Published online Sep 16, 2023. doi: 10.4253/wjge.v15.i9.574
Peer-review started: March 20, 2023
First decision: June 19, 2023
Revised: July 25, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 16, 2023
Processing time: 177 Days and 1.3 Hours
Endoscopic ultrasound guided gallbladder drainage (EUS-GBD) is being increasingly used in practice (either as a bridge to cholecystectomy in high-risk patients or as destination therapy in non-surgical patients). Stents are used to create a conduit between the lumen of the gallbladder (GB) and the intestinal lumen through the gastric or enteric routes. Among the various types of stents used, cautery-enhanced lumen apposing metallic stents (LAMS) may be associated with fewer adverse events (AEs).
To compare the clinical success, technical success, and rate of AEs between transgastric (TG) and trans-enteric [transduodenal (TD)/transjejunal (TJ)] approach to GB drainage. Further, we analyzed whether using cautery enhanced stents during EUS-GBD impacts the above parameters.
Study was registered in PROSPERO (CRD42022319019) and comprehensive literature review was conducted. Manuscripts were reviewed for the data collection: Rate of AEs, clinical success, and technical success. Random effects model was utilized for the analysis.
No statistically significant difference in clinical and technical success between the TD/TJ and TG approaches (P > 0.05) were noted. There was no statistically significant difference in the rate of AEs when comparing two-arm studies only. However, when all studies were included in the analysis difference was almost significant favoring the TD/TJ approach. When comparing cautery-enhanced LAMS with non-cautery enhanced LAMS, a statistically significant difference in the rate of AEs was observed when all the studies were included, with the rate being higher in non-cautery enhanced stents (14.0% vs 37.8%; P < 0.01).
As per our study results, TD/TJ approach appears to be associated with lower rate of adverse events and comparable efficacy when compared to the TG approach for the EUS-GBD. Additionally, use of cautery-enhanced LAMS for EUS-GBD is associated with a more favorable adverse event profile compared to cold LAMS. Though the approach chosen depends on several patient and physician factors, the above findings could help in deciding the ideal drainage route when both TG and TD/TJ approaches are feasible.
Core Tip: Endoscopic ultrasound guided gallbladder drainage (EUS-GBD) is increasingly used in management of gallbladder disease. EUS-GBD can be achieved using trans-gastric or trans-enteric (trans-duodenal or trans-jejunal) approach There are currently no randomized controlled trials comparing these two approaches. We performed a meta-analysis of the existing literature on EUS guided gallbladder drainage. Trans-enteric approach was observed to have a more favorable safety profile compared to trans-gastric approach. Further use of cautery enhanced lumen apposing metallic stents (LAMS) to achieve EUS-guided GBD was associated with lesser adverse effects when compared to use of non-cautery enhanced (cold) LAMS.
- Citation: Grover D, Fatima I, Dharan M. Comparison of trans-gastric vs trans-enteric (trans-duodenal or trans-jejunal) endoscopic ultrasound guided gallbladder drainage using lumen apposing metal stents. World J Gastrointest Endosc 2023; 15(9): 574-583
- URL: https://www.wjgnet.com/1948-5190/full/v15/i9/574.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i9.574
Acute cholecystitis is an acute inflammation of the gall bladder (GB) characterized by the clinical syndrome of right upper quadrant pain, fever, and leukocytosis. The primary underlying etiology is gallstones, but 5%-10% of cases may be due to acalculous cholecystitis[1]. Open or laparoscopic cholecystectomy is the definitive treatment; however, many patients with acute cholecystitis are not good surgical candidates due to comorbidities. Percutaneous gallbladder drainage (PT-GBD) has emerged as an alternative but is limited by complications such as recurrent cholecystitis, bile peritonitis, puncture-induced hemorrhage, drain site pain, and infection[2]. PT-GBD is a temporizing treatment modality that can be a bridge to surgery until patient’s clinical status to improves. Often patients are unable to undergo surgery and are left with a permanent percutaneous drain[3,4].
Endoscopic ultrasound guided gallbladder drainage (EUS-GBD) is a minimally invasive alternative to PT-GBD. It is preferred due to its comparable clinical and technical success and minimal adverse events (AEs)[5]. Even though EUS-GBD initially was an alternative to surgery, the current indications have expanded to include: Destination therapy in poor surgical candidates, bridging to cholecystectomy, conversion of PT-GBD to EUS-GBD, alternative to failed PT-GBD or failed ET-GBD (endoscopic trans-papillary GBD) or alterative to failed EUS-guided biliary drainage (EUS-BD)[3]. Drainage of the GB is done by either transduodenal (TD)/transjuojenal (TJ) or transgastric (TG) approach. This allows for decompression of the GB by bypassing the obstruction. The best transluminal access to achieve gallbladder drainage has not been well established. In addition, there is limited literature comparing trans-gastric and trans-enteric (TD/TJ) approaches for EUS-GBD[6-8]. With the increasing use of EUS-GBD, it becomes important to ascertain the best trans-luminal approach to gallbladder drainage. Hence, we undertook a study to analyze the available evidence on this topic.
EUS-GBD can be achieved using plastic stents, self-expandable metal stents (SEMS) and lumen apposing metallic stents (LAMS). LAMS maintains a strong seal, reducing the risk of bile leakage and stent migration. Hence, LAMS is ideal for EUS-GBD[9-11]. The novel cautery-enhanced LAMS (hot) is being increasingly used and has the advantage of completing the procedure in one step without additional exchanges and reducing the need for fluoroscopic assistance compared to non-cautery enhanced (cold) LAMS[12,13]. The design of the stent with flares at both ends may mitigate risk of stent migration[14]. Our study aimed to compare the clinical success, technical success, and rate of AEs between the approaches used for the site of puncture and the type of LAMS used for EUS-GBD.
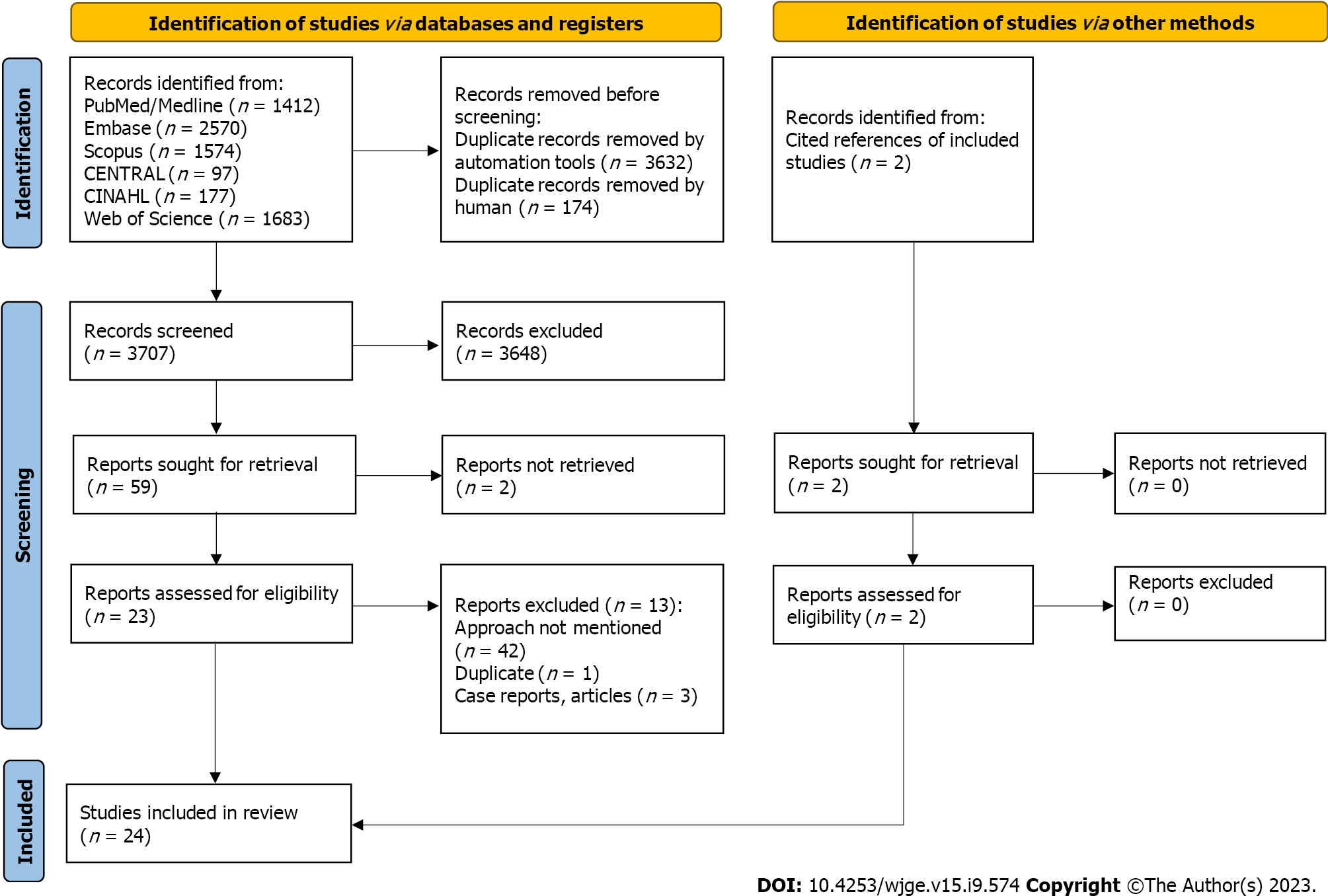
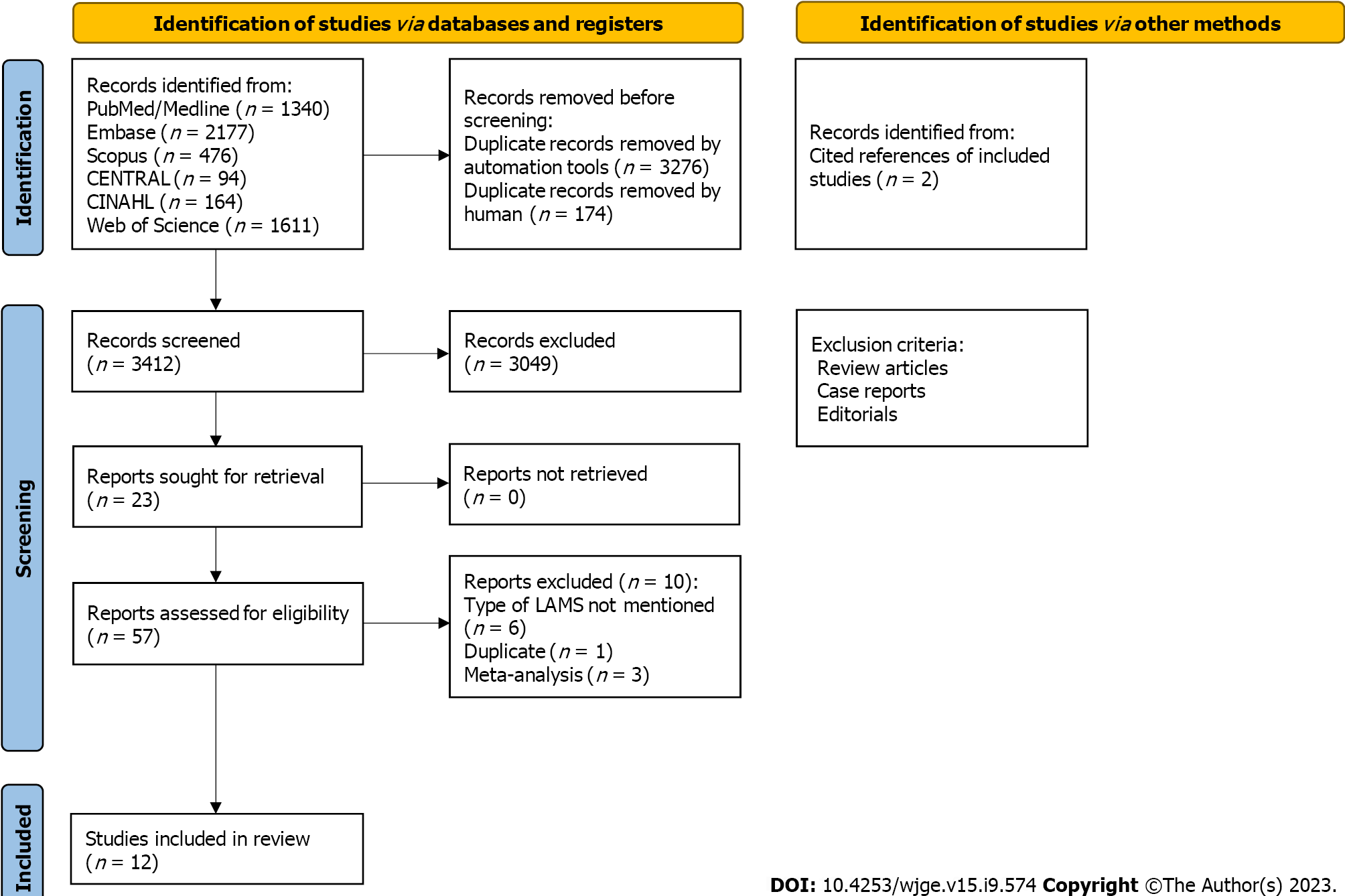
The study was registered in The International Prospective Register of Systematic Reviews (PROSPERO), and a comprehensive literature search was done on PubMed, Embase, Scopus, CENTRAL, CINAHL, and Web of Science. The literature search was done using words: “Ultrasonography”, “Endosonography”, “Endosonograph”, “Endoscopic ultrasound”, “lumen apposing metal stent”, “LAMS”, “transgastric”, “transduodenal”, hot” or “cold” AND “Gallbladder diseases”, Gallbladder”, “biliary”, “cholecyst”, AND “drainage”, “drain”. The references of the included studies were thoroughly reviewed. In total, 3707 studies were screened. For the comparison of TG and TD/TJ approach, twenty-four met the inclusion criteria (Figure 1). The inclusion criteria included randomized or nonrandomized controlled clinical trials and prospective and retrospective studies. Due to a lack of data, abstracts presented at conferences and case series (with four or more patients) were also included in the study. Reviews, meta-analyses, animal studies, letters from the editor, case reports, opinion articles, and editorials were not included. The other exclusion criteria included animal studies and studies in languages other than English. No age and gender restrictions were applied. A post-hoc analysis was done to compare the cautery-enhanced (hot) vs non-cautery enhanced approach and only twelve studies met the above-mentioned inclusion criteria (Figure 2). Manuscripts were reviewed for the data collection: Rate of AEs, clinical success, and technical success. The search was conducted again couple of days prior to the submission of this manuscript for the emerging data. Random effects models were estimated using Comprehensive Meta-Analysis (Biostat Inc.) software. A P value of less than 0.05 was considered significant.
The analysis was done using two methods. Method 1 included analysis of studies with patients in both arms only and method 2 included analyses of ALL studies. Due to limited data, we could not analyze the baseline characteristics and specific AEs. In the majority of the studies, technical success was defined as adequate access and drainage of the GB with the placement of the LAMS stent. Clinical success was described as a decline in serum bilirubin levels in patients with obstructive jaundice to 10% of the initial levels and improvement of cholestatic parameters in those without jaundice.
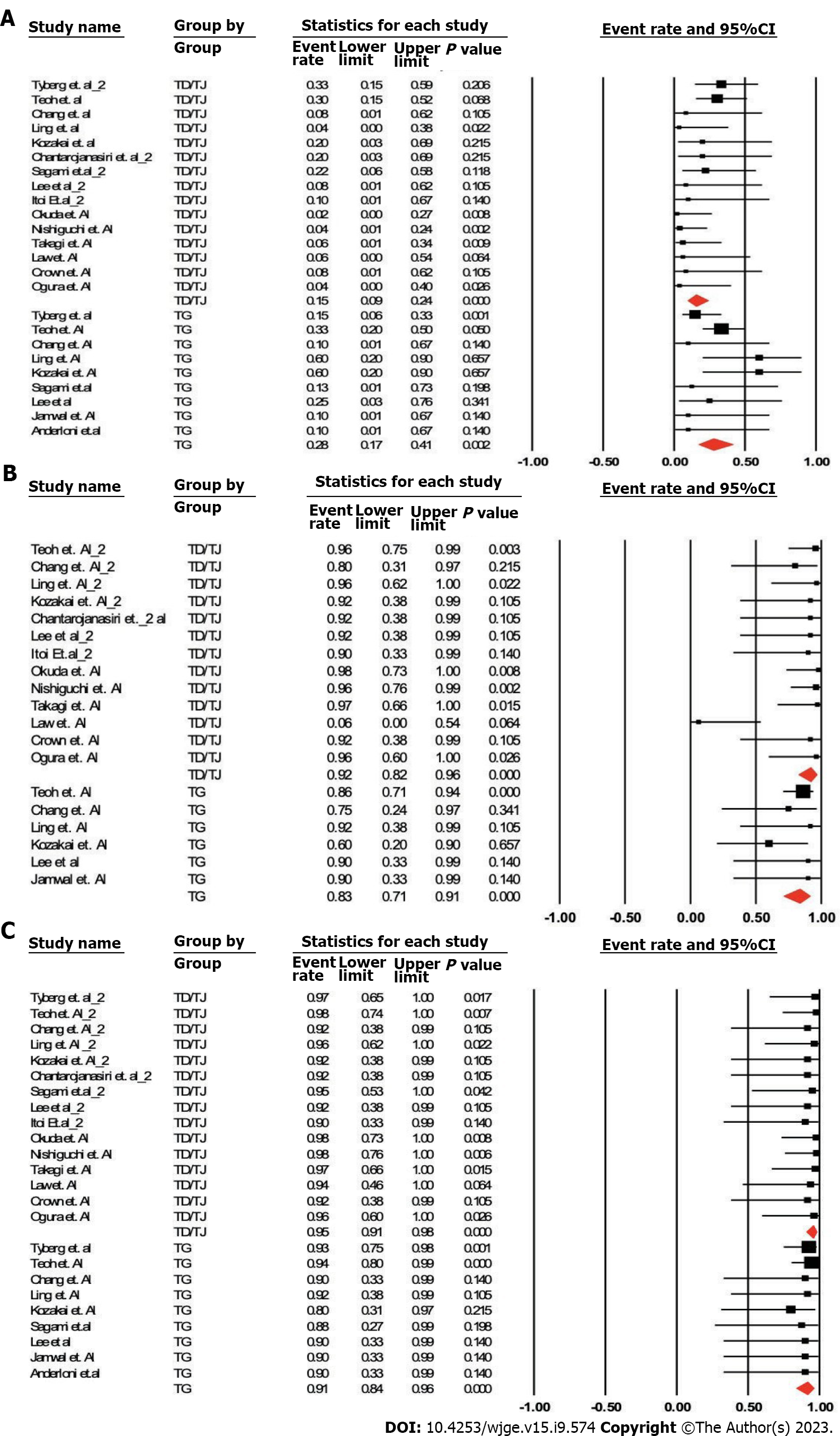
Analyses were done by two methods. Pooled odds ratios for AE, clinical success and technical success were calculated (Table 1, Figure 3).
| n of studies | Pooled odds ratio (TG vs TD/TJ) or AE (%) | 95%CI | P value | |
| Method 1: Including studies with patients in both arms only | ||||
| Adverse events | 6 | 1.58 | 0.46-5.45 | 0.47 |
| Clinical success | 3 | 0.30 | 0.06-1.48 | 0.14 |
| Technical success | 3 | 0.30 | 0.05-1.89 | 0.20 |
| Method 2: Including all studies | ||||
| Adverse events | 9 vs 15 | 27.5% vs 15.2% | (17.1%-41.1%) vs (9.5%-23.6%) | 0.07 |
| Clinical success | 6 vs 13 | 83.3% vs 91.7% | (71.0%-91.0%) vs (82.4%-96.3%) | 0.16 |
| Technical success | 9 vs 15 | 91.3% vs 95.6% | (83.6%-95.6%) vs 90.7%-97.7%) | 0.22 |
Method 1: Analysis of studies with patients in both arms only. TG vs TD/TJ: Pooled odds ratio (95% confidence interval), P value: AEs (6 studies): 1.58 (0.46-5.45), P = 0.47; clinical success (3 studies): 0.30 (0.06-1.48), P = 0.14; and technical success (3 studies): 0.30 (0.05-1.89), P = 0.20.
Method 2: Analysis of all studies (in total 15 using TD/TJ approach and 9 using TG approach). TG vs TD/TJ: AEs (Studies: 9 vs 15): 27.5% (17.1%-41.1%) vs 15.2% (9.5%-23.6%), P = 0.07; clinical success (Studies: 6 vs 13): 83.3% (71.0%-91.0%) vs 91.7% (82.4%-96.3%), P = 0.16; and technical success (Studies: 9 vs 15): 91.3% (83.6%-95.6%) vs 95.3% (90.7%-97.7%), P = 0.22.
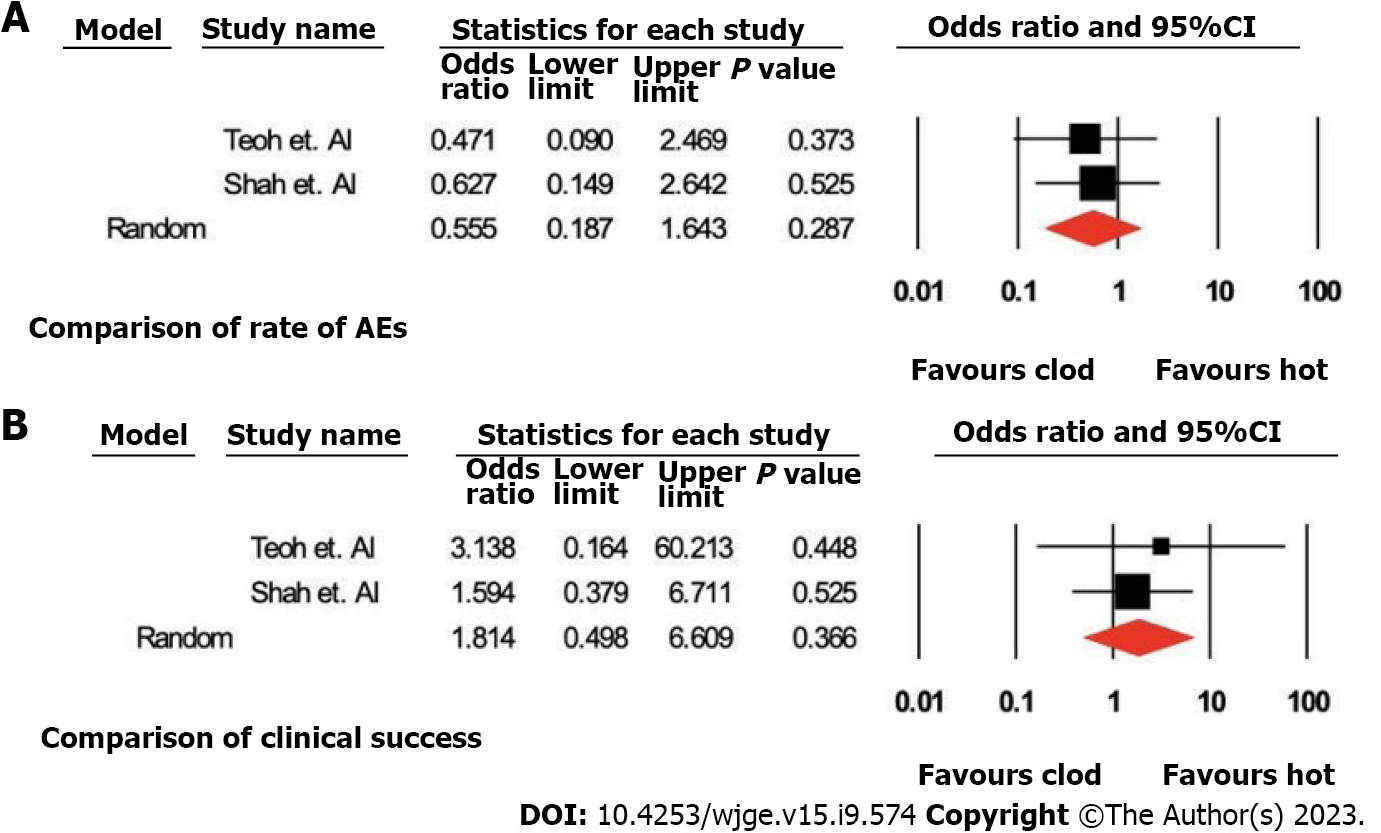
Analyses were done by two methods. Pooled odds ratios for AE, clinical success and technical success were calculated (Table 2, Figure 4).
| n of studies (cautery-enhanced vs non- cautery enhanced) | Pooled odds ratio (cautery-enhanced vs non-cautery enhanced) or AEs (%) | 95%CI | P value | |
| Method 1: Including studies with patients in both arms only | ||||
| Adverse events | 2 | 0.55 | 0.19-1.64 | 0.28 |
| Clinical success | 2 | 1.81 | 0.50-6.61 | 0.37 |
| Method 2: Including all studies | ||||
| Adverse events | 9 vs 3 | 14.0% vs 37.8% | (9.1%-21.0%) vs (26.5%- 50.6%) | 0 |
| Clinical success | 11 vs 3 | 89.9% vs 93.4% | (86.1%-92.7%) vs (72.8%-90.3%) | 0.12 |
| Technical success | 11 vs 3 | 94.4% vs 93.8% | 26.5%-50.6% | 0.82 |
Method 1: Analysis of studies with patients in both arms only. Cautery-enhanced vs non-cautery enhanced-pooled odds ratio (95% confidence interval); P value: AEs (2 studies): 0.55 (0.19-1.64), P = 0.28; clinical success (2 studies): 1.81 (0.50 to 6.61; P = 0.37). There was only one study that compared the technical success of both the arms.
Method 2: Including all the studies (in total 9 using cautery-enhanced LAMS and 3 using non-cautery enhanced LAMS). Cautery-enhanced vs non-cautery-enhanced-pooled percentage (IQR), P value: AEs (Studies: 9 vs 3): 14% (9.1%-21.0%) vs 37.8% (26.5%-50.6%), P ≤ 0.001; Clinical success (Studies: 11 vs 3): 89.9% (86.1%-92.7%) vs 93.4% (72.8%-90.3%), P = 0.12, and technical success (Studies: 11 vs 3): 94.4% (91.3%- 96.4%) vs 93.8% (86.3% vs 97.3%), P = 0.82.
Traditionally, early laparoscopic cholecystectomy is the gold standard treatment for cholecystitis[1,13]. In patients who are poor surgical candidates due to comorbidities, conservative management or percutaneous drainage of the GB is recommended. In the CHOCOLATE trial, for patients with a high APACHE II score, urgent laparoscopic cholecystectomy was associated with a reduced hospital length of stay, complications, and reinterventions compared to percutaneous drainage[15]. Hence, patients with cholecystitis should undergo cholecystectomy even in emergent conditions if well tolerated. Cholecystectomy has the additional advantage of obviating the future risk of recurrent cholecystitis.
Recent studies have reported EUS-GBD as a minimally invasive alternative to percutaneous drainage for cholecystitis in patients who are poor surgical candidates[16]. EUS-GBD has been studied for technical success, clinical success, and rate of AEs comparable to standard percutaneous techniques with decreased tube-related complications, including tube dislodgement, migration, obstruction and peri-tubal leakage[2,16,17]. In a meta-analysis by Luk et al[17], EUS-GBD was reported to have comparable technical and clinical success; however, EUS-GBD was associated with lower post-procedure adverse events, shorter hospital stays, and fewer reinterventions and readmissions compared to PT-GBD.
The efficacy and feasibility of EUS-GBD using different stents, including plastic stents, SEMS, and LAMS has been well documented[7,18-20]. Stent placement can result in complications such as stent migration, occlusion, bleeding, bile leak and pneumoperitoneum and associated morbidity. Plastic double pigtail stents are commonly associated with bile leaks, bile peritonitis and stent migration. SEMS provides the advantage of longer stent patency and prevents bile leakage but risk of stent migration remains. Both plastic stents and SEMS do not maintain apposition to seal the gap and reliably form a fistula. LAMS is considered an ideal stent due to its ability to make a firm seal with decreased complications[7,9,14].
The drainage of the GB is done by creating a cholecysto-enteric communication, via either TD/TJ or TG approach under the endoscopic/endosonographic guidance. In the TD approach, the retro-peritoneal duodenum is relatively immobile and thus provides a stable access site to the neck of GB which is the puncture site in this approach[6]. In addition, the inflamed GB wall may become adherent to the wall of the duodenum/jejunum lending further stability for access. Compared to stomach, the wall of duodenum/jejunum has less peristaltic activity which may decrease the risk of stent migration and stent occlusion due to tissue overgrowth[6,7]. Potential for reflux of food contest into the gallbladder may be lesser with TD approach resulting in reduced risk of stent occlusion or infection related to reflux[6,7]. The flow of food into the biliary system during EUS-GBD can lead to cholangitis or obstructive jaundice by occluding the stent. Due to the above reasons, TD/TJ is thought to be a safer option, but it has several limitations[6,7]. One of the limitations is the technical difficulty in accessing the neck of the GB, the puncture site in the TD/TJ approach. With TG approach the access point is usually the gallbladder body which provides a larger landing zone for deployment of the inner flange of the lumen apposing metal stent[6].
The thicker wall of the stomach may have larger perforating blood vessels (as compared to the duodenum) which can increase the risk of bleeding during transluminal access of the gallbladder. Management of delayed AEs may be easier with TG approach as the stomach is more accessible at laparoscopy compared to the duodenal bulb and thicker gastric wall permits more reliable wound closure[6]. Our study aimed to compare the clinical success, technical success, and rate of AEs between the approaches used for the site of puncture, i.e., TD/TJ vs TG.
Our meta-analysis showed no statistically significant difference in the clinical and technical success between the TD/TJ and TG approaches. In addition, we did not observe any statistically significant difference in the rate of AEs when comparing the two-arm studies only; however, the difference was almost significant (TG vs TD/TJ: 27.5% vs 15.2%, P = 0.07) when all the studies were analyzed with higher rates of AE noted with the TG approach. The commonly noted AEs with EUS-GBD include stent migration, stent occlusion, biliary peritonitis, pneumoperitoneum and recurrence of cholecystitis due to food impaction[18].
We also analyzed the above outcomes when deploying the cautery-enhanced (hot) vs non-cautery enhanced (cold) LAMS for EUS-GBD. The cautery-enhanced LAMS is a novel, fully covered, self-expanding stent with an electrocautery-enhanced delivery system ideal for EUS-GBD. Due to its “all-in-one” nature, the direct introduction of the device into the GB without prior placement of a guidewire eliminates the need for multiple steps and accessory exchanges[9]. The one step procedure with cautery-enhanced (hot) LAMS decreases the procedure time and the need for fluoroscopic assistance. The complications are further decreased by the hemostatic effect of cautery and absence of need for tract dilation likely reduces risk of bleeding and bile leak[12,13]. Deployment of non-cautery enhanced (cold) LAMS is wire guided and carries the risk of loss of wire access and attendant problems which do not apply to cautery enhanced LAMS. Hence, the cautery-enhanced LAMS is expected to have lesser rate of AEs as compared to non-cautery enhanced.
In our study, we did not observe any significant difference in the technical and clinical success between the cautery enhanced and non-cautery enhanced LAMS in EUS-GBD. No significant difference was observed in the rate of AEs between the two approaches when studies with both arms only were analyzed; however, a significant difference (P < 0.01) was noted in the rate of AEs when all the studies were included in the analysis, with the AEs being higher in non-cautery enhanced compared to cautery-enhanced LAMS.
There are several limitations to our study. The number of included studies was small due to data sparsity; thus, the results of this study might be underpowered. Additionally, the studies were done at several different centers, and the heterogeneity amongst various centers weakens the reliability of the results. Furthermore, since the number of studies was less than 10, publication bias is difficult to address. In addition to publication bias, selection bias, lead-time bias, and confounding factors cannot be excluded. Several studies reported their experience with EUS-GBD but did not aim to compare TD/TV vs TG approaches. With first method of analyzing studies with both arms, we were able to compare TD/TJ approach vs TG approach by the same operator. This helped reduce performance bias and data heterogeneity. However, these studies were not conducted with the primary aim of comparing both approaches. Some of the findings from our analysis did not reach statistical significance due to data sparsity. When we used the second method and included all studies in our analysis, due to the increase in available data, some findings were statistically significant. However (as some case series reported one approach only and pooled data included multiple operators) the analyzed data was quite heterogeneous. Given the sparse data and heterogeneity of the data we are unable to perform GRADE analysis and make recommendations based on GRADE methodology. Sufficiently powered randomized control trial should be done to compare the clinical outcomes of TD/TJ vs TG approach. Given the significantly reduced procedure time and ease of deployment of cautery enhanced LAMS for EUS-GBD, it is unlikely that a randomized controlled trial will be conducted to compare cautery enhanced vs and non-cautery enhanced LAMS to validate our findings.
Based on our study findings, cautery-enhanced LAMS deployment appears safer than non-cautery enhanced stent deployment for EUS-GBD. The TD/TJ approach may be associated with a more favorable AE profile with equal efficacy when compared to TG approach for EUS-GBD. While decision regarding approach to trans-luminal GB drainage depends on endoscopist preference and patient-specific anatomic considerations such as proximity of GB to the gastrointestinal tract lumen, it would be helpful to know which approach (trans-gastric vs trans-duodenal) has a favorable AEs profile especially when both approaches are feasible in a given patient. If EUS-GBD is a bridge to cholecystectomy, surgery appears feasible with both TG and TD/TJ approaches[21,22] but data regarding preferred approach is lacking[6].
Adequately powered RCTS are need to confirm the findings in our retrospective study.
Transduodenal endoscopic ultrasound guided gallbladder drainage (EUS-GBD) appeard to be safer than transgastric drainage. Hot lumen apposing metallic stents (LAMS) is better than cold LAMS.
As per out study transduodenal approach appeared to have a more favorable adverse event (AE) profile with comparable technical and clinical success when compared to transgastric approach. Cautery enhanced LAMS has a more favorable AE and shorter procedure time than cold LAMS.
Literature search was done using PubMed, Embase, Scopus, CENTRAL, CINAHL, and Web of Science database. The inclusion criteria included randomized or nonrandomized controlled clinical trials and prospective and retrospective studies. Due to a lack of data, abstracts presented at conferences and case series (with four or more patients) were also included in the study. Reviews, meta-analyses, animal studies, letters from the editor, case reports, opinion articles, and editorials were not included. The other exclusion criteria included animal studies and studies in languages other than English. No age and gender restrictions were applied. A post-hoc analysis was done to compare the cautery-enhanced (hot) vs non-cautery enhanced approach and only twelve studies met the above-mentioned inclusion criteria. Manuscripts were reviewed for the data collection: Rate of AEs, clinical success, and technical success. The search was conducted again couple of days prior to the submission of this manuscript for the emerging data. Random effects models were estimated using Comprehensive Meta-Analysis (Biostat Inc.) software. A P value of less than 0.05 was considered significant.
Compare trans-gastric vs trans-enteric EUS-GBD based on available literature. Compare AE profile, technical success and clinical success of both approaches. As a secondary outcome compare cautery enhanced LAMS use vs non-cautery enhanced (cold) LAMS.
Identify if any transgastric or transenteric EUS-GBD is better based on existing literature.
EUS-GB being increasingly used either as bridge to cholecystectomy or as destination therapy. GB can be accessed by transgastric or transenteric route. However, it is unclear if one approach is better than the other.
Marissa Iverson was responsible for the literature search and PRISMA flow sheet.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Guardado A, Spain; Wani I, India; Zharikov YO, Russia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Gallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA. 2022;327:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 182] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 2. | Molina H, Chan MM, Lewandowski RJ, Gabr A, Riaz A. Complications of Percutaneous Biliary Procedures. Semin Intervent Radiol. 2021;38:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Posner H, Widmer J. EUS guided gallbladder drainage. Transl Gastroenterol Hepatol. 2020;5:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | James TW, Baron TH. EUS-guided gallbladder drainage: A review of current practices and procedures. Endosc Ultrasound. 2019;8:S28-S34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Podboy A, Yuan J, Stave CD, Chan SM, Hwang JH, Teoh AYB. Comparison of EUS-guided endoscopic transpapillary and percutaneous gallbladder drainage for acute cholecystitis: a systematic review with network meta-analysis. Gastrointest Endosc. 2021;93:797-804.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Perez-Miranda M. Technical considerations in EUS-guided gallbladder drainage. Endosc Ultrasound. 2018;7:79-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 7. | Walter D, Teoh AY, Itoi T, Pérez-Miranda M, Larghi A, Sanchez-Yague A, Siersema PD, Vleggaar FP. EUS-guided gall bladder drainage with a lumen-apposing metal stent: a prospective long-term evaluation. Gut. 2016;65:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Teoh AYB, Serna C, Penas I, Chong CCN, Perez-Miranda M, Ng EKW, Lau JYW. Endoscopic ultrasound-guided gallbladder drainage reduces adverse events compared with percutaneous cholecystostomy in patients who are unfit for cholecystectomy. Endoscopy. 2017;49:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | van der Merwe SW, van Wanrooij RLJ, Bronswijk M, Everett S, Lakhtakia S, Rimbas M, Hucl T, Kunda R, Badaoui A, Law R, Arcidiacono PG, Larghi A, Giovannini M, Khashab MA, Binmoeller KF, Barthet M, Perez-Miranda M, van Hooft JE. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:185-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 275] [Article Influence: 91.7] [Reference Citation Analysis (3)] |
| 10. | Manta R, Mutignani M, Galloro G, Conigliaro R, Zullo A. Endoscopic ultrasound-guided gallbladder drainage for acute cholecystitis with a lumen-apposing metal stent: a systematic review of case series. Eur J Gastroenterol Hepatol. 2018;30:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kalva NR, Vanar V, Forcione D, Bechtold ML, Puli SR. Efficacy and Safety of Lumen Apposing Self-Expandable Metal Stents for EUS Guided Cholecystostomy: A Meta-Analysis and Systematic Review. Can J Gastroenterol Hepatol. 2018;2018:7070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Teoh AY, Perez-Miranda M, Kunda R, Lee SS, Irani S, Yeaton P, Baron TH, Sun S, Rerknimitr R, Moon JH, Holt B. 173 outcomes of an international multi-centered registry on eus-guided gallbladder drainage in patients that are unfit for cholecystectomy. Gastrointest Endosc. 2018;87:AB62. [DOI] [Full Text] |
| 13. | Mori Y, Itoi T, Baron TH, Takada T, Strasberg SM, Pitt HA, Ukai T, Shikata S, Noguchi Y, Teoh AYB, Kim MH, Asbun HJ, Endo I, Yokoe M, Miura F, Okamoto K, Suzuki K, Umezawa A, Iwashita Y, Hibi T, Wakabayashi G, Han HS, Yoon YS, Choi IS, Hwang TL, Chen MF, Garden OJ, Singh H, Liau KH, Huang WS, Gouma DJ, Belli G, Dervenis C, de Santibañes E, Giménez ME, Windsor JA, Lau WY, Cherqui D, Jagannath P, Supe AN, Liu KH, Su CH, Deziel DJ, Chen XP, Fan ST, Ker CG, Jonas E, Padbury R, Mukai S, Honda G, Sugioka A, Asai K, Higuchi R, Wada K, Yoshida M, Mayumi T, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: management strategies for gallbladder drainage in patients with acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Loozen CS, van Santvoort HC, van Duijvendijk P, Besselink MG, Gouma DJ, Nieuwenhuijzen GA, Kelder JC, Donkervoort SC, van Geloven AA, Kruyt PM, Roos D, Kortram K, Kornmann VN, Pronk A, van der Peet DL, Crolla RM, van Ramshorst B, Bollen TL, Boerma D. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high risk patients (CHOCOLATE): multicentre randomised clinical trial. BMJ. 2018;363:k3965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Tyberg A, Saumoy M, Sequeiros EV, Giovannini M, Artifon E, Teoh A, Nieto J, Desai AP, Kumta NA, Gaidhane M, Sharaiha RZ, Kahaleh M. EUS-guided Versus Percutaneous Gallbladder Drainage: Isn't It Time to Convert? J Clin Gastroenterol. 2018;52:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Luk SW, Irani S, Krishnamoorthi R, Wong Lau JY, Wai Ng EK, Teoh AY. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019;51:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Choi JH, Lee SS, Choi JH, Park DH, Seo DW, Lee SK, Kim MH. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Anderloni A, Buda A, Vieceli F, Khashab MA, Hassan C, Repici A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: a systematic review and pooled analysis. Surg Endosc. 2016;30:5200-5208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Jang JW, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Saumoy M, Tyberg A, Brown E, Eachempati SR, Lieberman M, Kunda R, Cosgrove N, Shah A, Siddiqui A, Gaidhane M, Sharaiha RZ, Kahaleh M. Cholecystectomy after endoscopic ultrasound guided gallbladder drainage? Gastrointest Endosc. 2017;85:AB481-AB482. [DOI] [Full Text] |
| 22. | Jang JW, Lee SS, Song TJ, Hyun YS, Park DY, Seo DW, Lee SK, Kim MH, Yun SC. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |