorders, malignant tumors, or other significant diseases; (2) Patients with significant functional impairments in vital organs, such as the liver or kidneys; (3) Individuals in special physiological states, including pregnancy or lactation; and (4) A history of intestinal resection surgery, intestinal fistulae, ileostomy, or disorders related to dietary intake.
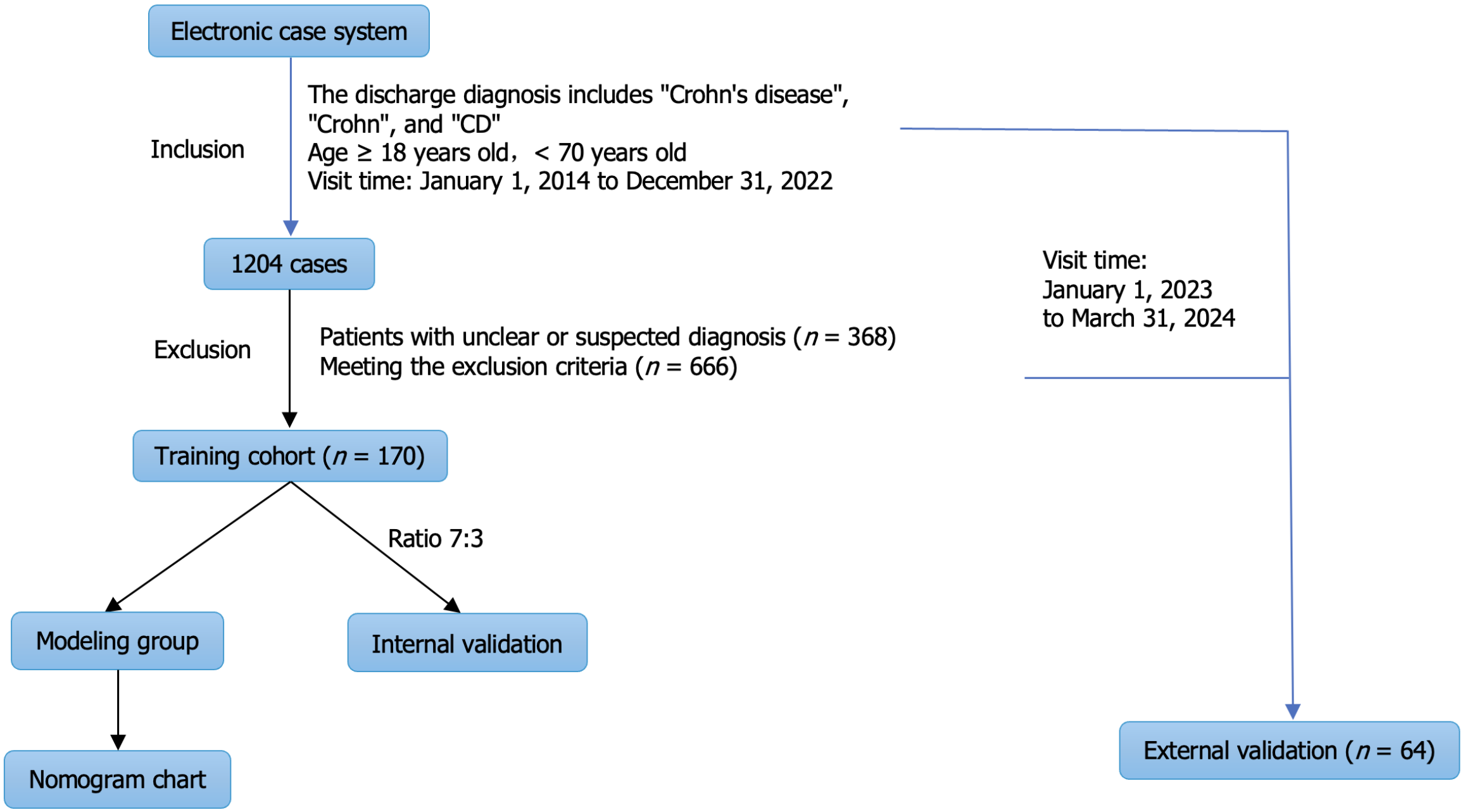
A total of 1204 patients were included in the electronic medical record system by searching for the keywords "Crohn's disease", "Crohn's", or "CD", while limiting the search by age and date of visit. Among these patients, 368 cases were confirmed to be non-CD through follow-up and were excluded from the study. The remaining 666 cases were also excluded for failing to meet the established criteria. Specifically, among these exclusions, 226 cases had only one hospitalization record, 173 did not undergo colonoscopy at the first visit, 52 did not undergo colonoscopy at the second visit, 82 had intestinal resection, 39 had enterostomy, 40 were complicated with severe infection, 22 were complicated with abnormal coagulation function, 15 were complicated with severe liver and kidney dysfunction, and 10 were complicated with rheumatism and immune system diseases. Seven cases were complicated with malignant tumors (Figure 1).
We collected a comprehensive set of general characteristics for each patient, which included gender, age, weight, BMI, smoking and drinking history, and any history of gastrointestinal surgery. Additionally, we recorded whether the patient was newly diagnosed with CD, the presence of perianal lesions at the time of initial diagnosis, and the endoscopic findings. We also gathered a range of laboratory indicators during both the initial and follow-up visits, including ESR, Hb, hematocrit, ALB, A/G, high sensitivity CRP (hsCRP), and FIB.
To ensure consistency and accuracy, all blood samples were collected from patients while they were on an empty stomach, either on the day of admission or the following morning. Hb, hematocrit, and ESR were analyzed using an automated analyzer. ALB, A/G, and hsCRP were accurately measured using the ROCHE COBAS 8000 system. Specifically, ALB concentrations were determined using the bromocresol green method, while total protein levels were assessed through the biuret method. A/G ratios were calculated by dividing ALB by the difference between total protein and ALB. For hsCRP measurements, the latex immunoturbidimetric method provided by Shushui Medical Technology Suzhou Co., Ltd. was employed. Finally, FIB levels were quantified using the STAGO magnetic bead coagulation (Clauss) method. Moreover, the changing amplitude in each laboratory indicator was calculated using the formula: [(Value at follow-up - value at initial diagnosis)/value at initial diagnosis] × 100%.
Endoscopic evaluations, including colonoscopy, enteroscopy, and capsule endoscopy, were conducted by highly skilled and experienced endoscopists. During the colonoscopy, careful attention was given to advancing the scope at least 20 centimeters into the terminal ileum. In cases where standard endoscopes faced challenges in navigating narrow passages, ultra-thin endoscopes were utilized as an alternative. If even the ultra-thin endoscopes were unable to pass successfully, the procedure was promptly terminated. The simplified endoscopic score for CD (SES-CD) evaluates five segments of the gastrointestinal tract: The terminal ileum, right colon, transverse colon, left colon, and rectum. It assesses four aspects: Ulcer size, ulcerated surface area, segment involvement, and intestinal stenosis. The scores for each segment are summed to produce a final score, which can range from 0 to 56.
Two senior endoscopists independently calculated the SES-CD score, and the final score was determined by taking their average. In cases where their scores significantly differed, a unified score was assigned after a thorough discussion. To minimize bias, the endoscopists remained unaware of other information, such as patient age, ESR, and hs-CRP levels. Endoscopic improvement was defined as a decrease of 50% or more in the SES-CD score compared to the previous visit, or an SES-CD score of 2 or less, with no observed ulcers (including oral ulcers) during follow-up visits. In contrast, endoscopic non-improvement was defined as a decrease of less than 50% in the SES-CD score compared to the previous visit during follow-up.
We conducted data analyses using SPSS 27.0 and R software packages. For variables with a normal distribution, we report the mean ± SD and performed t-tests. For non-normally distributed variables, we present the median along with the interquartile range (IQR) and analyzed them using Mann-Whitney U tests. Categorical variables are expressed as percentages and were examined using either χ2 tests or Fisher's exact tests. To identify risk factors, we performed binary logistic regression analysis, incorporating significant variables into a model using the “rms” package in R, which allowed us to create a nomogram chart. We assessed model accuracy with receiver operating characteristic (ROC) curves, evaluated the consistency between the predicted results of the model and the actual observed results using calibration curves, and evaluated the application value of the model in practical clinical decision-making using decision curve analysis (DCA). Any missing data points were excluded from the analysis, and we set the significance level at 0.05.
A total of 170 patients were included in the study, including 87 with endoscopic improvement and 83 without. At the time of initial diagnosis, 30 patients underwent small intestine endoscopy, 2 underwent capsule endoscopy, and 138 underwent colonoscopy. During the follow-up period, 30 patients had small intestine endoscopy, none had capsule endoscopy, and 40 underwent colonoscopy. We defined CD activity index (CDAI) < 150 points or a decrease of ≥ 70 points from baseline as clinical improvement, and found a significant difference between the two (P = 0.009). Almost all patients (97.7%) with endoscopic improvement showed clinical improvement (Table 1). The median age of the patients was 28 (IQR: 21-33) years old, the median BMI was 19.15 (IQR: 17.68-21.15) kg/m², the median disease duration was 6 (IQR: 2-24) months, and the median interval time was 6 (IQR: 4-10) months. Significant differences between the two groups were observed in age, interval time, and the changes in ESR, Hb, ALB, A/G, CRP, and FIB (P < 0.05). However, no significant differences were found regarding gender or disease course (P > 0.05). Due to the subjective nature of the clinical improvement, this study did not include it in the binary logistic regression analysis. Binary logistic stepwise regression analysis indicated that the changing amplitudes of ALB and FIB were independent risk factors for endoscopic improvement in patients with CD (Table 2).
| Item | Endoscopic improvement | P value | |
| Yes (n = 87) | No (n = 83) | ||
| Sex | 0.199 | ||
| Man | 72 | 62 | |
| Woman | 15 | 21 | |
| Age (years) | 26 (20-32) | 29 (23-34) | 0.042 |
| BMI (kg/m2) | 19.03 (17.57-20.98) | 19.31 (17.92-21.26) | 0.843 |
| Disease course (months) | 6 (2-12) | 11 (3-24) | 0.051 |
| Interval (months) | 5 (4-8) | 7 (4-13) | 0.029 |
| Clinical improvement | 0.0091 | ||
| Yes | 85 | 72 | |
| No | 2 | 11 | |
| First diagnosis | 0.384 | ||
| Yes | 63 | 55 | |
| No | 24 | 28 | |
| Smoking | 0.5361 | ||
| Yes | 7 | 4 | |
| No | 80 | 79 | |
| Drinking | 0.4881 | ||
| Yes | 0 | 1 | |
| No | 87 | 82 | |
| History of gastrointestinal surgery | 0.162 | ||
| Yes | 41 | 48 | |
| No | 46 | 35 | |
| Perianal lesions | 0.126 | ||
| Yes | 51 | 36 | |
| No | 27 | 32 | |
| Age (A; years) | 0.027 | ||
| < 17 (A1) | 0 | 0 | |
| 17-40 (A2) | 81 | 68 | |
| > 40 (A3) | 6 | 15 | |
| CD location (L) | 0.839 | ||
| Terminal ileum (L1) | 8 | 9 | |
| Colon (L2) | 27 | 28 | |
| Ileocolon (L3) | 52 | 46 | |
| CD behavior (B) | 0.4111 | ||
| Inflammatory (B1) | 69 | 60 | |
| Structuring (B2) | 17 | 20 | |
| Penetrating (B3) | 1 | 3 | |
| Medication | 0.0941 | ||
| Without medication | 8 | 2 | |
| 5-ASA | 10 | 19 | |
| Corticosteroids | 3 | 5 | |
| Immunosuppressants | 5 | 6 | |
| Enteral nutrition | 3 | 3 | |
| Biologics | 71 | 50 | |
| Others | 2 | 3 | |
| Changing amplitude of indicators (%) | |||
| BMI | 2.86 (-2.38-15.04) | 0.88 (-2.44-6.09) | 0.173 |
| ESR | -80.00 (-90.00 to -50.00) | -33.33 (-66.67-16.67) | < 0.001 |
| Hb | 9.57 (1.24-23.75) | 3.08 (-2.72-10.69) | 0.002 |
| A/G | 31.45 (15.03-55.77) | 5.15 (-8.22-21.55) | < 0.001 |
| ALB | 11.49 (4.69-26.63) | 1.29 (-4.50-10.30) | < 0.001 |
| CRP | -93.54 (-98.59 to -52.73) | -49.65 (-85.56-13.37) | < 0.001 |
| FIB | -36.24 ± 22.95 | -8.98 ± 31.12 | < 0.001 |
| B | SE | Wald χ2 | P value | OR | 95%CI | |
| Changing amplitude of ALB | 4.522 | 1.372 | 10.856 | < 0.001 | 92.009 | 6.246-1355.351 |
| Changing amplitude of FIB | -3.938 | 0.866 | 20.689 | < 0.001 | 0.019 | 0.004-0.106 |
| Constant | -1.407 | 0.323 | 18.964 | < 0.001 | 0.245 |
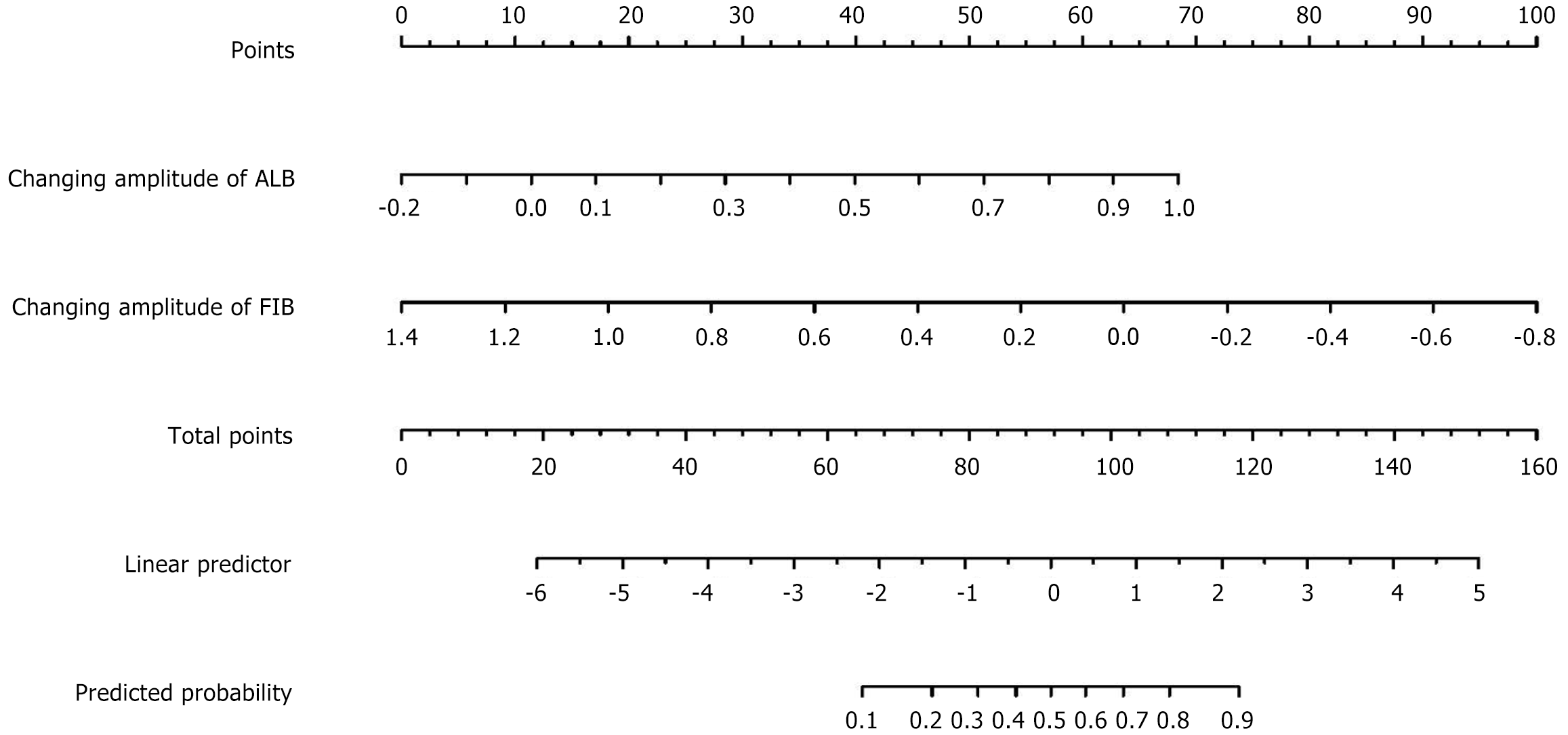
To develop a predictive model for endoscopic improvement in patients with CD, we randomly divided the training group into a modeling group and an internal validation group in a 7:3 ratio. We incorporated independent risk factors identified through binary logistic regression analysis into the model and created a nomogram to visualize the impact of each variable. This nomogram assigns scores that sum to a total score, reflecting the probability of endoscopic improvement. This tool assists clinicians in assessing and predicting endoscopic outcomes for CD patients (Figure 2).
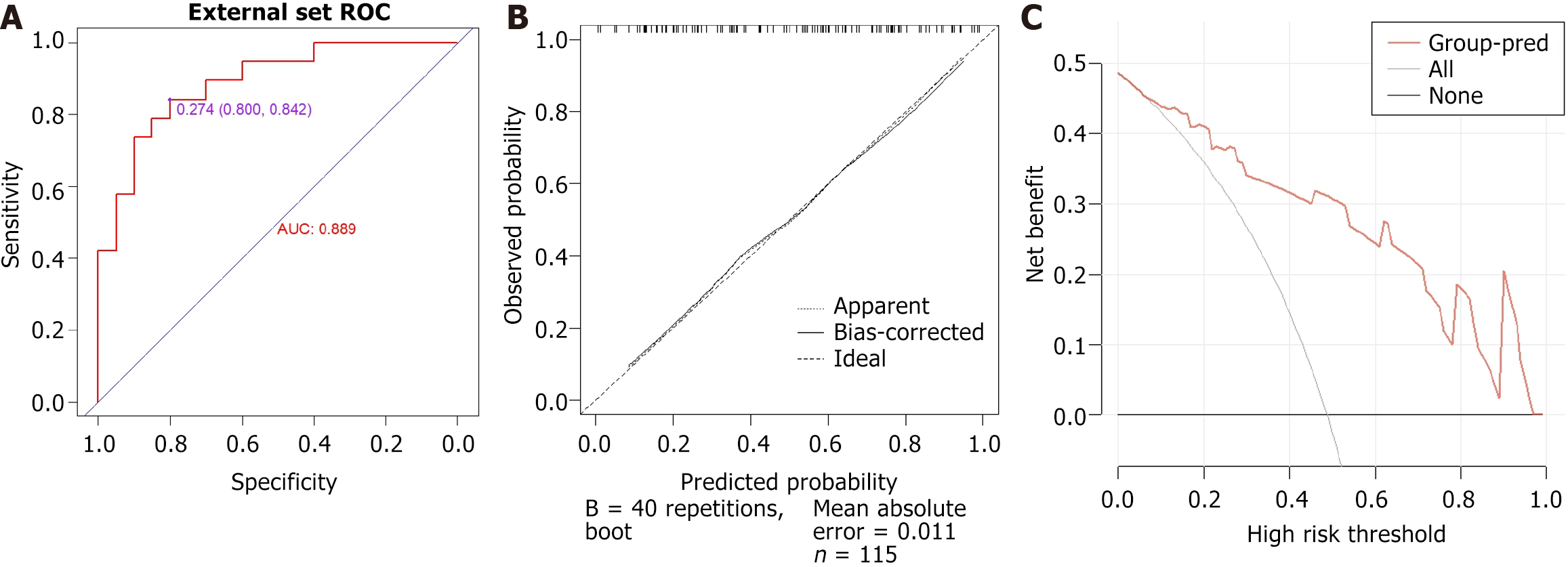
The ROC curve for the training group, which predicts endoscopic improvement during follow-up visits, is illustrated in Figure 3. The curve has an area under the curve (AUC) of 0.802 [95% confidence interval (CI): 0.736-0.869], with an optimal cutoff point established at 0.484. At this threshold, the sensitivity and specificity were 75.6% and 72.8%, respectively. Furthermore, a calibration curve was created, demonstrating that the deviation correction line closely aligns with the ideal line. The average absolute error of the calibration curve is as low as 0.011 (Figure 3).
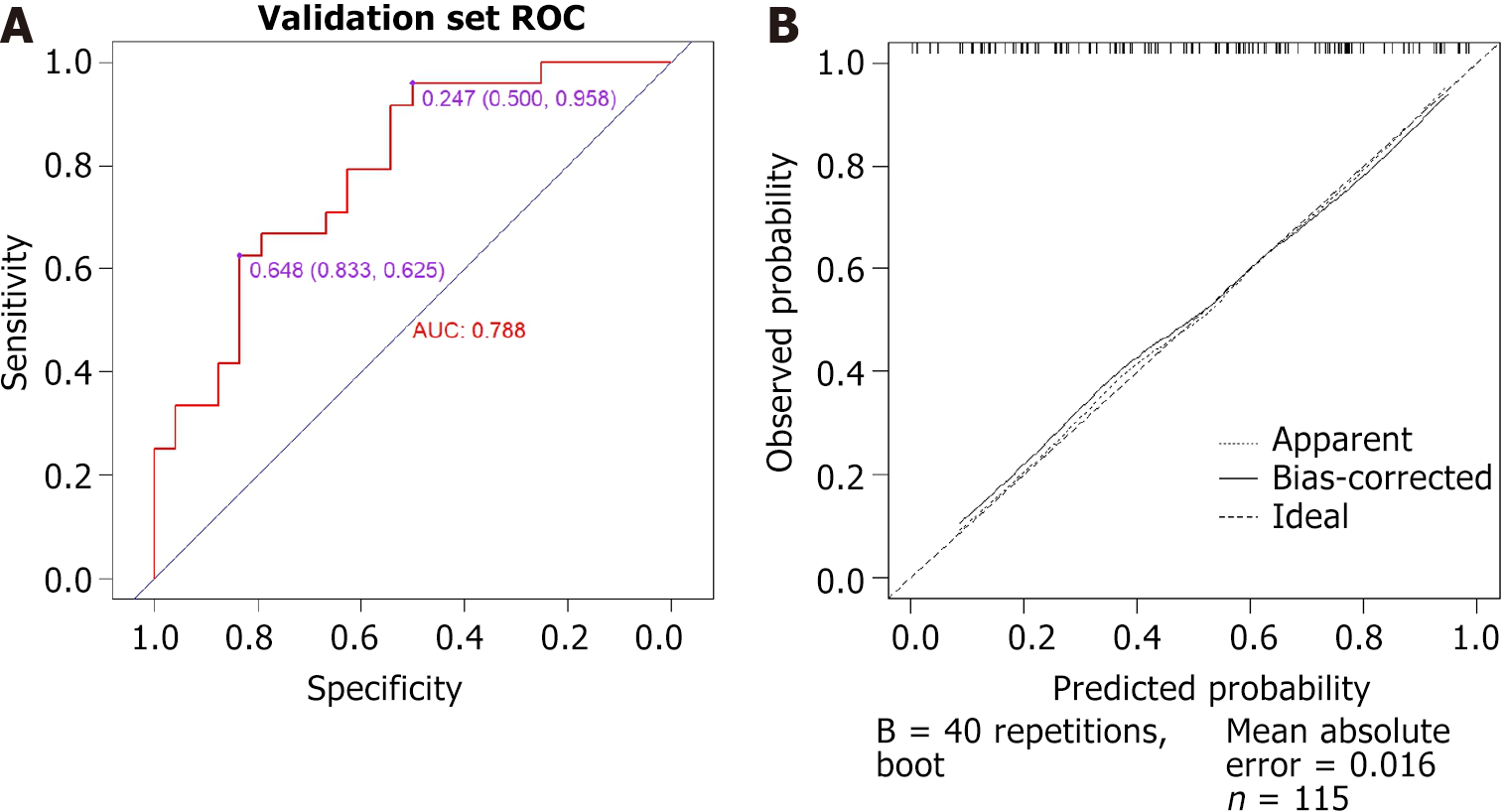
The ROC curve for predicting endoscopic improvement during follow-up visits in the internal validation group is presented in Figure 4, with an AUC of 0.788 (95%CI: 0.660-0.916). There are two optimal cutoffs identified: The first is 0.648, which corresponds to a sensitivity of 62.5% and specificity of 83.3%. The second cutoff is 0.247, associated with a sensitivity of 95.8% and specificity of 50.0%. Additionally, the calibration curve indicates that the deviation correction line for the internal validation group closely aligns with the ideal line, demonstrating a high level of accuracy. The average absolute error of the calibration curve is 0.016 (Figure 4).
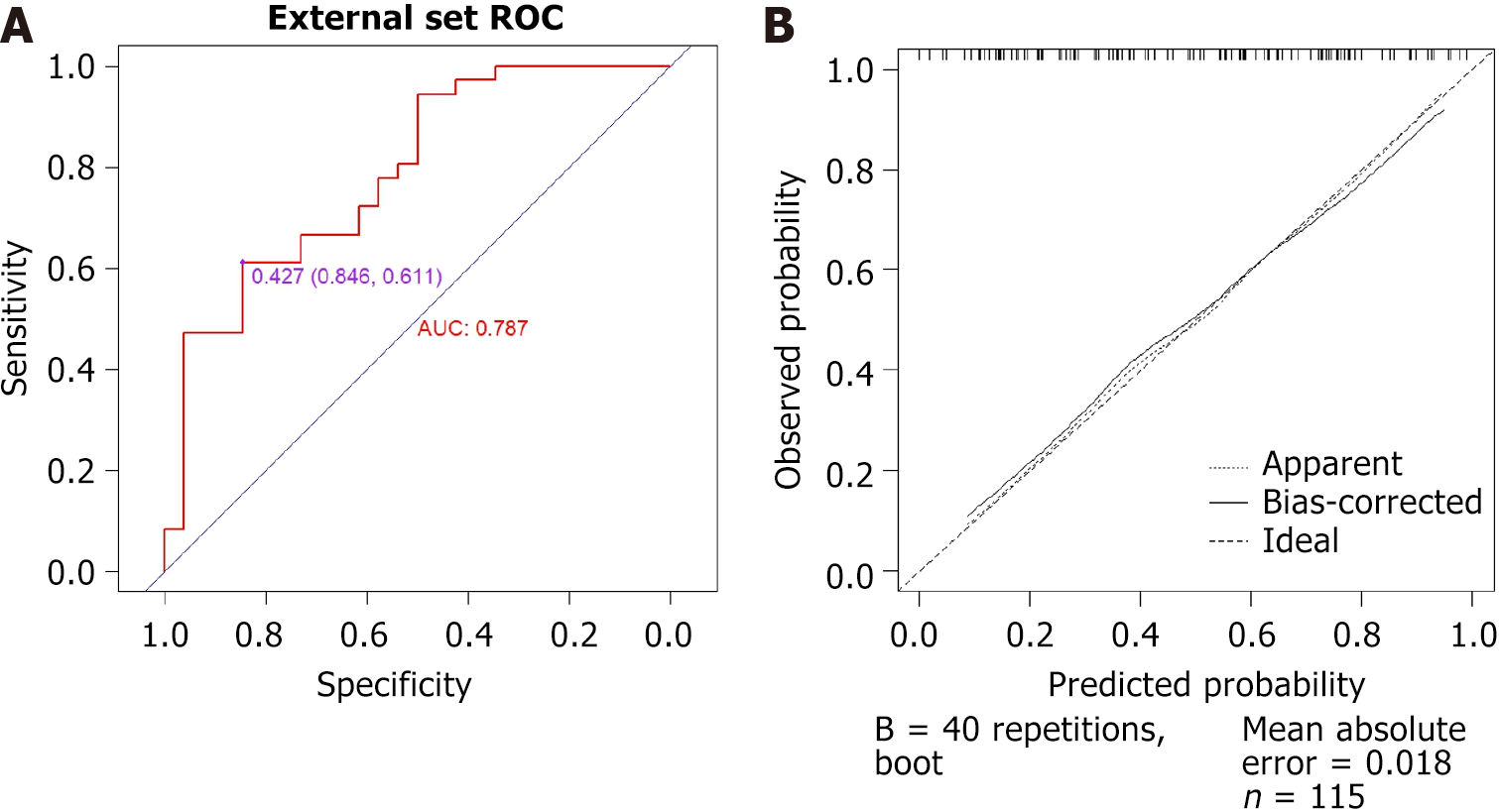
Additionally, patients who visited between January 2023 and March 2024 were included as an external validation group. A total of 64 cases were considered (7 cases were excluded due to difficulties in endoscopy passing through intestinal stenosis, which resulted in missing SES-CD scores). This group included 53 males and 11 females. There were no statistically significant differences in gender, age, or interval time between the training and external validation groups (P > 0.05). The ROC curve for external validation, which aimed to predict endoscopic improvement during follow-up visits, yielded an AUC of 0.787 (95%CI: 0.672-0.903) with an optimal cutoff value of 0.427. The corresponding sensitivity and specificity were 61.1% and 84.6%, respectively. Furthermore, a calibration curve was drawn, demonstrating that the deviation correction line of the training group closely overlaps with the ideal line, and the average absolute error for the calibration curve was 0.018 (Figure 5).
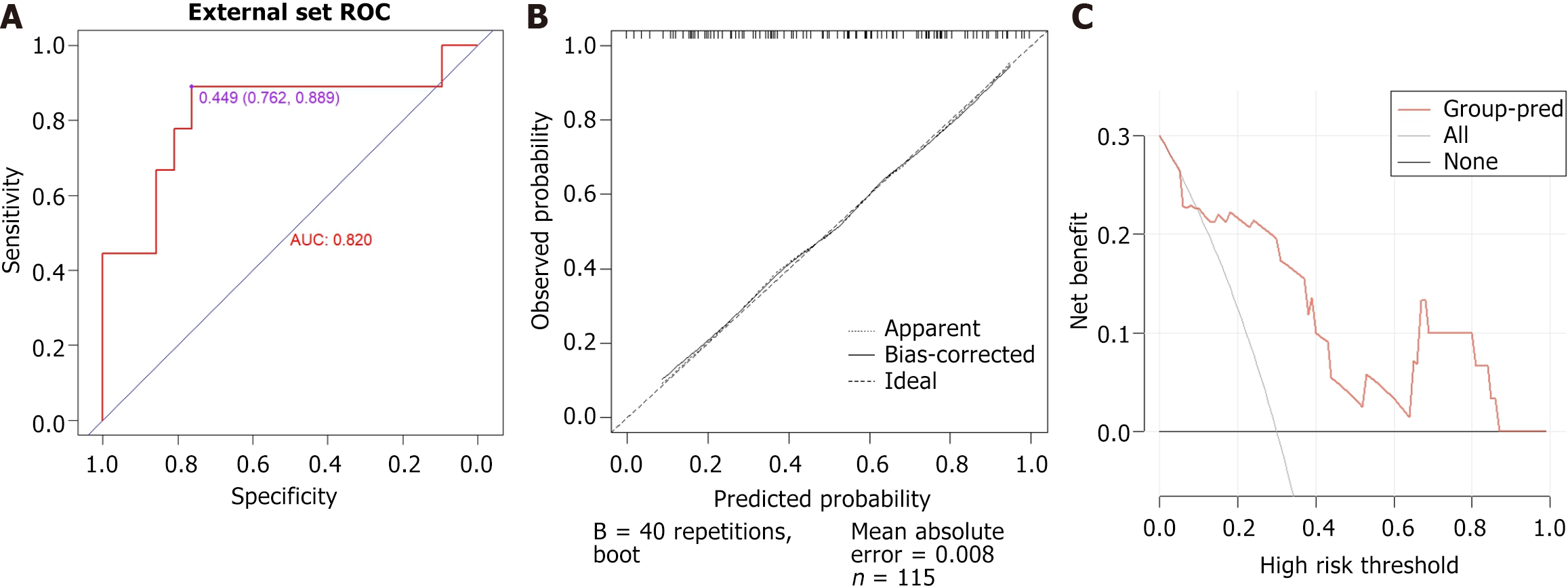
The DCA curves for the training, internal validation, and external validation groups were plotted based on the nomogram model (Figure 6). The DCA curve demonstrates the potential clinical net benefits at various risk thresholds, which are calculated using the model's sensitivity and specificity. Intervening in patients whose assessed risks fall within the threshold range provides greater net benefits than either intervening in all patients or not intervening at all. DCA shows that, at an appropriate risk threshold, the model offers a higher clinical benefit in predicting improvements in endoscopic activity compared to previous visits for patients with CD (Figure 6).
Table 1 shows a significant difference in the interval time between the two groups in the training group (P = 0.029). To further validate the applicability and robustness of the nomogram model, we divided the training group into three groups for sensitivity analysis based on interval time: Those within six months, those from six months to one year, and those exceeding one year.
When the interval time was within 6 months, 92 patients were included, with 56 from the endoscopic improvement group and 40 from the endoscopic non-improvement group. The AUC is 0.754 (95%CI: 0.656-0.852) and the average absolute error of the calibration curve is 0.018 (Figure 7).
Forty-two patients were revisited within six months to one year, including 20 from the endoscopic improvement group and 22 from the endoscopic non-improvement group. The AUC is 0.889 (95%CI: 0.789-0.990) and the average absolute error of the calibration curve is 0.011 (Figure 8).
Thirty-two patients revisited for more than one year, including 11 cases in the endoscopic improvement group and 21 cases in the endoscopic non-improvement group. The AUC is 0.820 (95%CI: 0.6187-1) and the average absolute error for the calibration curve is 0.008 (Figure 9).
Serological indicators, including platelet-related parameters[28], platelet-to-lymphocyte ratio[29], and neutrophil-to-lymphocyte ratio[30], play a crucial role in evaluating inflammation and dynamics of CD. However, frequent endoscopic examinations can be inconvenient for the management of CD patients. To address this issue, we proposed utilizing changes in specific serological markers as a predictive tool for assessing endoscopic activity in CD patients. This approach could enable non-invasive monitoring of disease progression, thereby improving patient comfort and facilitating clinical decision-making.
In this study, we predicted endoscopic improvement in patients with CD by analyzing fluctuations in serological indicators between visits. We utilized the SES-CD, which is a practical and straightforward scoring system[31]. According to the guidelines set by the International IBD Research Organization, we defined endoscopic response as either a reduction of 50% or more in the SES-CD score from the baseline or an SES-CD score of 2 or less[32]. Based on these criteria, patients who experienced a decline of 50% or more in their SES-CD score or had an SES-CD score of 2 or less during follow-up were classified as having achieved endoscopic improvement. Those who did not meet these benchmarks were categorized as non-improvement cases.
The study aimed to develop a non-invasive predictive model for endoscopic improvement in CD patients using changes in serological markers. Our research demonstrated that the changing amplitudes in ALB and FIB are independent predictors of endoscopic improvement in CD. These biomarkers, which are closely related to CD inflammation[24,33], changed by endoscopic improvement. The combined assessment of the changing amplitudes in ALB and FIB accurately predicted endoscopic improvement, as validated by both internal and external evaluations. The DCA confirmed the model's clinical relevance. However, further large-scale prospective studies are needed to refine the predictive model and optimize the management strategies for patients with CD.
Our study excluded subjective indicators, such as patients' clinical symptoms and CDAI scores, to mitigate biases and enhance practicality, accuracy, and clinical applicability. The model's predictive capabilities were rigorously validated through prospective assessment, demonstrating its reliability and effectiveness in real-world scenarios. However, limitations include a single-center retrospective design, a modest sample size, and potential selection bias. Future multi-center, large-scale trials are necessary to validate the model's reliability. Furthermore, the model's applicability in children, pregnant women, patients with intestinal resection, and those with severe infections is still uncertain and requires further investigation.
In summary, our findings underscore the significant potential of ALB and FIB changes between two visits to predict endoscopic improvement in CD patients accurately. This novel approach holds promise in guiding clinical decision-making and minimizing the need for invasive endoscopic procedures, particularly for patients with endoscopic contraindications.
The authors thank all members of the Department of Gastroenterology for assistance in various aspects of this work.
| 1. | Fow J, Grossman S. A comprehensive guide to patient-focused management strategies for Crohn disease. Gastroenterol Nurs. 2007;30:93-8; quiz 98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Roda G, Chien Ng S, Kotze PG, Argollo M, Panaccione R, Spinelli A, Kaser A, Peyrin-Biroulet L, Danese S. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 593] [Article Influence: 118.6] [Reference Citation Analysis (0)] |
| 3. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1853] [Article Influence: 185.3] [Reference Citation Analysis (1)] |
| 4. | Marchi M, Alboni S, Fabbrizi A, Feltri L, Galli G, Guicciardi A, Mancini S, Mattei G, Minarini A, Perrone D, Rioli G, Roncucci L, Sena P, Ferrari S. Prevalence of metabolic syndrome and of symptoms of anxiety and depression in patients undergoing colonoscopy. Eur psychiatr. 2017;41:S318-S319. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Mendall M, Harpsøe MC, Kumar D, Andersson M, Jess T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish National Birth Cohort. PLoS One. 2018;13:e0190600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Marino M, Picci A, Terrosu G, Macchini F, Vadala' di Prampero SF, Bulajic MM, Zilli M. Tu1968 Body Mass Index As New Risk Factor for Postoperative Endoscopic Recurrence in Crohn's Disease? Gastroenterol. 2016;150:S993. [DOI] [Full Text] |
| 8. | Brown P, Clark T, Dowson G, Warren L, Hamlin J, Hull M, Subramanian V. Relationship of Body Mass Index to Clinical Outcomes after Infliximab Therapy in Patients with Crohn's Disease. J Crohns Colitis. 2016;10:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Darwin L, Wall C, Su H, Gearry RB. P365 Weight gain after initiation of adalimumab in patients with Crohn’s disease. J Crohns Colitis. 2022;16 Suppl 1:i371. [DOI] [Full Text] |
| 10. | İnanç N, Fırat YY, Başmısırlı E, Çapar AG. Nutrient Intake of Crohn's Patients: Is There Consistency between Crohn's Disease Activity Index, Subjective Global Assessment and Body Mass Index? Iran J Public Health. 2021;50:2584-2592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817-1826.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 12. | Brignola C, Campieri M, Bazzocchi G, Farruggia P, Tragnone A, Lanfranchi GA. A laboratory index for predicting relapse in asymptomatic patients with Crohn's disease. Gastroenterology. 1986;91:1490-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Rieder F, Paul G, Schnoy E, Schleder S, Wolf A, Kamm F, Dirmeier A, Strauch U, Obermeier F, Lopez R, Achkar JP, Rogler G, Klebl F. Hemoglobin and hematocrit levels in the prediction of complicated Crohn's disease behavior--a cohort study. PLoS One. 2014;9:e104706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Magro F, Estevinho MM, Catalano G, Patita M, Arroja B, Lago P, Rosa I, Tavares-de-sousa H, Ministro P, Roseira J, Cancela E, Sousa P, Portela F, Correia L, Moreira P, Santiago M, Dias S, Afonso J, Danese S, Peyrin-biroulet L, Dias CC. P734 A single measurement of fecal calprotectin, particularly if combined with hemoglobin and C-reactive protein levels, predicts Crohn’s disease prognosis - a prospective study. J Crohns Colitis. 2023;17 Suppl 1:i863-i863. [DOI] [Full Text] |
| 15. | Penna FGC, Rosa RM, Pereira FH, Cunha PFS, Sousa SCS, Ferrari TCA, Cara C, Ferrari MLA. Combined evaluation of fecal calprotectin and C-reactive protein as a therapeutic target in the management of patients with Crohn's disease. Gastroenterol Hepatol. 2021;44:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Słowińska-Solnica K, Pawlica-Gosiewska D, Gawlik K, Owczarek D, Cibor D, Pocztar H, Mach T, Solnica B. Serum inflammatory markers in the diagnosis and assessment of Crohn's disease activity. Arch Med Sci. 2021;17:252-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Zhao H, Liu H, Qi W, Liu W, Ye L, Cao Q, Ge X, Zhou W, Wang X. Postoperative Ratio of C-Reactive Protein to Albumin as a Predictive Marker in Patients with Crohn's Disease Undergoing Bowel Resection. Gastroenterol Res Pract. 2021;2021:6629608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zhou FS, Gao N, Sun X, Jiang XY, Chen JJ, Mao QQ, Zhong L. C-reactive protein/abumin ratio is a useful biomarker for predicting the mucosal healing in the Crohn disease: A retrospective study. Medicine (Baltimore). 2021;100:e24925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Qin G, Tu J, Liu L, Luo L, Wu J, Tao L, Zhang C, Geng X, Chen X, Ai X, Shen B, Pan W. Serum Albumin and C-Reactive Protein/Albumin Ratio Are Useful Biomarkers of Crohn's Disease Activity. Med Sci Monit. 2016;22:4393-4400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Shivashankar R, Aberra F, Mendoza Ladd AH, Lichtenstein GR. Effect of Serum Albumin Levels on Efficacy of Vedolizumab in Patients with Crohn's Disease. Gastroenterol. 2017;152:S407-S408. [DOI] [Full Text] |
| 21. | Irwin J, Lord A, Ferguson E, Simms LA, Hanigan K, Montoya CA, Radford-Smith G. A Method Using Longitudinal Laboratory Data to Predict Future Intestinal Complication in Patients with Crohn's Disease. Dig Dis Sci. 2023;68:596-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Shiga H, Abe I, Onodera M, Moroi R, Kuroha M, Kanazawa Y, Kakuta Y, Endo K, Kinouchi Y, Masamune A. Serum C-reactive protein and albumin are useful biomarkers for tight control management of Crohn's disease in Japan. Sci Rep. 2020;10:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Li C, Wang W, Wang J, Li J, Qian S, Cai C, Liu Y. Serum Albumin to Globulin Ratio is Associated with the Presence and Severity of Inflammatory Bowel Disease. J Inflamm Res. 2022;15:1907-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Chen XF, Zhao Y, Guo Y, Huang ZM, Huang XL. Predictive value of fibrinogen in identifying inflammatory bowel disease in active stage. BMC Gastroenterol. 2021;21:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Peng Z, Xu D, Li Y, Liu X, Li F, Peng Y. A novel role of prognostic nutritional index in predicting the effectiveness of infliximab in Crohn's disease. Ann Med. 2023;55:2236011. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Hirayama H, Morita Y, Imai T, Takahashi K, Yoshida A, Bamba S, Inatomi O, Andoh A. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn's disease. BMC Gastroenterol. 2022;22:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Padysz M, Banasik J, Gąsiorowska A. P305 Platelet parameters evaluation as a non-invasive marker of inflammation in Crohn’s disease. J Crohns Colitis. 2019;13:S252-S253. [DOI] [Full Text] |
| 29. | Ben Jeddi H, Kchir H, Hassine A, Issaoui D, Chaabouni H, Maamouri N. P215 The mean platelet volume compared with other serum biomarkers: is it predictive of activity of Crohn’s disease? J Crohns Colitis. 2019;13 Suppl 1:S203-S203. [DOI] [Full Text] |
| 30. | Feng JR, Qiu X, Wang F, Chen PF, Gao Q, Peng YN, Lin X, Liu Q, Liu J, Zhao Q, Li J. Diagnostic Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Crohn's Disease. Gastroenterol Res Pract. 2017;2017:3526460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A, Mary JY, Colombel JF, Rutgeerts P. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1309] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 32. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1399] [Article Influence: 139.9] [Reference Citation Analysis (115)] |
| 33. | Cabral VL, de Carvalho L, Miszputen SJ. [Importance of serum albumin values in nutritional assessment and inflammatory activity in patients with Crohn's disease]. Arq Gastroenterol. 2001;38:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |