Published online Mar 16, 2023. doi: 10.4253/wjge.v15.i3.103
Peer-review started: September 25, 2022
First decision: January 3, 2023
Revised: January 11, 2023
Accepted: February 8, 2023
Article in press: February 8, 2023
Published online: March 16, 2023
Processing time: 171 Days and 8.6 Hours
Gastric neuroendocrine neoplasms (gNENs) are a rare type of gastric neoplasm, even if their frequency is increasing according to the latest epidemiologic revisions of the main registries worldwide. They are divided into three main subtypes, with different pathogeneses, biological behaviors, and clinical characteristics. GNEN heterogeneity poses challenges, therefore these neoplasms require different management strategies. Update the knowledge on the endoscopic treatment options to manage g-NENs. This manuscript is a narrative review of the literature. In recent years, many advances have been made not only in the knowledge of both the pathogenesis and the molecular profiling of gNENs but also in the endoscopic expertise towards innovative treatment options, which proved to be less aggressive without losing the capa
Core Tip: Gastric neuroendocrine neoplasms (gNENs) are a rare form of gastric neoplasia, although their incidence is increasing worldwide according to recent epidemiological reviews of large registries. The heterogeneity of gNENs poses a challenge, and therefore these neoplasms require different treatment strategies. Among the possible treatment options, the endoscopic approach is increasingly used and progressively improved, with different techniques available, ranging from classical polypectomy (cold or hot snare) to endoscopic mucosal resection (both with “en bloc” and piecemeal techniques), endoscopic submucosal dissection and endoscopic full-thickness resection. In this manuscript, we have summarized all new endoscopic techniques for the treatment of gastric neuroendocrine tumors.
- Citation: Massironi S, Gallo C, Laffusa A, Ciuffini C, Conti CB, Barbaro F, Boskoski I, Dinelli ME, Invernizzi P. Endoscopic techniques for gastric neuroendocrine tumors: An update. World J Gastrointest Endosc 2023; 15(3): 103-113
- URL: https://www.wjgnet.com/1948-5190/full/v15/i3/103.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i3.103
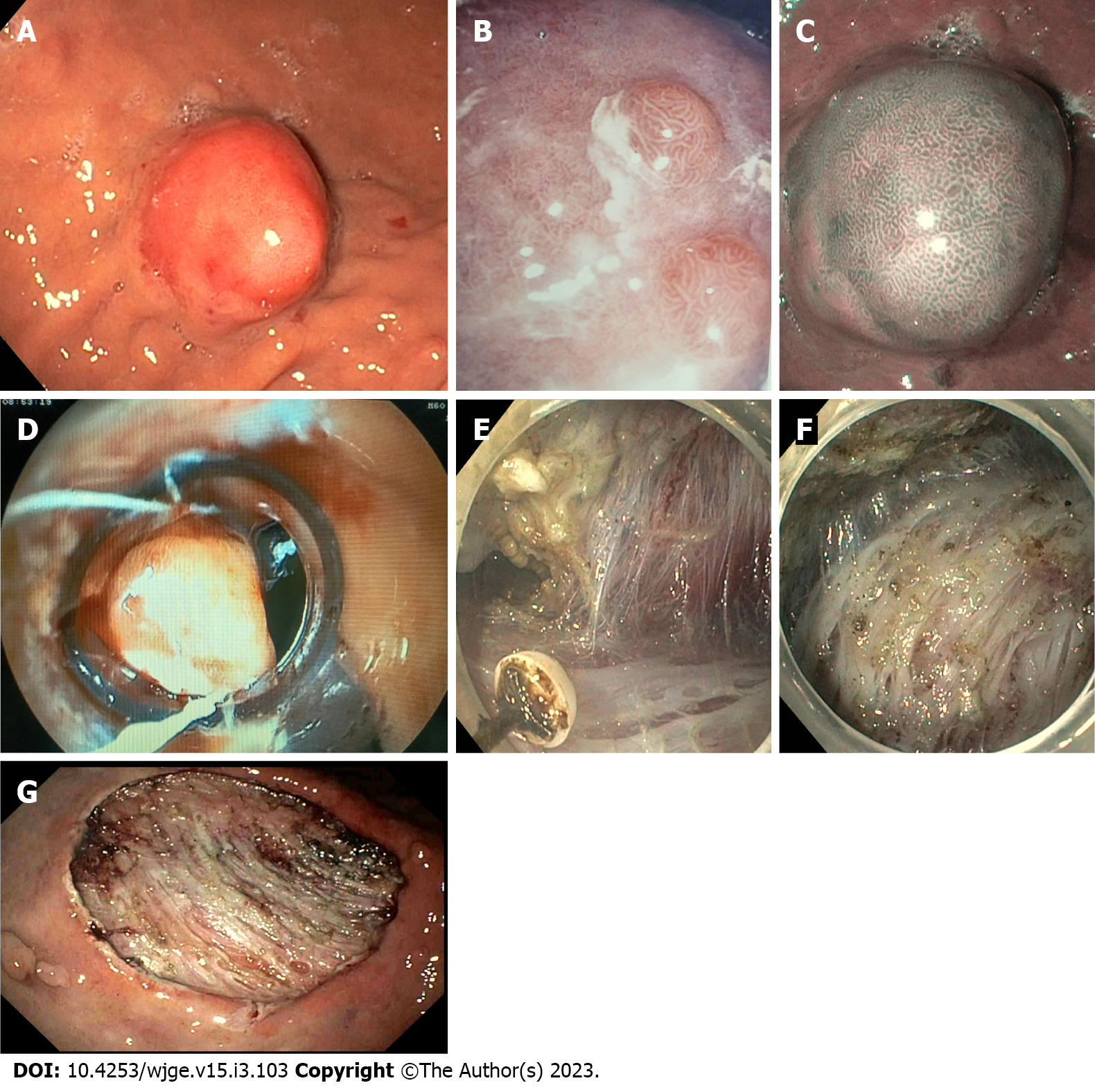
Gastric neuroendocrine neoplasms (gNENs) are heterogeneous tumors whose incidence has increased rapidly recently due to improved recognition and awareness of neuroendocrine neoplasms as distinct tumor types[1]. Representing approximately 1%-2% of all gastrointestinal (GI) malignancies[2], they are still a rare type of tumor, even if they constitute the most frequent localization of digestive NENs, accounting for 20% of all enteric neuroendocrine tumors in selected countries, followed by rectal NENs[3-5]. In addition to the European Neuroendocrine Tumors Society (ENETS) grading system that all NENs follow, based on the degree of differentiation and the Ki67 index (i.e., well-differentiated G1, G2 and G3, and poorly differentiated G3 neoplasms), gNENs are also divided into three main clinical types with different etiology and pathophysiology, as well as different prognosis and treatment strategy[6]: Type 1 gNENs are associated with chronic autoimmune atrophic gastritis (CAAG); type 2 gNENs are associated with gastrinoma/MEN-1 syndrome; in contrast; type 3 gNENs are not associated with any related pathology because they are usually sporadic[3,7]. Type 1 tumors represent the majority of gNENs and account for approximately 70%-80%[8]; they are usually detected through an upper GI endoscopy, and they mainly appear as small, multiple, located in the gastric body or fundus. They are composed of enterochromaffin-like (ECL) cells, that are usually confined to the mucosal or submucosal layers of the gastric wall[6] (Figure 1A-C); as for their etiopathogenesis, they are known to be an epiphenomenon of hypergastrinemia due to CAAG[9,10], while the role of PPI is more controversial[11]. Patients with CAAG, therefore, have an increased incidence of gNENs[12], and for this reason, they should undergo endoscopic surveillance with a variable interval[13].
Since type 1 gNENs are associated with a risk of metastasis of less than 5%, a conservative approach based on endoscopic resection (ER) and follow-up is preferred to surgery for small neoplasms greater than 5 mm in diameter and not infiltrating the muscularis propria[14,15], although there is no evidence of a significant superiority of ER over surveillance alone in terms of prognosis and recurrence in case of these small lesions[16]. According to ENETS guidelines, a EUS staging is recommended for lesions > 10 mm to determine the exact depth of tumor infiltration, its size and echogenicity, to assess loco-regional lymph node involvement, and thus to confirm the appropriateness of ER[17,18]. Nevertheless, the accuracy of EUS in staging submucosal lesions appears to be only 45% when compared with the histologic diagnosis after complete ER[19]. Therefore, accurate staging is often not possible until the lesion has been removed, as histology remains the gold standard for determining tumor differentiation, infiltration of the deep resection margins, and lymphatic vessel invasion[9].
Type 2 gNENs represent the smallest proportion of all gNENs, accounting for only 5%-6% of them; like type 1 neoplasms, they arise from ECL cells, and they are often small, multiple, and polypoid. They also represent an epiphenomenon of the trophic effect induced by hypergastrinemia on the gastric mucosa, but in this case hypergastrinemia is due to preexisting gastro-entero-pancreatic gastrinoma; type 2 gNENs are therefore associated with Zollinger-Ellison Syndrome (ZES), particularly in the context of multiple endocrine neoplasia type 1 (MEN-1) syndrome[6]. To date, there is no complete agreement among international guidelines regarding the timing of endoscopic surveillance of gNEN in patients diagnosed with gastrinoma[20-22]. Although approximately 10%-30% of cases are diagnosed at a metastatic stage, type 2 gNENs are relatively benign tumors[23], and therefore, the same therapeutic approach is taken as for type 1 gNENs[17,24], even if the definitive treatment is removal/treatment of primary gastrinoma; for this purpose, EUS is useful to detect the associated primary duodenal/ pancreatic lesion[16].
Type 3 gNENs, which account for approximately 14%-25% of all gNENs, are usually larger, sporadic single lesions, with a greater tendency to infiltrate and metastasize[14]. They are not associated with hypergastrinemia. Because of their aggressiveness, surgery represents the therapeutic strategy of choice, with total or subtotal gastrectomy together with lymphadenectomy being the standard treatment, as for gastric adenocarcinoma. ER may be a reasonable alternative only in selected cases of small (< 10 mm) G1/G2 (Ki-67 < 5%) type 3 gNENs that have been completely endoscopically resected (R0) and that have no risk factors for metastatic disease[25,26].
Different endoscopic techniques have been described to approach gNENs, and the majority of them proved to be radical[27]. Conventional approaches, such as polypectomy and traditional endoscopic mucosal resection (EMR) with mucosal lifting and hot snare resection, have recently been compared with new techniques, such as modified EMR, endoscopic submucosal dissection (ESD) and endoscopic full-thickness resection (EFTR), which are more invasive options, but with higher radicality rates. The rationale behind this shift trend towards new techniques lies in the increasingly clear evidence of the existence of well-differentiated gNENs that are already metastatic at the diagnosis.
This narrative review aims to describe in detail various proposed techniques for gNENs resection, even including latest technical tips.
This manuscript is a narrative review of the literature. We performed a systematic research in PubMed, Medline and Embase databases using the terms “gastric neuroendocrine neoplasms” and “endoscopy” or “endoscopic treatment”, and we selected original articles, with English written abstract available.
Epidemiologically, most detected gNENs lesions are < 10 mm in diameter[9], so that the most common and simple endoscopic treatment, especially when they are < 5 mm, is excisional biopsy, which has an overall diagnostic, staging, and therapeutic role[28]. For lesions > 5 mm, endoscopic treatment should be performed if a therapeutic goal R0 can be achieved, and it can be performed with polypectomy or with more technically demanding endoscopic procedures, such as EMR, ESD, or EFTR[29].
Cold snare polypectomy is a simple procedure in which the lesion is resected with a snare[29]. The endoscopist advances the snare sheath, opens the snare, and encircles the polyp; then, the nurse slowly closes the snare until the lesion is trimmed, capturing 1-2 mm of normal tissue around the polyp. This technique can be performed without lifting the polyp. However, cold snare polypectomy can be performed also with fluid injection into the submucosal layer (e.g., saline), to lift the gNEN and then cut with the snare using the same technique. In this second option, more normal tissue around the lesion can be captured to achieve a R0 resection. However, in this case, single-layer snares are preferable to conventional ones, because of their higher mechanical cutting power[30]. Cold snare polypectomy provides margins without coagulation artifacts[31]. Potential complications with this technique include bleeding, which is usually controlled by applying clips after the incision, or perforation, which is very rare[32,33].
Hot snare polypectomy is very similar to the cold snare technique[29], but in this case the snare not only cuts mechanically, but it also applies electrocoagulation when it is completely closed around the lesion. In this way, even larger lesions can be removed en bloc. Hot snare polypectomy is mostly used for lesions > 10 mm, pedunculated, or for flat lesions, which are actually very rare among NENs.
Traditional EMR: EMR is the technical term for the snare resection after an appropriate lifting of the lesion. There are many solutions that can be injected into the submucosal layer to obtain it; glycerol and saline solution are most used. For the resection of larger (> 10 mm) or flat lesions, EMR, as mentioned earlier, has a lower rate of incomplete resection, compared with cold or hot snare polypectomy[29]. The aim of EMR in gNENs is the en bloc R0 resection. However, although some studies show that EMR can achieve a high percentage of free resection margins in the smallest and most superficial lesions, conventional EMR sometimes cannot provide effective R0 resection, because many lesions already have submucosal involvement at the time of detection[9].
Anchored EMR: Anchored EMR is a very similar technique to conventional EMR: After lifting the submucosal layer, the endoscopist places the snare tip on the normal tissue surrounding the lesion and performs a small incision using the electrocoagulation. The tip of the snare is then inserted into the small incision and thus anchored into the tissue, and this allows the rest of the snare to open more stably around the lesion, better guaranteeing en bloc resection[34,35].
Cap band EMR: Cap band EMR is a technique mainly used for esophageal or cardial lesions[36]. After aspirating the lesion into the transparent cap of a band ligation set (DuetteTM Multi-band Mucosectomy Device®, Cook Medical, Bloomington, IN, United States), an elastic band is placed around the base of the lesion. Resection can then be performed with an appropriate snare closed below the mucosectomy band[37] (Figure 1D). The DuetteTM Multi-band Mucosectomy Device allows the en bloc EMR of small lesions. For larger lesions, this system allows only piece-meal resections, which limits the pathologist’s capability to evaluate the lateral margins[36].
A recent study compared traditional EMR with cap-band EMR for removal of gastric submucosal lesions, including some gNEN, and showed a similar en bloc resection rate, which was 97% for conventional saline- mucosectomy, and 100% for cap band mucosectomy technique[38].
Under-water EMR: Under-water EMR is performed without lifting the lesion with any solution, but using the ability of water to lift the lesion[39]. Filling the lumen with water, it allows the lesion to be lifted[40]. The complications are comparable to those of conventional EMR[39]. This technique has been shown to be more effective than traditional EMR for en bloc resection of colonic lesions[41], including rectal NENs[42]. However, to date, only a few cases of underwater EMR in gNENs have been described[43].
Overall, EMR is a safe, cost-effective, and technically simple procedure. However, its major limitation is associated to the size of the lesion, which often forces the endoscopist to perform a piece-meal resection, especially for lesions larger than 10 mm in diameter, with the risk of a lower rate of radical excision. According to recent studies, complete resection is achieved with EMR in 52%-84% of cases[44,45]. Nevertheless, there is limited evidence to date on the role of piece-meal resection in NENs. In a study that included 14 gNENs between 10-20 mm, treated with EMR, complete resection was not achieved in six cases. However, no recurrence occurred in any of them after 5-year follow-up[46]. Moreover, EMR often removes an amount of submucosal tissue insufficient to accurately define lymphatic vessel invasion, making an accurate histopathologic assessment impossible[46,47]. In addition, it should be considered that neuroendocrine tumors are usually not confined to the mucosa but they frequently invade the submucosal layer[48,49].
In case of incomplete resection, a second endoscopic procedure is more difficult due to fibrosis, with a higher risk of perforation. Hybrid techniques such as EMR/ESD or ESD alone, can better achieve R0 resection in larger lesions.
This technique, developed in Japan about 20 years ago for the endoscopic treatment of early gastric cancer, allows en bloc ER, regardless of tumor size, including the submucosal layer underneath the lesion, thus increasing the chance of histologically complete resection[50]. In addition, examination of a substantial amount of submucosal tissue allows accurate determination of lymphatic invasion and histologic grading, which may guide subsequent therapeutic decisions[47,51]. ESD is technically more demanding than EMR and it is associated with longer procedure times and higher risk of complications (bleeding and perforation). It consists of a delineating a circumferential excision zone around the lesion by using an electrocauterization knife, followed by the creation of a cushion under the lesion by the injecting of a viscous solution, and thus performing a dissection underneath the submucosal layer under direct visualization[46,52] (Figure 1D-G).
In 2012, an initial study by Chen et al[51] about the role of ESD in the management of gNENs examined 33 cases, including 22 type 1 and 11 type 3 gNENs. Histopathologic examination revealed a 100% complete resection rate, with horizontal and vertical negative margins and no lymphovascular invasions in all cases. Only one patient experienced delayed bleeding which could be controlled endoscopically, and no perforation was reported. Additional surgery was indicated for type 3 gNENs larger than 10 mm (7 cases), but only one patient agreed to undergo surgery. During a median follow-up of 28.9 months, two local recurrences occurred both of which were successfully treated by ESD. No lymph node metastases (LNM), or distant metastases were observed in any patient[51].
Two studies have examined the efficacy of ESD compared with EMR in the treatment of type 1 gNENs. The first was a small study of 13 lesions by Sato et al[53], that found a superiority of ESD in achieving complete resection with 100% negative horizontal and vertical margins, whereas positive vertical margins occurred in 66.7% of cases in the EMR group. A subsequent retrospective study by Kim et al[47] performed on 87 small lesions (< 10 mm in diameter) confirmed these results: The histological rate of complete resection was higher in the ESD group (94.9%) than in the EMR group (83.3%), mainly because the vertical margins were significantly less affected in patients who underwent ESD (2.6% vs 16.7%, P = 0.038). This is explained as EMR removes less submucosal tissue than ESD and for larger lesions only piece-meal resection is possible with higher risk of incomplete resection. Regarding safety, the bleeding rate was similar in both groups, but perforation occurred in one patient in the ESD group; all complications were successfully managed endoscopically[47]. Despite these findings, pooled data analysis of a recent systematic review by Panzuto et al[54] aiming at determining the best endoscopic technique (ESD, EMR, or polypectomy) in the management of type 1 gNENs did not show clear superiority of ESD over EMR in terms of efficacy and safety, with similar complete resection rates (97.4% and 92.3%, respectively) and complication rates (11.7% and 5.4%, respectively). Nevertheless, ESD demonstrated a lower risk of recurrence.
Regarding type 3 gNENs, studies reporting ESD are mainly focused on finding a proper indication for ER. In 2013, Kwon et al[23] retrospectively collected data from 50 patients with type 3 gNENs less than 20 mm in size, who were endoscopically treated by EMR (41 patients) or ESD (9 patients). Complete pathologic resection was achieved in 80.4% of all cases. ESD showed a lower complete resection rate than EMR (66.7% and 85.4%, respectively), probably due to larger average size of lesions in the ESD group. Lymphovascular invasion associated with larger tumor size was observed in 3 cases, although no statistical significance was found; all 3 patients subsequently underwent surgical resection. In the remaining patients, no local or distant recurrence was observed during the median follow-up period of 46 mo, even in the case of incomplete resection. This study concluded that ER should be considered as initial treatment for type 3 gNENs smaller than 20 mm and confined to the submucosal layer[23]. However, another South Korean study by Min et al[55] reported that type 3 G2 and G3 gNENs had aggressive features with frequent metastases regardless of tumor size and depth of invasion. In this study only one patient had a LNM 68 mo after a complete ESD of a type 3 G1 gNEN of 19 mm, so the authors suggested that only for type 3 G1 gNENs no larger than 15 mm surgical wedge resection or ER (EMR or ESD) can be considered as a valid option in the absence of lymphovascular invasion[55]. A 2020 Japanese multicenter retrospective study analyzed data from 144 patients with type 3 gNENs who underwent primary surgical (81) or ER (63 in total, 53 treated by ESD, 10 treated by EMR). In the second group, 15 patients required additional surgery because of lymphovascular invasion, positive vertical margin, and/or G2 grading; of the remaining patients only one developed LNM and liver metastases during a median follow-up of 32 mo. In this study, LNM occurred in 16.1% of cases and was observed in one patient with a 6 mm type 3 G1 gNEN. Given the risk of LNM, authors concluded that gastrectomy with lymph node dissection is recommended for all type 3 gNENs, even for small low grading tumors; however, given the overall and recurrence-free survival superior to 90%, ER for type 3 G1 gNENs ≤ 10 mm in size confined to submucosa could be an alternative therapeutic option despite the risk of LNM[56]. Conversely, Li et al[57] published a retrospective study reporting 33 ER (ESD and EMR) of G1-G2 type 3 gNENs, with no local recurrence, LNM or distant metastases during a median follow-up period of 36 mo, and concluded that ER is safe and effective for G1-G2 type 3 gNENs confined to the submucosa and smaller than 20 mm. However, as mentioned before, no one of these studies was aimed to demonstrate the efficacy of ESD in this setting or its superiority over EMR, and therefore further studies are needed. Furthermore, no randomized controlled trials comparing EMR and ESD in gNENs resection are to date available[58]. Data from a Chinese retrospective study analyzing efficacy and safety of different endoscopic techniques on any GI NEN, proved ESD to have a higher pathological complete resection rate compared to EMR[59].
EFTR, performed with the application of an over-the-scope-clip (OVESCO®, Tübingen, Germany), has been shown to be feasible, effective, and safe for small colorectal subepithelial tumors[60]. A multicenter retrospective study has shown that EFTR could be a rapid, effective, and safe alternative for the removal of rectal NEN < 20 mm[61]. Several studies investigated the role of EFTR in the management of gastric subepithelial tumors, but to date very few data are available on gNENs[62-67]. In the RESET trial, three gNENs with a size of < 15 mm were removed by using the gastric EFTR device, and R0 resection was obtained in all cases; no recurrence was detected at 3-mo follow up[67]. Anyway, further prospective, or controlled studies are needed to clarify whether EFTR has a standardized role in the treatment of gNEN.
Table 1 summarizes key information regarding the possible endoscopic therapeutic approaches for the different types of gNENs.
| Type 1 gNENs (any grade) | Type 2 gNENs (any grade) | Type 3 gNENs (G1) | Type 3 gNENs (G2, G3) | |
| Endoscopic presentation | Small, located in the gastric body or fundus, associated with CAAG | Small, multiple lesions, associated with gastrinoma (MEN1) | Larger, infiltrative, sporadic, single lesions | Larger, infiltrative, sporadic, single lesions |
| Risk of metastases | < 5% | 10%-30% | 50%-90% | 50%-90% |
| Suggested resection technique | < 5 mm: Endoscopic surveillance vs excisional biopsy | < 5 mm: Endoscopic surveillance vs excisional biopsy | < 5 mm: Excisional biopsy vs polypectomy | Surgery (regardless of the size) |
| 5-10 mm: Polypectomy vs EMR (traditional or modified) vs ESD (ESD lower risk of recurrence) | 5-10 mm: Polypectomy vs EMR (traditional or modified) vs ESD (ESD lower risk of recurrence) | 5-10 mm: Modified EMR vs ESD (no randomized trials) | ||
| > 10 mm: EUS (to make sure it is confined to the submucosal layer, without LNM) + modified EMR vs ESD (no randomized trials) | > 10 mm: EUS (to make sure it is confined to the submucosal layer, without LNM) + modified EMR vs ESD (no randomized trials) | > 10 mm: Surgery vs EUS + ESD (possible role of EFTR) |
Endoscopic surveillance after endoscopic treatment of gNENs has never been validated in prospective studies[68,69], so it is mainly based on histology. If resection margins are positive or indeterminate, the patient should undergo gastroscopy after 3-6 mo. If macroscopic residual disease is detected, a second and more aggressive endoscopic treatment is recommended. Otherwise, taking a biopsy from the scar is suggested[70].
After R0 ER of type 1 gNEN, follow-up with an upper GI endoscopy is recommended every 6-12 mo in the first three years, and annually thereafter; after ER of type 2 or 3 gNENs, annually follow-up is suggested[70]. According to an Italian prospective study, a specific timing has also been proposed for type 1 gNENs based on the tumor recurrence rate[71].
GNENs include different subtypes of neoplasms with distinct management and prognoses. After proper evaluation of size, site, morphology, and clinical context, different endoscopic techniques have been shown to be appropriate to treat GI localized neoplasms. To simplify, small lesions, especially when < 5 mm, can be radically resected by excisional biopsy or, if pedunculated, by polypectomy (cold or hot snare); > 5 mm type 1 and 2 (G1, G2, and G3) gNENs, and for type 3 (G1), if confined to the submucosal layer and without LNM or distant metastases, the therapeutic goal of R0 could be achieved by both modified EMR techniques (anchored, cap band and under-water EMR) and ESD; ESD might be preferred over EMR for larger lesions, > 10 mm in diameter, but no randomized controlled trials are yet available to confirm this. Larger type 3 G2/G3 gNENs should undergo surgery. Endoscopic ultrasound might achieve a more standardized role in the therapeutic diagram of gastric neuroendocrine lesions. Further randomized, controlled head-to-head studies with homogeneous and stratified patients are needed.
Pietro Invernizzi and Sara Massironi are members of the European Reference Network on Hepatological Diseases (ERN RARE LIVER), and they thank AMAF Monza ONLUS and AIRCS for the unrestricted research funding.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Neuroendocrine Tumor Society (ENETS), No. 699.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scopel M, Italy; Sun D, China; Yuan HJ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2484] [Article Influence: 310.5] [Reference Citation Analysis (4)] |
| 2. | Das S, Dasari A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr Oncol Rep. 2021;23:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 4. | Borbath I, Garcia-Carbonero R, Bikmukhametov D, Jimenez-Fonseca P, Castaño A, Barkmanova J, Sedlackova E, Kollár A, Christ E, Kaltsas G, Kos-Kudla B, Maasberg S, Verslype C, Pape UF. The European Neuroendocrine Tumour Society registry, a tool to assess the prognosis of neuroendocrine neoplasms. Eur J Cancer. 2022;168:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Xue L, Cai Y, Chen W, Chen S, Xue P. Clinical Spectrum and Endoscopic Treatment of Gastrointestinal Carcinoid Tumour. J Coll Physicians Surg Pak. 2022;32:1330-1333. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (8)] |
| 7. | Panzuto F, Campana D, Massironi S, Faggiano A, Rinzivillo M, Lamberti G, Sciola V, Lahner E, Manuzzi L, Colao A, Annibale B. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: A multicentre retrospective study. Dig Liver Dis. 2019;51:1456-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (11)] |
| 9. | Scherübl H, Cadiot G. Early Gastroenteropancreatic Neuroendocrine Tumors: Endoscopic Therapy and Surveillance. Visc Med. 2017;33:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rossi RE, Invernizzi P, Mazzaferro V, Massironi S. Response and relapse rates after treatment with long-acting somatostatin analogs in multifocal or recurrent type-1 gastric carcinoids: A systematic review and meta-analysis. United European Gastroenterol J. 2020;8:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Cavalcoli F, Zilli A, Conte D, Ciafardini C, Massironi S. Gastric neuroendocrine neoplasms and proton pump inhibitors: fact or coincidence? Scand J Gastroenterol. 2015;50:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 12. | Miceli E, Vanoli A, Lenti MV, Klersy C, Di Stefano M, Luinetti O, Caccia Dominioni C, Pisati M, Staiani M, Gentile A, Capuano F, Arpa G, Paulli M, Corazza GR, Di Sabatino A. Natural history of autoimmune atrophic gastritis: a prospective, single centre, long-term experience. Aliment Pharmacol Ther. 2019;50:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 14. | Putzer D, Schullian P, Jaschke W, Bale R. NEN: Advancement in Diagnosis and Minimally Invasive Therapy. Rofo. 2020;192:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Panzuto F, Massironi S, Partelli S, Campana D, Rinzivillo M, Invernizzi P, Andreasi V, Lamberti G, Falconi M. Gastro-entero-pancreatic neuroendocrine neoplasia: The rules for non-operative management. Surg Oncol. 2020;35:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | O'Toole D, Palazzo L. Endoscopy and Endoscopic Ultrasound in Assessing and Managing Neuroendocrine Neoplasms. Front Horm Res. 2015;44:88-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zilli A, Arcidiacono PG, Conte D, Massironi S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: lights and shadows. Dig Liver Dis. 2018;50:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 19. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 907] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 21. | Rydzewska G, Cichocki A, Ćwikła JB, Foltyn W, Hubalewska-Dydejczyk A, Kamiński G, Lewczuk A, Nasierowska-Guttmejer A, Nowakowska-Duława E, Pilch-Kowalczyk J, Sowa-Staszczak A, Kos-Kudła B; Consensus Conference; Polish Network of Neuroendocrine Tumours. Gastroduodenal neuroendocrine neoplasms including gastrinoma - management guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2013;64:444-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kaltsas G, Caplin M, Davies P, Ferone D, Garcia-Carbonero R, Grozinsky-Glasberg S, Hörsch D, Tiensuu Janson E, Kianmanesh R, Kos-Kudla B, Pavel M, Rinke A, Falconi M, de Herder WW; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology. 2017;105:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 26. | Massironi S, Campana D, Partelli S, Panzuto F, Rossi RE, Faggiano A, Brighi N, Falconi M, Rinzivillo M, Delle Fave G, Colao AM, Conte D. Heterogeneity of Duodenal Neuroendocrine Tumors: An Italian Multi-center Experience. Ann Surg Oncol. 2018;25:3200-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Massironi S, Conte D, Rossi RE. Somatostatin analogues in functioning gastroenteropancreatic neuroendocrine tumours: literature review, clinical recommendations and schedules. Scand J Gastroenterol. 2016;51:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Carvão J, Dinis-Ribeiro M, Pimentel-Nunes P, Libânio D. Neuroendocrine Tumors of the Gastrointestinal Tract: A Focused Review and Practical Approach for Gastroenterologists. GE Port J Gastroenterol. 2021;28:336-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 30. | Rutter MD, Jover R. Personalizing Polypectomy Techniques Based on Polyp Characteristics. Clin Gastroenterol Hepatol. 2020;18:2859-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | de Benito Sanz M, Hernández L, Garcia Martinez MI, Diez-Redondo P, Joao Matias D, Gonzalez-Santiago JM, Ibáñez M, Núñez Rodríguez MH, Cimavilla M, Tafur C, Mata L, Guardiola-Arévalo A, Feito J, García-Alonso FJ; POLIPEC HOT-COLD Study Group. Efficacy and safety of cold versus hot snare polypectomy for small (5-9 mm) colorectal polyps: a multicenter randomized controlled trial. Endoscopy. 2022;54:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Horiuchi A, Ikuse T, Tanaka N. Cold snare polypectomy: Indications, devices, techniques, outcomes and future. Dig Endosc. 2019;31:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Ortigão R, Weigt J, Afifi A, Libânio D. Cold versus hot polypectomy/endoscopic mucosal resection-A review of current evidence. United European Gastroenterol J. 2021;9:938-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Oh CK, Cho YS, Lee SH, Lee BI. Anchoring endoscopic mucosal resection versus conventional endoscopic mucosal resection for large nonpedunculated colorectal polyps: a randomized controlled trial. Endoscopy. 2023;55:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Pioche M, Wallenhorst T, Lepetit H, Lépilliez V, Rivory J, Legros R, Rostain F, Bianchi L, Charissoux A, Hervieu V, Moreno-Garcia M, Robinson P, Saurin JC, Ponchon T, Viprey M, Roche L, Subtil F, Jacques J. Endoscopic mucosal resection with anchoring of the snare tip: multicenter retrospective evaluation of effectiveness and safety. Endosc Int Open. 2019;7:E1496-E1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Alzoubaidi D, Graham D, Bassett P, Magee C, Everson M, Banks M, Novelli M, Jansen M, Lovat LB, Haidry R. Comparison of two multiband mucosectomy devices for endoscopic resection of Barrett's esophagus-related neoplasia. Surg Endosc. 2019;33:3665-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Soehendra N, Seewald S, Groth S, Omar S, Seitz U, Zhong Y, de Weerth A, Thonke F, Schroeder S. Use of modified multiband ligator facilitates circumferential EMR in Barrett's esophagus (with video). Gastrointest Endosc. 2006;63:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Karaca C, Daglilar ES, Soyer OM, Gulluoglu M, Brugge WR. Endoscopic submucosal resection of gastric subepithelial lesions smaller than 20 mm: a comparison of saline solution-assisted snare and cap band mucosectomy techniques. Gastrointest Endosc. 2017;85:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Nett A, Binmoeller K. Underwater Endoscopic Mucosal Resection. Gastrointest Endosc Clin N Am. 2019;29:659-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Spadaccini M, Fuccio L, Lamonaca L, Frazzoni L, Maselli R, Di Leo M, Galtieri PA, Craviotto V, D'Amico F, Hassan C, Repici A. Underwater EMR for colorectal lesions: a systematic review with meta-analysis (with video). Gastrointest Endosc. 2019;89:1109-1116.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Li P, Ma B, Gong S, Zhang X, Li W. Underwater endoscopic mucosal resection for colorectal lesions: a meta-analysis. Surg Endosc. 2021;35:3003-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, Massironi S. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol. 2022;28:1123-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (5)] |
| 43. | Kono Y, Sakae H, Okada H. Underwater endoscopic mucosal resection for gastric polyp. Dig Endosc. 2018;30:525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Zhong DD, Shao LM, Cai JT. Endoscopic mucosal resection vs endoscopic submucosal dissection for rectal carcinoid tumours: a systematic review and meta-analysis. Colorectal Dis. 2013;15:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Sivandzadeh GR, Ejtehadi F, Shoaee S, Aminlari L, Niknam R, Taghavi AR, Geramizadeh B, Hormati A, Safarpour AR, Bagheri Lankarani K. Endoscopic mucosal resection: still a reliable therapeutic option for gastrointestinal neuroendocrine tumors. BMC Gastroenterol. 2021;21:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014;2014:253860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 48. | Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 355] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 49. | Grozinsky-Glasberg S, Alexandraki KI, Angelousi A, Chatzellis E, Sougioultzis S, Kaltsas G. Gastric Carcinoids. Endocrinol Metab Clin North Am. 2018;47:645-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Chen WF, Zhou PH, Li QL, Xu MD, Yao LQ. Clinical impact of endoscopic submucosal dissection for gastric neuroendocrine tumors: a retrospective study from mainland China. ScientificWorldJournal. 2012;2012:869769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | de Mestier L, Lorenzo D, Fine C, Cros J, Hentic O, Walter T, Panis Y, Couvelard A, Cadiot G, Ruszniewski P. Endoscopic, transanal, laparoscopic, and transabdominal management of rectal neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2019;33:101293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | Sato Y, Takeuchi M, Hashimoto S, Mizuno K, Kobayashi M, Iwafuchi M, Narisawa R, Aoyagi Y. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013;60:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 54. | Panzuto F, Magi L, Esposito G, Rinzivillo M, Annibale B. Comparison of Endoscopic Techniques in the Management of Type I Gastric Neuroendocrine Neoplasia: A Systematic Review. Gastroenterol Res Pract. 2021;2021:6679397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Min BH, Hong M, Lee JH, Rhee PL, Sohn TS, Kim S, Kim KM, Kim JJ. Clinicopathological features and outcome of type 3 gastric neuroendocrine tumours. Br J Surg. 2018;105:1480-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Hirasawa T, Yamamoto N, Sano T. Is endoscopic resection appropriate for type 3 gastric neuroendocrine tumors? Dig Endosc. 2021;33:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Li YL, Qiu XD, Chen J, Zhang Y, Li J, Xu JM, Wang C, Qi ZR, Luo J, Tan HY. Clinicopathological characteristics and prognosis of 77 cases with type 3 gastric neuroendocrine tumours. World J Gastrointest Oncol. 2020;12:1416-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | O'Toole D, Kianmanesh R, Caplin M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology. 2016;103:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Sun W, Wu S, Han X, Yang C. Effectiveness of Endoscopic Treatment for Gastrointestinal Neuroendocrine Tumors: A Retrospective Study. Medicine (Baltimore). 2016;95:e3308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H, Neuhaus H, Albers D, Birk M, Thimme R, Probst A, Faehndrich M, Frieling T, Goetz M, Riecken B, Caca K. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67:1280-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (2)] |
| 61. | Meier B, Albrecht H, Wiedbrauck T, Schmidt A, Caca K. Full-thickness resection of neuroendocrine tumors in the rectum. Endoscopy. 2020;52:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356-3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015;47:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Kappelle WFW, Backes Y, Valk GD, Moons LMG, Vleggaar FP. Endoscopic full-thickness resection of gastric and duodenal subepithelial lesions using a new, flat-based over-the-scope clip. Surg Endosc. 2018;32:2839-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Meier B, Schmidt A, Glaser N, Meining A, Walter B, Wannhoff A, Riecken B, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors with the gFTRD-system: a prospective pilot study (RESET trial). Surg Endosc. 2020;34:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 69. | Hirai M, Matsumoto K, Ueyama H, Fukushima H, Murakami T, Sasaki H, Nagahara A, Yao T, Watanabe S. A case of neuroendocrine tumor G1 with unique histopathological growth progress. World J Gastrointest Endosc. 2013;5:605-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 71. | Merola E, Sbrozzi-Vanni A, Panzuto F, D'Ambra G, Di Giulio E, Pilozzi E, Capurso G, Lahner E, Bordi C, Annibale B, Delle Fave G. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012;95:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |