Published online Jun 16, 2024. doi: 10.4253/wjge.v16.i6.282
Revised: March 13, 2024
Accepted: May 6, 2024
Published online: June 16, 2024
Processing time: 134 Days and 8.9 Hours
The escalating prevalence of gastrointestinal cancers underscores the urgency for transformative approaches. Current treatment costs amount to billions of dollars annually, combined with the risks and comorbidities associated with invasive surgery. This highlights the importance of less invasive alternatives with organ preservation being a central aspect of the treatment paradigm. The current standard of care typically involves neoadjuvant systemic therapy followed by surgical resection. There is a growing interest in organ preservation approaches by way of minimizing extensive surgical resections. Endoscopic ablation has proven to be useful in precursor lesions, as well as in palliative cases of unrese
Core Tip: Endoscopic ablation of precancerous lesions is widely accepted in the luminal gastrointestinal tract. In unresectable gastrointestinal malignancy, the palliative role of endoscopic ablation is growing. Recently, the prospect of endoscopic ablation is expanding to include downstaging previously unresectable disease, as well as contributing to non-surgical complete clinical response as an adjunct therapy. The prospect for synergy in improving overall survival while balancing the quest for organ and function preservation warrants methodical investigations.
- Citation: Soliman YY, Soliman M, Reddy S, Lin J, Kachaamy T. Organ and function preservation in gastrointestinal cancer: Current and future perspectives on endoscopic ablation. World J Gastrointest Endosc 2024; 16(6): 282-291
- URL: https://www.wjgnet.com/1948-5190/full/v16/i6/282.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i6.282
Gastrointestinal cancers are a diverse group of malignancies making up over one-quarter of all cancer cases, and arise from the esophagus, stomach, liver, biliary tract, pancreas, small intestine, colon, and rectum[1]. Collectively, these cancers are a major global health burden due to their significant morbidity, mortality, and cost burden.
The National Comprehensive Cancer Center (NCCN) is a not-for-profit alliance of leading cancer centers in the United States with the vision of defining and advancing high-quality, high-value, patient-centered cancer care. NCCN clinical practice guidelines and recommendations are commonly referenced in cancer care. Surgical resection is generally recommended for most locoregional gastrointestinal cancers without targetable mutations as first-line of treatment. In cases of borderline resectable and/or locally advanced disease, neoadjuvant treatment is considered[2-12]. This approach has been more promising in esophagogastric and colorectal cancers[13-16] than in pancreaticobiliary cancers[17-20]. This difference is at least in part due to the lack of unanimous commensurate improvement in overall survival with the improvement in complete histopathologic resection rates in pancreaticobiliary cancers. Furthermore, post-operative complications are significant considerations even when curative resections are achieved[21,22].
There has been growing interest in organ preservation approaches. Total neoadjuvant therapy (TNT) followed by a “watch and wait” strategy has been increasingly studied. The esophagus and the rectum tend to be major areas of interest. For example, the CROSS trial reported no viable tumor cells in 23% of patients with adenocarcinoma and 49% of squamous cell carcinoma of the esophagus who received neoadjuvant chemoradiotherapy followed by esophagectomy[23]. The surgery as needed for oesophageal cancer (SANO) trial reported non-inferiority in overall survival (OS) and improved short-term health-related quality of life of neoadjuvant chemoradiation with active surveillance and surgery as needed vs standard surgery[24]. In rectal cancer, the OPRA trial found that organ preservation was possible in half of the patients who underwent TNT[25]. In the other half with disease recurrence who ultimately needed to undergo surgery, there was no statistically significant difference in disease-free survival post-operatively. International consensus groups establish a framework for studying and standardizing the topic[26]. Other systemic treatment strategies for complete clinical response continue to be proposed and studied[27]. Therefore, organ and function preservation approaches encompass minimally invasive systemic therapy, radiation therapy, minimally invasive surgeries, and endoscopic resection of accessible tumors < T1b[2,5,6,12].
In cases where complete endoscopic or minimally invasive resection is not feasible, endoscopic ablation can have a role in disease mitigation. This is particularly in palliation of advanced disease by debulking an obstructive mass that is refractory to systemic and locoregional therapy. Endoscopic ablation adds to the growing repertoire of palliative endoscopic modalities[28]. Therefore, in addition to treating pre-malignant and early-stage lesions, endoscopic ablation has the potential for palliation as well as integration in organ preservation and down-staging disease strategies. We review endoscopic ablation modalities that have been considered in the continuum of gastrointestinal cancers.
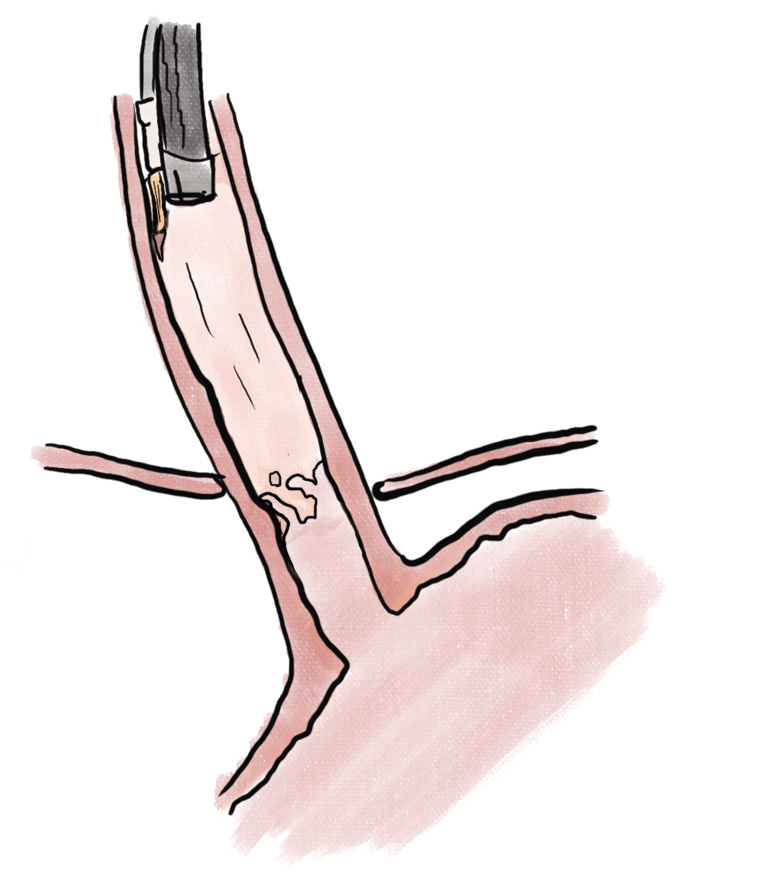
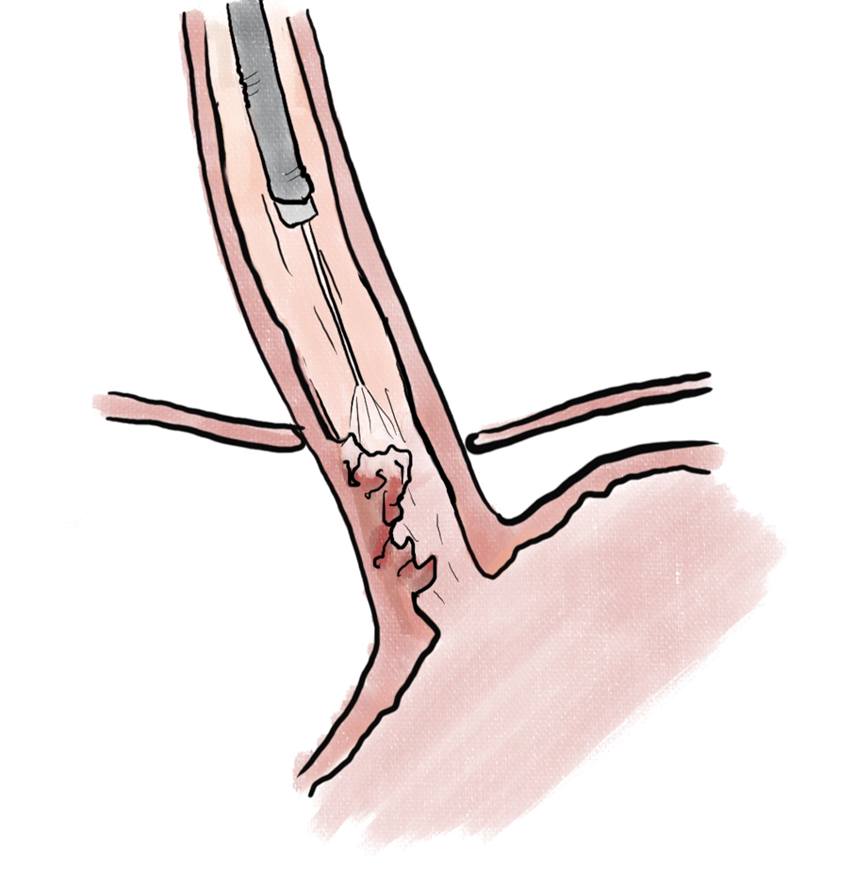
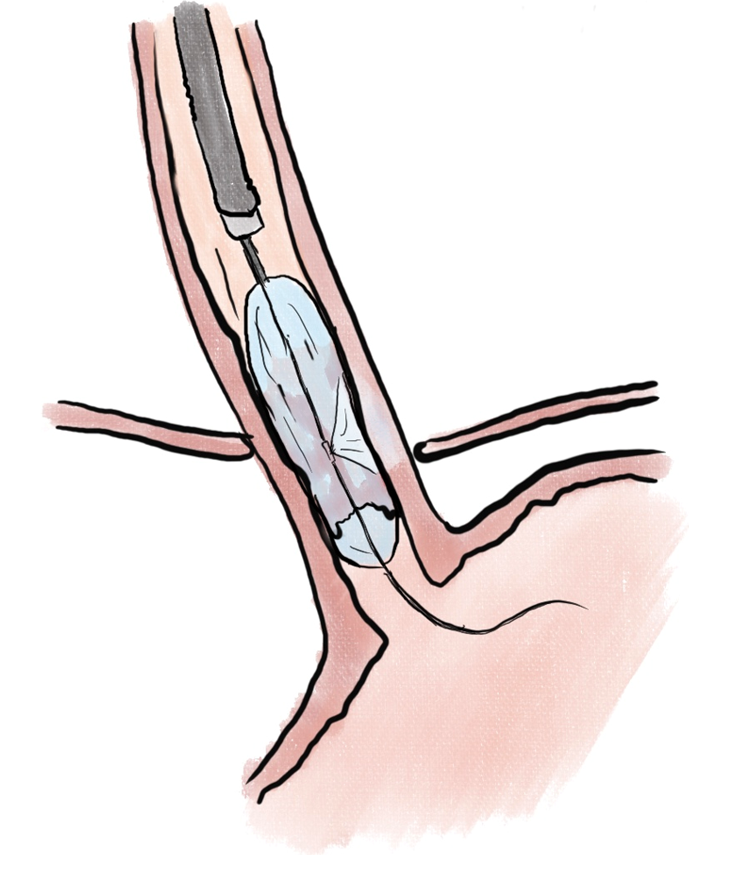
Endoscopic ablation of dysplastic Barrett esophagus with radiofrequency ablation (RFA) (Figure 1) has been shown to have high rate of complete eradication of dysplasia and intestinal metaplasia, as well as a reduced risk of disease progression to adenocarcinoma[29]. Liquid nitrogen spray cryotherapy (LNSC) (Figure 2) and balloon cryotherapy (BC) (Figure 3) have been shown to eradicate dysplasia at rates of approximately 80%[30]. In patients with intramucosal adenocarcinoma related to Barrett, both RFA and LNSC were found to be effective[31]. In early esophageal squamous cell neoplasia, RFA and BC have been found to be safe and effective with repeat treatments as necessary until eradication of the neoplasia[32-34]. On the palliative front, in a prospective multi-center study of 55 patients with inoperable esophageal cancer, LNSC has been found to improve dysphagia and quality of life after a mean of 3.2 treatments[35].
Moreover, a pilot study of a single session of neoadjuvant LNSC in 21 patients reported improvement in dysphagia in 71% of patients. Among 9 patients with locally advanced cancer who completed chemoradiation, 6 had negative mucosal biopsies, with 5 having clinical complete response[36]. These studies on cryotherapy had low serious adverse events and are promising. The potential of synergy of endoscopic ablation with systemic treatment warrants further studies.
Gastric dysplasia has been shown to be readily eradicated with endoscopic RFA in no more than 3 sessions 8 wk apart[37]. Argon Plasma Coagulation (APC) has also been shown to be comparable to RFA for eradicating low-grade intraepithelial neoplasia, though RFA was touted to be more appropriate in larger lesions that are flat, whereas APC was favored in smaller and more protrusive lesions[38]. Endoscopic laser therapy has also been successfully used in eradicating intramucosal gastric cancer, with up to 75% of patients found to have no residual tumor on laparotomy[39]. In residual gastric tumors following endoscopic resection, APC achieved curative ablation in 73.2% after a single session, and up to 86.6% following an extra session of APC[40].
In subepithelial gastrointestinal tumors (GIST), case reports of endosonographic fine needle injections (FNI) for ablation with ethanol[41] and RFA[42] have been successful in cases where patients either rejected or had prohibitive comorbidities to surgical resection. In this class of tumors, appropriately selected neoadjuvant and/or adjuvant tyrosine kinase inhibitors tend to have a favorable effect in disease control as well[15].
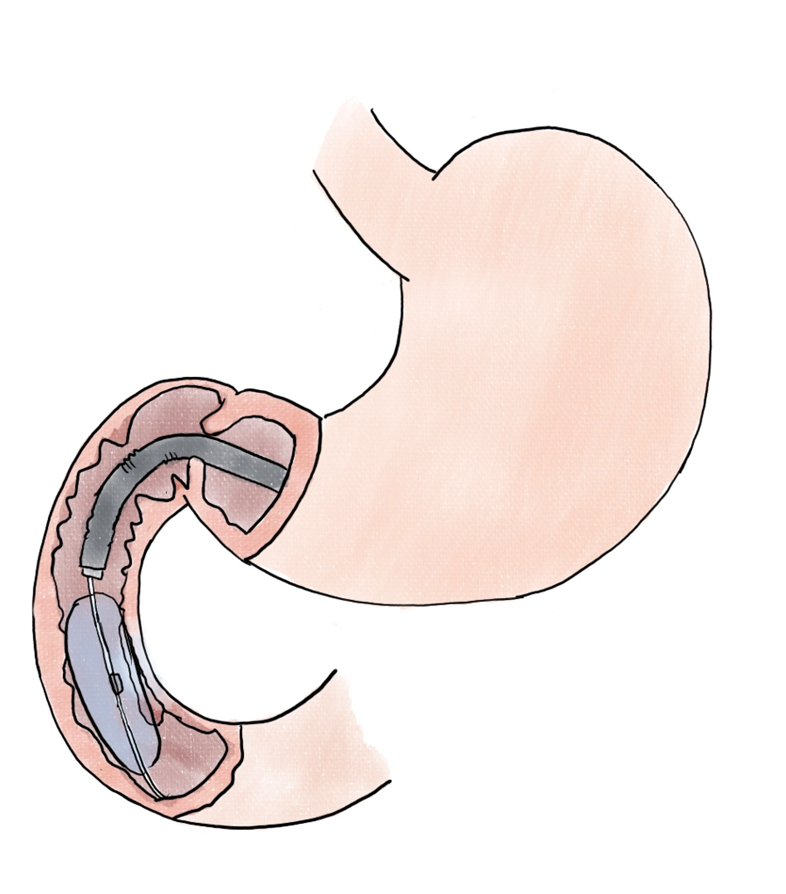
Whereas endoscopic resection is the mainstay in mucosal disease, the complexity of duodenal anatomy and endoscopic positioning may necessitate adjunct modalities. In non-ampullary adenomas, endoscopic cryoballoon ablation (Figure 4) was successful in 12 of 17 (71%) of cases, with 13 of 17 (76.5%) having had previous attempts at endoscopic resection[43].
In ampullary tumors that were removed via snare papillectomy, 18 of 86 (20.9%) were found to have < 20 mm intraductal extension without evidence of metastatic disease. These were treated with intraductal electrocautery ablation using a 6 Fr cystotome, with a 100% success rate and no recurrence over a median follow-up of 20 months. Among these patients, 8 of 18 (44%) had evidence of high-grade dysplasia or adenocarcinoma, which were eradicated[44]. Intraductal RFA was prospectively studied among multiple centers in France for treatment of residual adenoma following papillectomy. Twenty patients were identified, with each undergoing a single session of RFA. Successful eradication was 85% at 6 months and 70% at 12 months[45]. The long-term effects of RFA were examined in 29 patients who underwent intraductal RFA as an adjunct modality following papillectomy. Eradication of dysplasia was demonstrated in 93% of patients within 1-2 months, and 76% of patients in long-term follow-up up to a median of 776 d[46]. These data samples are promising given that the traditional alternate approach is a pancreaticoduodenectomy[11].
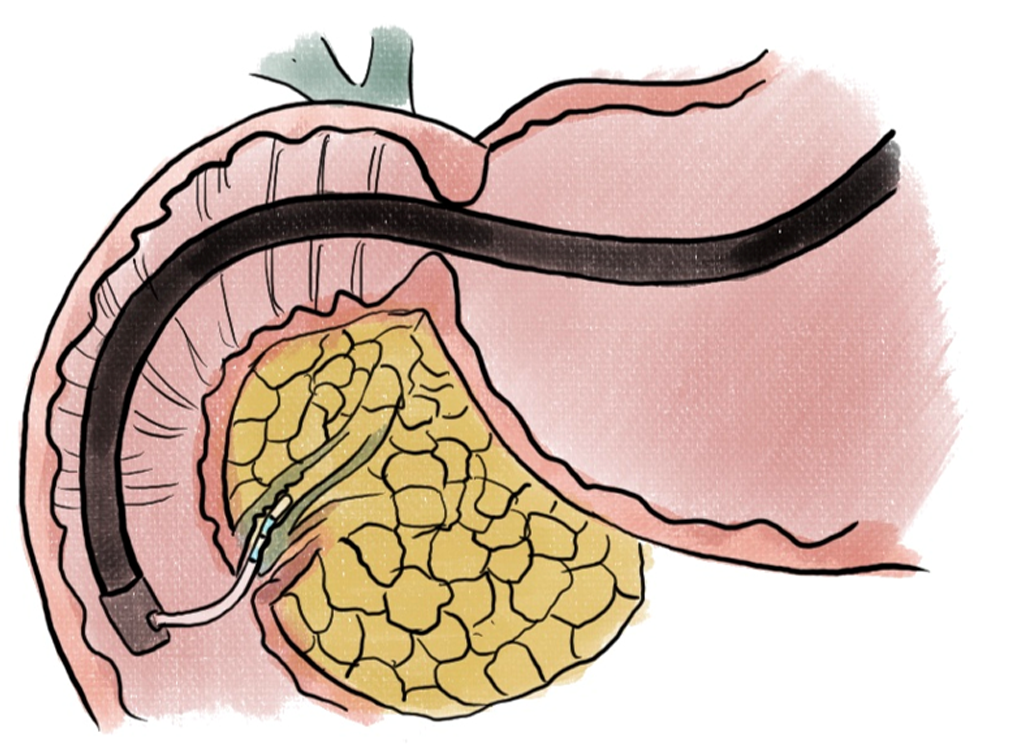
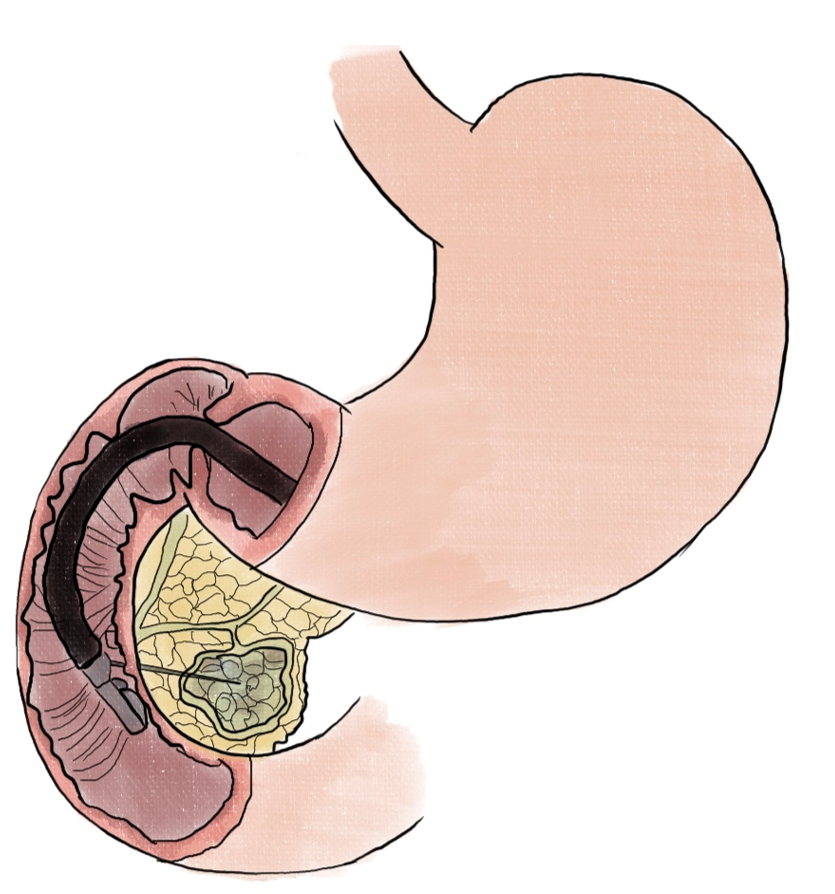
Pancreatic neuroendocrine tumors (PNETs) have been prime candidates for endoscopic therapies given the prospect of avoiding surgical resections. Endoscopic ultrasound-guided ethanol ablation (EUS-EA) (Figure 5) was found to result in PNET ablation in 24 of 40 (60%) patients with tumors < 2 cm in diameter[47].
With RFA, a multicenter prospective study noted complete necrosis of PNETs to be 7 of 15 (46.7%) in 6 months and 6 of 11 (54.5%) in 12 months. Importantly, all 13 patients with functional PNETs had complete clinical response[48]. In another prospective multicenter study, 12 of 14 (86%) PNETs had complete resolution at 12-month follow-up[49]. This study also included 17 pancreatic cystic neoplasms - 16 of which were intraductal pancreatic mucinous neoplasia. At 12 months, 11 had complete response and disappeared on subsequent imaging[49].
For locally advanced pancreatic adenocarcinoma (PDAC), RFA was correlated with tumor regression (> 50% reduction in size) in 6 of 10 patients who had a range of 1-4 sessions of treatment. One patient who had portal confluence encasement, as well as abutment of the celiac axis, common hepatic and mesenteric arteries had a significant tumor reduction and underwent a standard pancreaticoduodenectomy[50]. In a phase II randomized controlled trial, EUS-guided thermal ablation (Figure 6) has also been shown to be numerically promising in 6-month progress-free survival in locally advanced PDAC, though statistically not significant[51]. More recently, a small study incorporating EUS FNI of large surface area microparticle paclitaxel with standard of care for locally advanced pancreatic cancer found that 8 of 10 patients became resectable[52].
Ablative therapies of pancreatic tumors have profound implications. On the PNET and cystic neoplasm fronts, the prospect of organ and function preservation is tremendous in lieu of surgical resections. On the PDAC front, the potential of downstaging disease from unresectable to resectable carries remarkable implications for the overall treatment of pancreatic cancer.
Endobiliary ablation has been primarily utilized for palliative therapy in cholangiocarcinoma (CCA). A randomized trial of 65 patients with unresectable extrahepatic CCA (eCCA) who underwent biliary stenting and RFA had a significantly longer overall mean survival of 13.2 months vs 8.3 months, with a longer mean stent patency of 6.8 months vs 3.4 months, and with no significant difference in adverse events[53]. This has been corroborated in a multicenter randomized controlled trial with 174 patients randomized in a 1:1 ratio - each undergoing 2 index endoscopic interventions. The median overall survival was higher in the RFA group at 14.3 vs 9.2 months. Post-procedural Karnofsky performance scores were higher in the RFA group until 9 months. Acute cholecystitis was more frequent in the RFA group, but other adverse events were comparable[54]. In another study in patients with hilar biliary obstruction, 30 patients underwent endobiliary RFA for ingrowth occlusion after self-expandable metal stent placement. Technical success was possible in 28 of 30 (93.3%) of patients. Clinical success was achieved in 20 of the 28 patients who underwent RFA (71.4%), while recurrent biliary obstruction occurred in 9 of 20 (45%) of patients in a median of 163 d[55]. Another endobiliary ablative modality includes photodynamic therapy (PDT). A randomized trial of 43 patients who underwent endobiliary PDT alone vs in combination with S-1 (an oral anticancer drug combining a fluorouracil prodrug with an inhibitor of fluorouracil phosphorylation). A higher 1-year survival rate of 76.2% vs 32%, higher overall survival at 17 months vs 8 months, as well as higher progression-free survival at 10 months vs 2 months was noted in the combination group compared to PDT. There were no significant differences in adverse events or quality of life in either group[56].
Endobiliary ablation of CCA is typically limited to extrahepatic and hilar disease. The available data are supportive of a synergy of systemic treatment and endobiliary ablation in further improving survival[57].
Endoscopic ablation in colorectal disease has been largely used in the context of precursor polyps as an adjunct modality to endoscopic resection. Endoscopic thermal ablation of the margins of large colon polyps has a lower recurrence rate 1.4% vs 27.1% in incomplete thermal ablation[58]. Endoscopic argon plasma coagulation (APC) of recurrent adenomatous tissue successfully eradicated polyps in 9 of 11 (82%) of patients[59]. Moreover, a multicenter study found that APC of polyp margin and base after EMR was associated with significantly lower recurrent adenomatous tissue than margin-only ablation (0.9% vs 8.8%)[60].
In the early 2000s, endoscopic laser photoablation was used to treat large sessile polyps with low- or high-grade dysplasia. Follow-up after 28 months with 4.3 treatment sessions per polyp resulted in complete eradication of 61% of polyps[61]. The application for palliation of bleeding and obstruction in rectal cancer has not been as promising[62].
RFA for palliation of rectosigmoid tumors was studied in 12 patients who were planned for surgical resection. In the 10 patients who had a specimen resected, 82% of the tumor mass was noted to be destroyed attributable to ablation[63]. In patients with inoperable cancer, a phase I study of 10 procedures of endoscopic calcium electroporation in 6 patients who needed palliation had no adverse events. Five of the six patients reported symptom relief. One patient, who received concurrent systemic therapy was noted to have complete clinical response on follow-up 12 wk thereafter[64]. In such cases, the potential transition from a purely palliative role to a possible adjunct modality for organ preservation warrants further analysis and evaluation.
Endoscopic ablation encompasses a broad spectrum of modalities and techniques. The roles and applications are further stratified by disease process and anatomy of the target organ. Ablation of precancerous lesions in the luminal gastrointestinal tract has been a proven premise in cancer prevention and organ preservation. In the pancreas, the utility of endosonographic ablation in cystic neoplasms and PNETs has the potential of avoiding surgical resection - thereby highlighting the prospect of organ preservation. In cases of locally advanced or unresectable pancreatic adenocarcinoma, the tangible outcome is downstaging disease to a resectable stage thereby targeting improvement in overall survival. Studies on the latter category continue to emerge. Localized tumor injection and ablation as adjuncts to systemic therapy continue to have merit given the poor survival rates in pancreatic cancer. Furthermore, the costs and comorbidities of total esophagectomy, pancreaticoduodenectomy and abdominoperineal resections reinforce the need for organ preservation strategies.
It is important to reframe gastrointestinal malignancy into a systemic process that can elicit an immune response, which can be leveraged in treating the underlying disease. In addition to the debulking effect from local ablation, the targeted destruction of the local tumor can induce the exposure of cellular debris - a source of tumor antigens. Coupled with necrosis and a proinflammatory environment, antigen-presenting cells including dendritic cells infiltrate that environment and amplify the systemic immune response[65]. Furthermore, beyond triggering an immune response, a phase I clinical trial reported changes in cancer-associated gene expression after endoscopic ablation with calcium electroporation[66]. Therefore, the systemic implications of endoscopic ablation may go beyond the local effects of debulking and potentiating an immune response. For example, preclinical predictive numerical models utilizing confocal endomicroscopy have been shown to correlate with the degree of thermal injury as reflected by a ratio between damage depth over mucosa and submucosa thickness[67]. The optimal modality and dosimetry of ablation continue to be areas of active inquiry, especially with a paradigm shift to organ preservation such as in the SANO and OPRA trials[24,25].
Finally, multi-cancer early detection tests have the prospect of identifying early-stage cancers that otherwise have no screening protocols. Early detection may expand organ preservation treatment options including neoadjuvant ablation. This may have a significant impact in diseases where resection may be difficult, such as pancreaticobiliary malignancy. Ultimately, balancing such paradigm shifts with standard-of-care practices entails multidisciplinary discussions centered around the patient’s unique circumstances and preferences.
| 1. | Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond). 2021;41:1137-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 2. | Chiorean EG, Chiaro MD, Tempero MA, Malafa MP, Benson AB, Cardin DB, Christensen JA, Chung V, Czito B, Dillhoff M, Donahue TR, Dotan E, Fountzilas C, Glazer ES, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Masood A, Moravek C, Nakakura EK, Narang AK, Nardo L, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Truty MJ, Vollmer C, Wolff RA, Wolpin BM, Rn BM, Lubin S, Darlow SD. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:753-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Hecht JR, Hoffe S, Hubbard J, Hunt S, Hussan H, Jeck W, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Maratt J, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Williams G, Algieri F, Gurski L, Stehman K. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:653-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 5. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 950] [Article Influence: 237.5] [Reference Citation Analysis (16)] |
| 6. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 954] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 7. | von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Poppe M, Schuetze S, Shabason J, Spraker MB, Zimel M, Bergman MA, Sundar H, Hang LE. NCCN Guidelines® Insights: Gastrointestinal Stromal Tumors, Version 2.2022. J Natl Compr Canc Netw. 2022;20:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 571] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 9. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 689] [Article Influence: 172.3] [Reference Citation Analysis (0)] |
| 10. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 405] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 11. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 12. | Ajani JA, Barthel JS, Bentrem DJ, D'Amico TA, Das P, Denlinger CS, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Mulcahy MF, Orringer MB, Osarogiagbon RU, Posey JA, Sasson AR, Scott WJ, Shibata S, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD; National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 13. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4600] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 14. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1639] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 15. | Vassos N, Jakob J, Kähler G, Reichardt P, Marx A, Dimitrakopoulou-Strauss A, Rathmann N, Wardelmann E, Hohenberger P. Preservation of Organ Function in Locally Advanced Non-Metastatic Gastrointestinal Stromal Tumors (GIST) of the Stomach by Neoadjuvant Imatinib Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 16. | Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, Palmer A, Seligmann J, Laurberg S, Murakami K, West N, Quirke P, Gray R; FOxTROT Collaborative Group. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 207] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 17. | Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Fietkau R, Ghadimi M, Grützmann R, Wittel UA, Jacobasch L, Uhl W, Croner RS, Bechstein WO, Neumann UP, Waldschmidt D, Boeck SH, Moosmann N, Reinacher-Schick AC, Golcher H, Adler W, Semrau S, Kallies A, Hecht M, Tannapfel A, Oettle H. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: First results of the CONKO-007 trial. JCO. 2022;40 (16_suppl):4008-4008. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ghaneh P, Palmer D, Cicconi S, Jackson R, Halloran CM, Rawcliffe C, Sripadam R, Mukherjee S, Soonawalla Z, Wadsley J, Al-Mukhtar A, Dickson E, Graham J, Jiao L, Wasan HS, Tait IS, Prachalias A, Ross P, Valle JW, O'Reilly DA, Al-Sarireh B, Gwynne S, Ahmed I, Connolly K, Yim KL, Cunningham D, Armstrong T, Archer C, Roberts K, Ma YT, Springfeld C, Tjaden C, Hackert T, Büchler MW, Neoptolemos JP; European Study Group for Pancreatic Cancer. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 157] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 20. | Seufferlein T, Uhl W, Kornmann M, Algül H, Friess H, König A, Ghadimi M, Gallmeier E, Bartsch DK, Lutz MP, Metzger R, Wille K, Gerdes B, Schimanski CC, Graupe F, Kunzmann V, Klein I, Geissler M, Staib L, Waldschmidt D, Bruns C, Wittel U, Fichtner-Feigl S, Daum S, Hinke A, Blome L, Tannapfel A, Kleger A, Berger AW, Kestler AMR, Schuhbaur JS, Perkhofer L, Tempero M, Reinacher-Schick AC, Ettrich TJ. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)-a randomized phase II trial of the AIO pancreatic cancer group. Ann Oncol. 2023;34:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 87] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 21. | Johnston SA, Louis M, Churilov L, Ma R, Marhoon N, Bui A, Christophi C, Weinberg L. The financial burden of complications following rectal resection: A cohort study. Medicine (Baltimore). 2020;99:e20089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Löfgren A, Åkesson O, Johansson J, Persson J. Hospital costs and health-related quality of life from complications after esophagectomy. Eur J Surg Oncol. 2021;47:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4075] [Article Influence: 313.5] [Reference Citation Analysis (0)] |
| 24. | van der Wilk B, Eyck BM, Wijnhoven BPL, Lagarde SM, Rosman C, Noordman BJ, Valkema MJ, Coene PP, Dekker JWT, Hartgrink H, Heisterkamp J, Kouwenhoven EA, Nieuwenhuijzen GAP, Pierie JEN, van Sandick J, Sosef MN, Spaander M, van der Zaag E, Steyerberg EW, van Lanschot J. LBA75 Neoadjuvant chemoradiotherapy followed by surgery vs active surveillance for oesophageal cancer (SANO-trial): A phase-III stepped-wedge cluster randomised trial. Ann Oncol. 2023;34:S1317. [DOI] [Full Text] |
| 25. | Verheij FS, Omer DM, Williams H, Lin ST, Qin LX, Buckley JT, Thompson HM, Yuval JB, Kim JK, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Guillem JG, Temple L, Goodman KA, Segal NH, Cercek A, Yaeger R, Nash GM, Widmar M, Wei IH, Pappou EP, Weiser MR, Paty PB, Smith JJ, Wu AJ, Gollub MJ, Saltz LB, Garcia-Aguilar J. Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol. 2024;42:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 130] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 26. | Fokas E, Appelt A, Glynne-Jones R, Beets G, Perez R, Garcia-Aguilar J, Rullier E, Smith JJ, Marijnen C, Peters FP, van der Valk M, Beets-Tan R, Myint AS, Gerard JP, Bach SP, Ghadimi M, Hofheinz RD, Bujko K, Gani C, Haustermans K, Minsky BD, Ludmir E, West NP, Gambacorta MA, Valentini V, Buyse M, Renehan AG, Gilbert A, Sebag-Montefiore D, Rödel C. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol. 2021;18:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 27. | Zhou L, Yu G, Wen R, Jia H, Zhang T, Peng Z, Fan H, Pan A, Yu Y, Zhu X, Gong H, Gao X, Lou Z, Zhang W. Neoadjuvant chemoradiation therapy combined with immunotherapy for microsatellite stable ultra-low rectal cancer (CHOICE II): study protocol of a multicentre prospective randomised clinical trial. BMJ Open. 2023;13:e069793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Soliman YY, Kundranda M, Kachaamy T. Endoscopic Palliative Therapies for Esophageal Cancer. Gastrointest Endosc Clin N Am. 2024;34:91-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 29. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, Jobe BA, Eisen GM, Fennerty MB, Hunter JG, Fleischer DE, Sharma VK, Hawes RH, Hoffman BJ, Rothstein RI, Gordon SR, Mashimo H, Chang KJ, Muthusamy VR, Edmundowicz SA, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Falk GW, Kimmey MB, Madanick RD, Chak A, Lightdale CJ. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 972] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 30. | Dhaliwal L, Iyer PG. State of the Art: Management of Barrett's Esophagus Related Dysplasia and Neoplasia-Which Patient and What Therapy? Foregut. 2021;1:68-77. [DOI] [Full Text] |
| 31. | Thota PN, Arora Z, Dumot JA, Falk G, Benjamin T, Goldblum J, Jang S, Lopez R, Vargo JJ. Cryotherapy and Radiofrequency Ablation for Eradication of Barrett's Esophagus with Dysplasia or Intramucosal Cancer. Dig Dis Sci. 2018;63:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | He S, Bergman J, Zhang Y, Weusten B, Xue L, Qin X, Dou L, Liu Y, Fleischer D, Lu N, Dawsey SM, Wang GQ. Endoscopic radiofrequency ablation for early esophageal squamous cell neoplasia: report of safety and effectiveness from a large prospective trial. Endoscopy. 2015;47:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Canto MI, Abrams JA, Künzli HT, Weusten B, Komatsu Y, Jobe BA, Lightdale CJ. Nitrous oxide cryotherapy for treatment of esophageal squamous cell neoplasia: initial multicenter international experience with a novel portable cryoballoon ablation system (with video). Gastrointest Endosc. 2018;87:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Ke Y, van Munster SN, Xue L, He S, Zhang Y, Dou L, Liu Y, Liu X, Li W, Lv N, Dawsey SM, Weusten BLAM, Bergman JJGHM, Wang G. Prospective study of endoscopic focal cryoballoon ablation for esophageal squamous cell neoplasia in China. Gastrointest Endosc. 2019;90:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Kachaamy T, Sharma N, Shah T, Mohapatra S, Pollard K, Zelt C, Jewett E, Garcia R, Munsey R, Gupta S, Rojas-DeLeon M, Gupta D, Kaul V, Pannala R, Vashi P. A prospective multicenter study to evaluate the impact of cryotherapy on dysphagia and quality of life in patients with inoperable esophageal cancer. Endoscopy. 2023;55:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Shah T, Kushnir V, Mutha P, Majhail M, Patel B, Schutzer M, Mogahanaki D, Smallfield G, Patel M, Zfass A. Neoadjuvant cryotherapy improves dysphagia and may impact remission rates in advanced esophageal cancer. Endosc Int Open. 2019;7:E1522-E1527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Leung WK, Tong DK, Leung SY, Chan FS, Tong TS, Ho RS, Chu KM, Law SY. Treatment of Gastric Metaplasia or Dysplasia by Endoscopic Radiofrequency Ablation: A Pilot Study. Hepatogastroenterology. 2015;62:748-751. [PubMed] |
| 38. | Wang N, Chai N, Li L, Li H, Zhai Y, Feng X, Liu S, Zhang W, Linghu E. Comparison of Endoscopic Radiofrequency Ablation and Argon Plasma Coagulation in Patients with Gastric Low-Grade Intraepithelial Neoplasia: A Large-Scale Retrospective Study. Can J Gastroenterol Hepatol. 2022;2022:2349940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Yasuda K, Mizuma Y, Nakajima M, Kawai K. Endoscopic laser treatment for early gastric cancer. Endoscopy. 1993;25:451-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Oh S, Kim SG, Choi JM, Jin EH, Kim JH, Im JP, Kim JS, Jung HC. Ablation of residual gastric tumor by argon plasma coagulation after endoscopic resection. Surg Endosc. 2017;31:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Hernández-Ludeña L, Consiglieri CF, Gornals JB. EUS-guided ethanol ablation therapy for gastric stromal tumors. Rev Esp Enferm Dig. 2018;110:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Bazaga Pérez de Rozas S, Gallardo Ramírez MA, García-Alonso FJ, Carbajo AY, Pérez-Miranda Castillo M, de la Serna Higuera C. Endoscopic ultrasound-guided radiofrequency ablation for management of gastric gastrointestinal stromal tumor. Endoscopy. 2019;51:E223-E224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Dbouk M, Brewer Guiterrez O, Trindade AJ, Diehl DL, Kwon RS, Thosani NC, Khara HS, Benias PC, Kerdsirichairat T, Canto MI. Initial multicenter experience with nitrous oxide cryoballoon for treatment of flat duodenal adenomas (with video). Gastrointest Endosc. 2021;93:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Pérez-Cuadrado-Robles E, Piessevaux H, Moreels TG, Yeung R, Aouattah T, Komuta M, Dano H, Jouret-Mourin A, Deprez PH. Combined excision and ablation of ampullary tumors with biliary or pancreatic intraductal extension is effective even in malignant neoplasms. United European Gastroenterol J. 2019;7:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Camus M, Napoléon B, Vienne A, Le Rhun M, Leblanc S, Barret M, Chaussade S, Robin F, Kaddour N, Prat F. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: a multicenter prospective study. Gastrointest Endosc. 2018;88:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Cho SH, Oh D, Song TJ, Park DH, Seo DW, Lee SK, Kim MH, Lee SS. Long-term Outcomes of Endoscopic Intraductal Radiofrequency Ablation for Ampullary Adenoma with Intraductal Extension after Endoscopic Snare Papillectomy. Gut Liver. 2023;17:638-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Choi JH, Park DH, Kim MH, Hwang HS, Hong SM, Song TJ, Lee SS, Seo DW, Lee SK. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc. 2018;30:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Figueiredo Ferreira M, Garces-Duran R, Eisendrath P, Devière J, Deprez P, Monino L, Van Laethem JL, Borbath I. EUS-guided radiofrequency ablation of pancreatic/peripancreatic tumors and oligometastatic disease: an observational prospective multicenter study. Endosc Int Open. 2022;10:E1380-E1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 50. | Thosani N, Cen P, Rowe J, Guha S, Bailey-Lundberg JM, Bhakta D, Patil P, Wray CJ. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) for advanced pancreatic and periampullary adenocarcinoma. Sci Rep. 2022;12:16516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Testoni SGG, Petrone MC, Reni M, Rossi G, Barbera M, Nicoletti V, Gusmini S, Balzano G, Linzenbold W, Enderle M, Della-Torre E, De Cobelli F, Doglioni C, Falconi M, Capurso G, Arcidiacono PG. Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Sharma NR, Lo SK, Hendifar A, Othman MO, Patel K, Mendoza-Ladd A, Verco S, Maulhardt HA, Verco J, Wendt A, Marin A, Schmidt CM, diZerega G. Response of Locally Advanced Pancreatic Cancer to Intratumoral Injection of Large Surface Area Microparticle Paclitaxel: Initial Report of Safety and Clinical Outcome. Pancreas. 2023;52:e179-e187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 54. | Gao DJ, Yang JF, Ma SR, Wu J, Wang TT, Jin HB, Xia MX, Zhang YC, Shen HZ, Ye X, Zhang XF, Hu B. Endoscopic radiofrequency ablation plus plastic stent placement versus stent placement alone for unresectable extrahepatic biliary cancer: a multicenter randomized controlled trial. Gastrointest Endosc. 2021;94:91-100.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 55. | Inoue T, Ibusuki M, Kitano R, Sakamoto K, Kimoto S, Kobayashi Y, Sumida Y, Nakade Y, Ito K, Yoneda M. Endoscopic radiofrequency ablation for ingrowth occlusion after bilateral metal stent placement for malignant hilar biliary obstruction: a prospective pilot study. Gastrointest Endosc. 2023;97:282-290.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 56. | Park DH, Lee SS, Park SE, Lee JL, Choi JH, Choi HJ, Jang JW, Kim HJ, Eum JB, Seo DW, Lee SK, Kim MH, Lee JB. Randomised phase II trial of photodynamic therapy plus oral fluoropyrimidine, S-1, versus photodynamic therapy alone for unresectable hilar cholangiocarcinoma. Eur J Cancer. 2014;50:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Inoue T, Yoneda M. Recent Updates on Local Ablative Therapy Combined with Chemotherapy for Extrahepatic Cholangiocarcinoma: Photodynamic Therapy and Radiofrequency Ablation. Curr Oncol. 2023;30:2159-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Sidhu M, Shahidi N, Gupta S, Desomer L, Vosko S, Arnout van Hattem W, Hourigan LF, Lee EYT, Moss A, Raftopoulos S, Heitman SJ, Williams SJ, Zanati S, Tate DJ, Burgess N, Bourke MJ. Outcomes of Thermal Ablation of the Mucosal Defect Margin After Endoscopic Mucosal Resection: A Prospective, International, Multicenter Trial of 1000 Large Nonpedunculated Colorectal Polyps. Gastroenterology. 2021;161:163-170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 59. | Tsiamoulos ZP, Bourikas LA, Saunders BP. Endoscopic mucosal ablation: a new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc. 2012;75:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 60. | Djinbachian R, Pohl H, Rex DK, Levenick JM, Pleskow DK, Wallace MB, Khashab M, Singh A, Melson J, Yang D, Gavrić A, von Renteln D. Thermal ablation after endoscopic mucosal resection of large colorectal polyps: not only the margins, but also the base? Gut. 2023;73:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Norberto L, Polese L, Angriman I, Erroi F, Cecchetto A, D'Amico DF. Laser photoablation of colorectal adenomas: a 12-year experience. Surg Endosc. 2005;19:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Rao VS, Al-Mukhtar A, Rayan F, Stojkovic S, Moore PJ, Ahmad SM. Endoscopic laser ablation of advanced rectal carcinoma--a DGH experience. Colorectal Dis. 2005;7:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Vavra P, Dostalik J, Zacharoulis D, Khorsandi SE, Khan SA, Habib NA. Endoscopic radiofrequency ablation in colorectal cancer: initial clinical results of a new bipolar radiofrequency ablation device. Dis Colon Rectum. 2009;52:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Broholm M, Vogelsang R, Bulut M, Stigaard T, Falk H, Frandsen S, Pedersen DL, Perner T, Fiehn AK, Mølholm I, Bzorek M, Rosen AW, Andersen CSA, Pallisgaard N, Gögenur I, Gehl J. Endoscopic calcium electroporation for colorectal cancer: a phase I study. Endosc Int Open. 2023;11:E451-E459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, Fry TJ, Wood BJ. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 66. | Egeland C, Balsevicius L, Gögenur I, Gehl J, Baeksgaard L, Garbyal RS, Achiam MP. Calcium electroporation of esophageal cancer induces gene expression changes: a sub-study of a phase I clinical trial. J Cancer Res Clin Oncol. 2023;149:16031-16042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Quero G, Saccomandi P, Kwak JM, Dallemagne B, Costamagna G, Marescaux J, Mutter D, Diana M. Modular laser-based endoluminal ablation of the gastrointestinal tract: in vivo dose-effect evaluation and predictive numerical model. Surg Endosc. 2019;33:3200-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |