Published online Feb 16, 2023. doi: 10.4253/wjge.v15.i2.19
Peer-review started: September 13, 2022
First decision: October 30, 2022
Revised: November 19, 2022
Accepted: February 1, 2023
Article in press: February 1, 2023
Published online: February 16, 2023
Processing time: 152 Days and 20.9 Hours
Rectal neuroendocrine tumors (rNETs) measuring less than 10 mm in diameter are defined as small rNETs. Due to the low risk of distant invasion and metastasis, endoscopic treatments, including modified endoscopic mucosal resection, en
Core Tip: Rectal neuroendocrine tumors (rNETs) measuring less than 10 mm in diameter are defined as small rNETs. Due to the low risk of distant invasion and metastasis, endoscopic treatments, including modified endoscopic mucosal resection, endoscopic submucosal dissection, and other transanal surgical procedures, are effective. This review article proposes a follow-up plan according to the size and histopathology of the tumor after operation.
- Citation: Ma XX, Wang LS, Wang LL, Long T, Xu ZL. Endoscopic treatment and management of rectal neuroendocrine tumors less than 10 mm in diameter. World J Gastrointest Endosc 2023; 15(2): 19-31
- URL: https://www.wjgnet.com/1948-5190/full/v15/i2/19.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i2.19
Among different types of rectal tumors, neuroendocrine tumors (NETs) are relatively rare. However, the incidence of rectal NETs (rNETs) has been on the rise in recent years, accounting for approximately 48% of gastrointestinal NETs[1]. Due to the increasing popularity of colonoscopy, 85% to 95% of rNET cases are diagnosed in the early stage of the disease[2]. rNETs usually occur locally and have a relatively low risk of distant metastasis and a relatively high five-year survival rate[3]. The World Health Organization graded the NETs according to histology features[4]. However, no consensus has been reached on the best diagnostic, treatment, and management approaches. More research is needed to determine how to fully evaluate the rNET stage, select the best surgical approaches, predict the disease prognosis, and formulate the follow-up strategies. Preoperative assessment of the tumor size, depth of invasion, and presence of distance metastasis is extremely important for the diagnosis and treatment of rNETs.
The risk factors for rNET metastasis include the tumor size, invasion of the muscularis propria, pathological classification (Ki-67 index and mitoses), vascular infiltration, and atypical endoscopic findings[5,6]. Tumor size is the most important factor for predicting the risk of rNET metastasis[7]. rNETs measuring less than 10 mm in diameter are defined as small rNETs, which are relatively indolent tumors. Because of their low risk of distant metastasis, most small rNETs are confined to the mucosa and submucosa and rarely infiltrate into the muscularis propria. In addition, small rNETs rarely have distant lymph node metastasis and can be clinically cured by endoscopic treatment[8]. rNETs are usually found in early stage, and pelvic radiotherapy is not required after localized resection of rNETs[9]. To date, common treatments for rNETs include endoscopic treatment and other transanal surgical procedures, both of which completely remove the rNET locally[10,11]. The follow-up strategies are determined according to the disease prognosis and postoperative pathological evaluation.
This review article introduces the characteristics and keys for the preoperative evaluation of rNETs; compares the characteristics of existing treatment methods for rNETs less than 10 mm in diameter; and summarizes the disease prognosis, follow-up strategies, clinical diagnoses, treatments, and management of rNETs.
Pretreatment evaluation of rNETs is very important for the selection of surgical approaches and prognosis prediction. The main content of preoperative evaluation of rNETs includes tumor staging and classification, endoscopic ultrasonography (EUS), imaging examinations such as magnetic resonance imaging (MRI), and histological examinations.
rNETs usually secrete glucagon and enteroglucagon instead of serotonin; thus, rNETs rarely lead to neuroendocrine tumor syndrome and do not arouse early attention[12]. rNETs are usually revealed unintentionally during colonoscopy. Patients with rNETs may have certain symptoms, such as changes in bowel habits, blood in stools, tenesmus, anal pain, and weight loss[12]. The relevant guidelines of the United States and Europe argue that the staging and histological evaluation of rNET are the basic factors for predicting disease prognosis[13,14], with the consensus of dividing NETs into grades 1, 2, and 3 (G1, G2, and G3) based on mitotic figures and Ki67 index[4]. The European Neuroendocrine Tumor Society (ENETS) and the Union for International Cancer Control (IUCC)/American Joint Committee on Cancer (UICC/AJCC) have also proposed the TNM classification of rNETs[15].
Tumor stage and size are important predictors of lymph node metastasis and affect the disease prognosis. A tumor diameter of 10 mm is used as a cutoff value for the assessment of the rNET prognosis. Only 1% of rNETs smaller than 10 mm in diameter have distant metastasis, while the metastasis rate of rNETs larger than 2 cm in diameter is 60%[16]. Thus, tumor size nearly accurately predicts the prognosis of the disease and is strongly correlated with the prognosis and survival rate. Tsang et al[9] in a single-center study of 91 rNET cases over 13 years showed that patients with rNETs of less than 10 mm in diameter had a 2% distant metastasis rate, while a study by Soga et al[17] of 1271 rNET patients reported a 5.5% metastasis rate among patients with rNETs less than 10 mm in diameter.
The 2016 ENETS guidelines proposed that the size and the depth of invasion of an rNET can be used to predict lymph node metastasis. rNETs smaller than 10 mm in diameter have a 3% chance of lymph node metastasis[16]. Good histological characteristics of rNETs include a low grade (G1) and no ev
Small rNETs also have a risk of metastasis. Any suspected malignancy should be fully evaluated for infiltration depth and disease stage. The typical rNET is a small and smooth sessile tumor appearing normal or yellow in color with a submucosal bulge, which is usually approximately 5 cm from the anal verge. According to their morphology, rNETs can be divided into the following categories: Type Ia rNETs are protruding lesions with an angle between the tumor and the periphery of less than 90°; type Ib rNETs are protruding lesions with an angle between the tumor and the periphery of 90° to 150°; type II rNETs involve flat or slightly raised lesions with an angle of greater than 150°; and type III rNETs present a collapsed surface or ulcerated lesions. Type I lesions are the most common, especially subtype Ib lesions. Incomplete resection of type II and type III lesions is more likely to occur[22]. A meta-analysis showed that the endoscopic G1-stage of rNETs less than 16 mm in diameter involved no typical endoscopic characteristics (e.g., central depression, ulcer, semi-ulcer, erosion, ulcer, and hyperemia) and were confined to the submucosa without lymphatic vascular infiltration, showing a high complete resection rate and good long-term prognosis in patients[23].
Tumor size, TNM stage, lymph node metastasis, and tumor classification of rNETs are significantly correlated with recurrence and survival outcomes in patients[24]. More and more pathological markers have been used as predictors of rNET prognosis. With the development of new technologies, the extensive application of gene technology and sequencing technology may provide more information and predict the prognosis of patients with rNETs[9].
EUS, together with imaging examinations and colonoscopy, provides important information for the selection of rNET treatment options. EUS also judges the size and depth of the tumor. An rNET appears as a smooth, uniform, hypoechoic submucosal mass under EUS that protrudes on the third layer and is covered by the second layer but often blurred above it. The judgment of the size and depth of rNETs by experienced radiologists is usually highly consistent with the final histological evaluation. EUS can well assess rNETs by accurately evaluating the tumor size, depth of invasion, and presence of lymph node metastasis in the perirectal space[5].
EUS and MRI complement the assessment of rNETs. MRI can well identify rNET and assist in the tumor staging[25]. MRI is sensitive to the assessment of lymph nodes. However, it is relatively easy to miss T1-stage rNETs using MRI, while EUS can accurately distinguish T1- and T2-stage rNETs but can hardly evaluate T4-stage rNETs[26]. Computed tomography (CT) can assess the fat, fascia, and lymph nodes around the rectum, supplementing MRI, thereby facilitating the assessment of distant metastases of rNETs. Moreover, MRI is necessary for T2, T3, T4 and nodal-positive tumors[27], especially to assess the involvement of other pelvic structures and liver[5].
The ENETS guidelines suggest that all endoscopists should conduct at least one biopsy and one EUS before the surgical resection of rNETs and should choose the resection approach for rNETs based on the pathological diagnosis, tumor stage, and tumor classification. However, in clinical practice, pathologists often accidentally discover the NETs after routine polypectomy, and the selection of resection approaches is often affected by the experience of surgeons and the conditions of surgical equipment. By combining the assessment of rNET stage and classification as well as the application of EUS, MRI, pathological examinations, and other examinations, a comprehensive evaluation of rNET before surgery is essential for the selection of surgical approaches and the prediction of disease prognosis.
The only cure for rNETs less than 10 mm in diameter is to completely remove the tumor locally. A localized resection of an rNET refers to clean or complete resection of the local tumor, which is evaluated by histopathological examination when the lateral and vertical margins are negative. Selection of rNET treatment methods should be based on the comprehensive diagnostic evaluation as aforementioned, with the goal of achieving the best tumor resection, i.e., with a clear edge and no residual tumor tissue. Tumor size is the simplest indicator for the prediction of rNET prognosis and is thus often used as an important reference for the selection of treatment approaches for rNETs. Minimally invasive endoscopic treatments for rNETs less than 10 mm in diameter with no vascular invasion and distant metastasis can achieve clinically curative outcomes. The relevant guidelines reported previously also recommend that endoscopic local resection of rNETs be the first choice for rNET treatment[5]. rNETs less than 10 mm in diameter carry a lower risk of metastasis, and the tumors can be completely resected locally by endoscopy or other transanal surgical procedures[10,11,28]. A study has shown that, compared to ordinary polypectomy, advanced endoscopic or surgical procedures better achieve a pathologically complete response[29]. In clinical practice, small rNETs are difficult to distinguish quickly from rectal polyps when they are first discovered. The surgeons thus often choose to adopt ordinary endoscopic rectal polyp resection methods, such as biopsy forceps. However, researchers do not recommend the use of endoscopic biopsy clamps for the removal of rNETs because the histological characteristics of these tumors that affect the complete resection rate of the tumor are not accurately revealed through this approach, increasing the risk of postoperative residual and local recurrence[15]. Some researchers have suggested that any suspicious rNETs that cannot be confirmed for the first time should be marked under endoscopy to facilitate the search for these lesions before the next treatment and should be subjected to further treatment after confirming the results of a full evaluation. In summary, if the tumor size and mucosal and submucosal changes are confusing, further and full evaluation is needed instead of simply resection methods.
Selection of the best surgical approaches among the endoscopic and surgical resection techniques is still under heated debate even after the full evaluation and assuming the suspicious lesion is an rNET by preoperative EUS. Transanal resection of rNETs removes the tumor within 8 cm from the anal verge and ensures a deep removal in the muscularis mucosa. However, the risk of aggressive surgery, i.e., rectal anterior resection, when treating rNETs less than 10 mm in diameter is greater than the benefit. Transanal endoscopic microsurgery (TEMS) is a localized resection approach of the lesion under a laparoscopic view through the anus, with the advantage of direct and complete removal of the lesion without worrying about perforation, and the resection wound is fully and surgically sutured under direct vision[8]. A previous study has shown that TEMS achieves relatively great short-term and long-term prognoses for rNETs, and this surgical treatment when applied for small rNETs has a greater chance of retaining the anus[30]. However, TEMS needs to be performed in an operating room, thus carrying expensive fees for the operation and anesthesia. Intubation and anesthesia have a relatively large impact on patients. Most importantly, postoperative fecal incontinence may occur if the lesion is close to the anal margin[31], especially for small rNETs less than 10 mm in diameter because TEMS may cause adverse effects in patients. Therefore, the surgical indications of TEMS should be strictly controlled[30]. Endoscopic treatment does not require general anesthesia, and it can be carried out in daytime operating rooms or outpatient clinics to avoid the risks of intubation and anesthesia and can save time and medical costs, rendering it more easily acceptable by patients.
Some scholars have proposed that G1 rNETs revealed under endoscopy are usually less than 16 mm in diameter, without irregular endoscopic findings (e.g., central depression, ulcers, and congestion), and are limited to the submucosa without LVI, suggesting a relatively high rate of complete resection and a good prognosis. Thus, endoscopic treatment is suitable for G1 rNETs and leads to better postoperative life compared to general surgery. Small rNETs less than 10 mm in diameter are limited to the submucosa and have no lymph node or distant metastasis, and, therefore, endoscopic treatment is the first choice for their treatment[32,33]. Since the development of endoscopic technology, the main surgical procedures for lesion resection are endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). Traditional EMR is technically simple, but it seems difficult to guarantee complete resection for rNETs with this approach. Therefore, various device-assisted improved EMR techniques have been derived and may be compared to ESD.
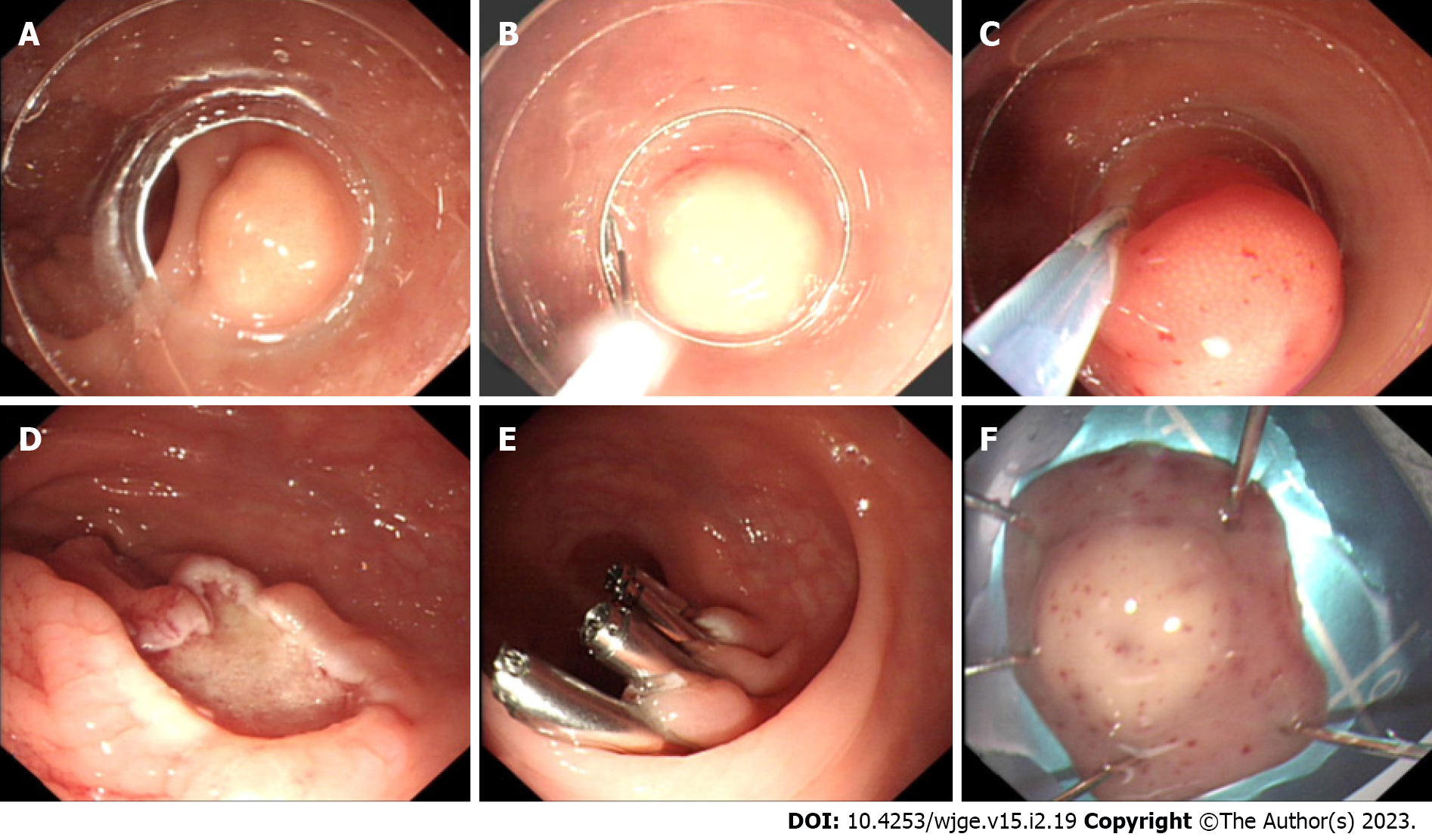
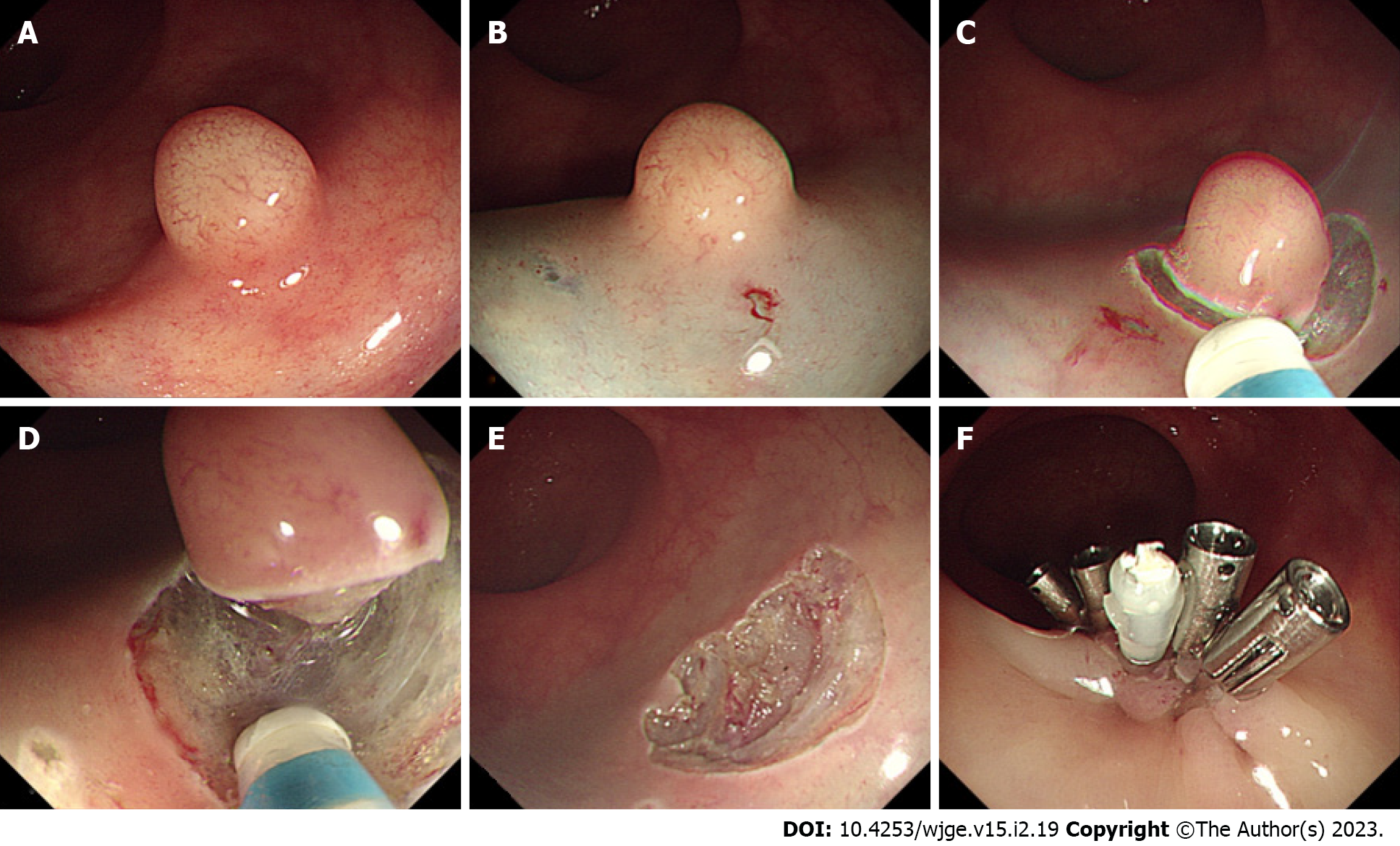
The main challenge of traditional EMR resection of rNETs is that the depth of vertical resection is not fully guaranteed, resulting in a positive vertical resection margin. Therefore, improved EMR is used to assist the device to fully attract and lift the lesion to ensure the depth of vertical resection. Cap-assisted EMR (EMR-C) (Figure 1), a transparent cap-assisted EMR approach, injects a water cushion under the tumor, i.e., placing a crescent snare in the transparent cap. After fully attracting the tumor to the transparent cap, the rNET is endoscopically removed using a snare, followed by clipping the wound with a hemostatic clip. EMR-C is ideal for relatively small rNETs[34]. Considering the effectiveness of treatment, operation duration, and surgical complications, a previous study has suggested that EMR-C may be the best endoscopic treatment for rNETs available[25]. The EMR-C procedure commonly used in our endoscopy center can also achieve a good resection effect for rNETs. However, further studies are needed to confirm whether the depth of the vertical resection margin is fully guaranteed when the water cushion is not injected before the resection.
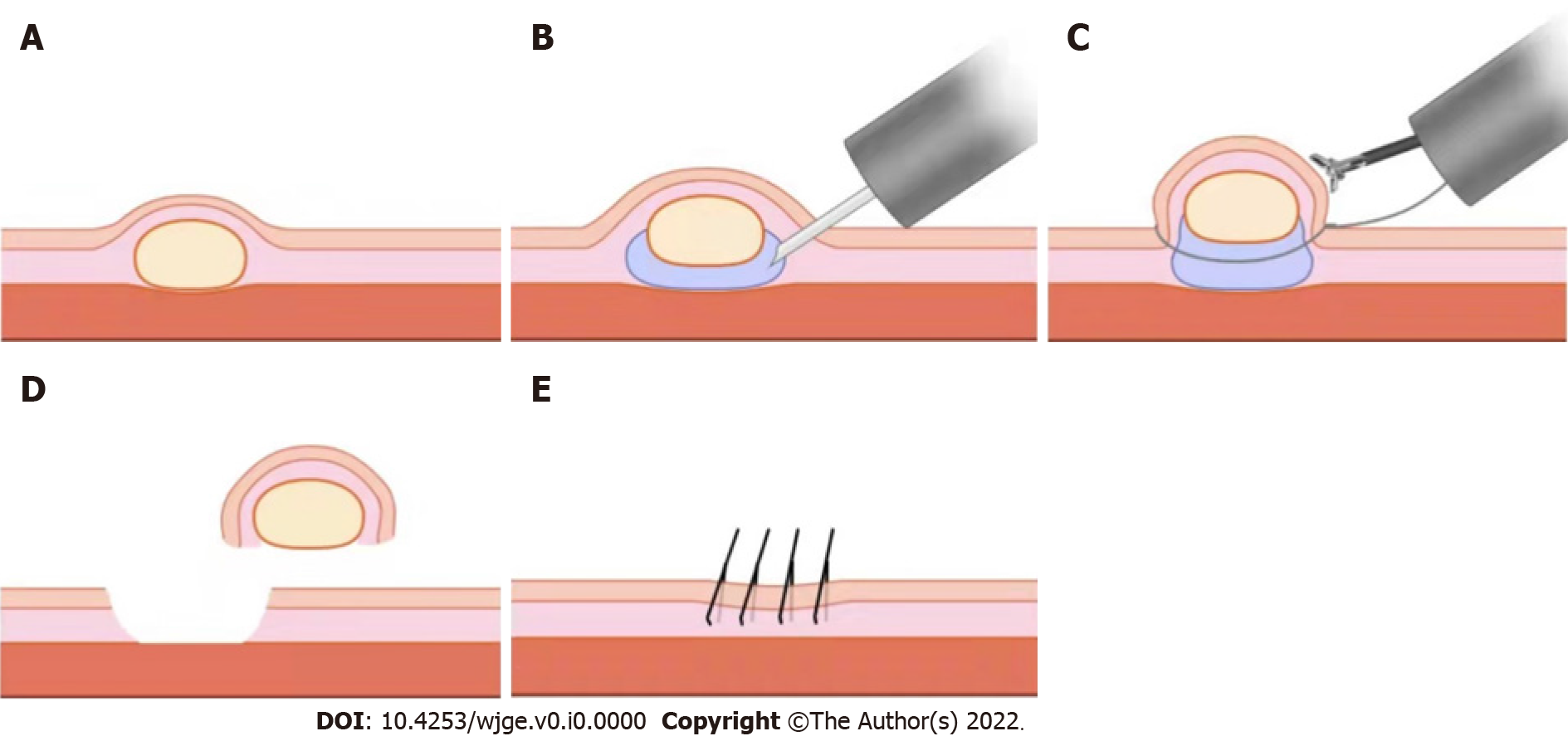
EMR using a dual-channel endoscope (EMR-D) is a simple, easy-to-learn, and effective technique, but it requires dual-instrument channel endoscopy[35], where one channel delivers the snare and the other channel delivers the forceps to lift the lesion before directly removing the lesion by the snare. The vertical depth of the resection can be fully ensured by the way of lifting[36] (Figure 2). Compared to ESD, EMR-D is technically simple, minimally invasive, and safer for the removal of small rNETs[36].
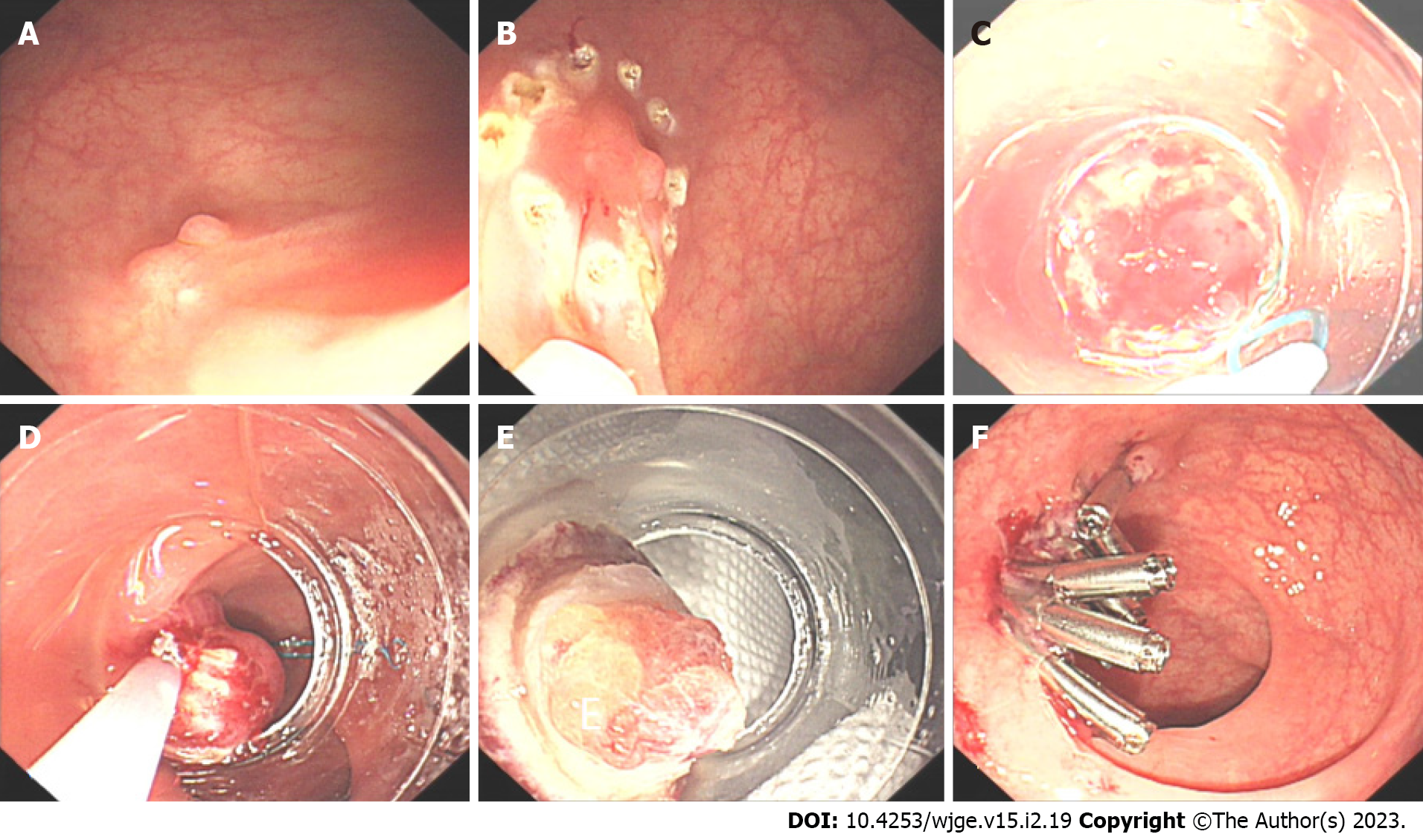
EMR with a ligation device (EMR-L) (Figure 3) improves the complete resection rate of rNETs[37-39]. Similarly, an injection is performed in the submucosa of the lesion to fully attract the tumor to the transparent cap before releasing the rubber ring from the ligation device to form a pseudo-polyp, which is followed by retracting the snare under the rubber ring, then electro-coagulating and resecting the tumor. Compared to traditional EMR, EMR-L more fully ensures the vertical depth of tumor resection due to the use of a snare and ligation device[40]. Traditional EMR is likely to cause incomplete resection of the lesion and crush the wound, which affects the pathological evaluation[35]. EMR-L improves these shortcomings of traditional EMR, resects without destroying or deforming the tumor, and moves the tumor further away from the lateral and vertical incisal margins[33]. In the treatment of initial lesions, when the tumor diameter is less than 5 mm and known to be an rNET, application of EMR technology, especially with the aid of a transparent cap or a ligature, usually achieves an ideal resection outcome[31].
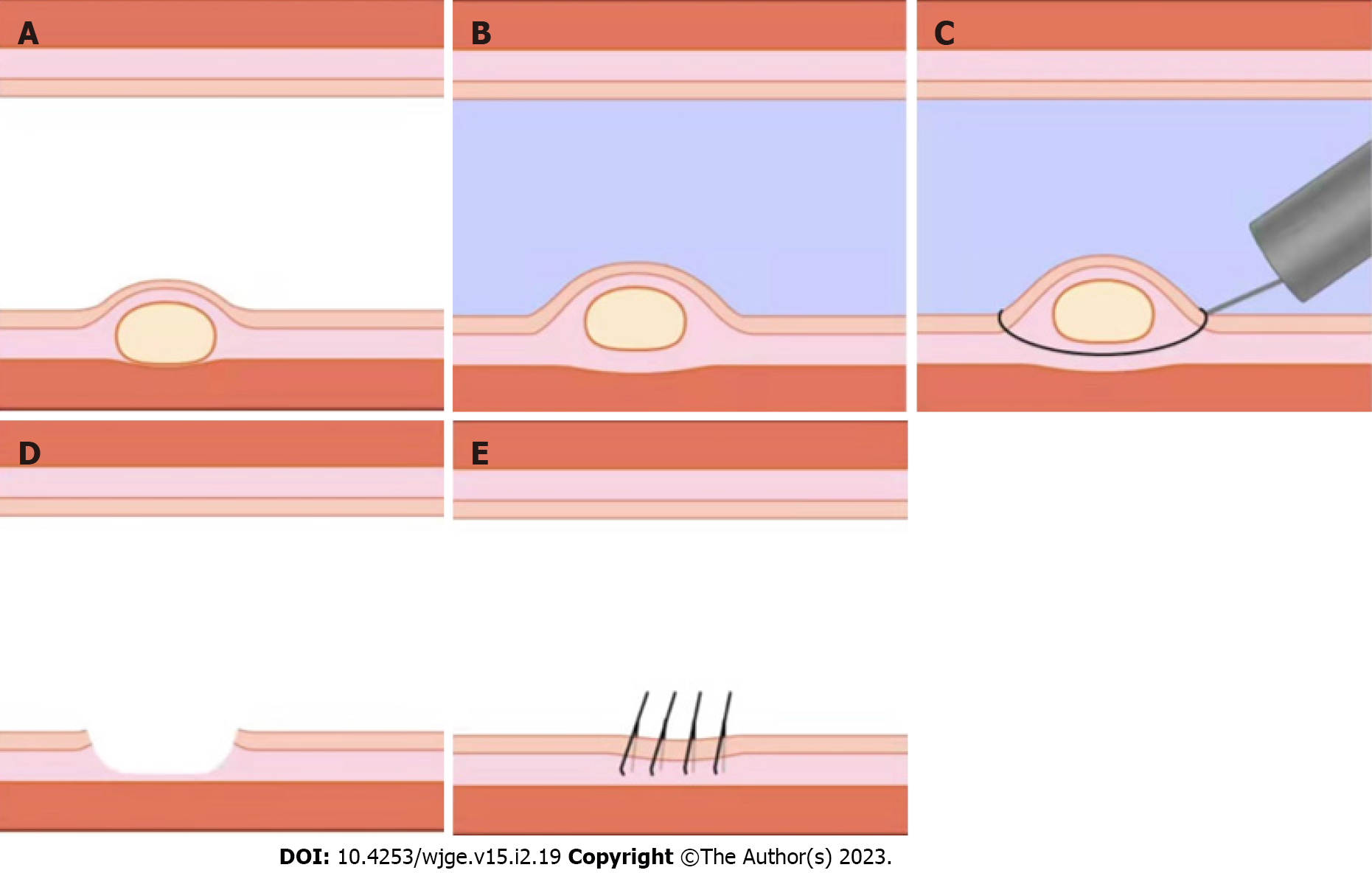
In addition, some scholars have proposed that underwater EMR (UEMR) (Figure 4) ensures a clean resection margin and safe removal of rNETs[32]. Here, the local intestinal tube is pumped and filled with water under endoscopy and without submucosal injection to float the tumor by the buoyancy of the water before electro-coagulating and resecting the tumor using a snare. However, some scholars have suggested that EMR electrocoagulation damages the edge of the specimen, which is not conducive to the judgment of the margin. Application of traction and magnification via underwater ESD may be better than UEMR[41,42].
ESD is commonly used for lesion resection, and it achieves radical treatment of local lesions, even in lesions involving the submucosa, retaining the muscle layer, i.e., preserving the local anatomy and function[43]. Patients with rNETs less than 10 mm in diameter have no lymph node or distant metastases, and a G1 rNET limited to the submucosa is an absolute indication for ESD[44] (Figure 5). The biological characteristics of rNETs are derived from lower crypts by growing deep into the submucosa, showing a subepithelial tumor–like growth pattern[33]. Due to the proximity to the muscularis propria, it is difficult to dissect the submucosa and is easy to result in a positive vertical margin[45]. Linked imaging mode is used to assist in identifying bleeding points during ESD surgery, and white light is used to avoid vascular damage[46]. For rNETs that are too small in size, it is challenging to use ESD to separate the submucosa from the muscularis propria. More approaches should be used, such as submucosal tunneling endoscopic resection, to improve the ESD resection of rNETs[33]. Some scholars have proposed that small rNETs can be removed by ESD using a pocketed-creation method with a hook knife to drill into the precut submucosa pocket of a transparent cap to expand the submucosa and finally complete the tumor resection[47].
Many studies have shown that there is no significant difference between modified EMR and ESD in the operation duration, en bloc resection, complete resection rate, complications, or recurrence rate[22,48]. ESD usually lasts for a long time and requires highly experienced surgeons for the operation. A meta-analysis suggested that EMR with attraction for the treatment of rNETs less than 10 mm in diameter achieves a higher complete resection rate, shorter operation duration, and similar complete resection and recurrence rates compared to ESD[49]. Compared to EMR, the recurrence rate of rNETs after ESD is lower, while the risk of perforation in rNET patients undergoing ESD is greater, and the requirements for the ESD operator are also higher[15]. Some investigators believe that the complete resection rate of rNETs by EMR-L is as high as that with ESD[45]. Some scholars carried out a retrospective study of rNET cases undergoing EMR-L and ESD resection and showed that these two types of surgery could be used to completely remove whole lesions in all cases, and the complete resection rate of EMR-L was higher than that of ESD, with the lateral and vertical resection margins being farther away from the tumor[33]. EMR-L also obtains a more sufficient distance of the vertical resection margin; further, it is easily performed and less time-consuming[50], and carries a lower risk of adverse events such as bleeding and perforation[45]. In addition, the incidence of low rectal perforation during EMR-L is lower. These findings suggest that EMR-L is more suitable for the treatment of rNETs than ESD. Comparison of different endoscopic and surgical techniques is listed in Table 1.
| Technique | Description | Advantages | Risks | Percentage of R0 resection and complication |
| Transanal resection | Removes the tumor at a higher position | Ensures a deep removal in the muscularis mucosa | For rNETs less than 10 mm, the risk is greater than the benefit | 96.8% R0 resection; urinary tract infection, subcutaneous emphysema, urinary tract infection[56] |
| Transanal endoscopic microsurgery (TEMS) | A localized resection under a laparoscopic view through the anus | Direct and complete removal of the lesion and the resection wound is fully and surgically sutured under direct vision | Expensive fees for the operation and anesthesia; postoperative fecal incontinence | 92.3% R0 resection, no complication[57] |
| Traditional EMR | Mucosal resection by electro-coagulation | Fast and convenient | Incomplete resection; crushed wound affects the pathological evaluation | 50% R0 resection, 7.1% complications[37] |
| Cap-assisted EMR (EMR-C) | Attracts the tumor to a cap and removes it using a crescent snare | Effective treatment, short operation duration | The depth of the vertical resection margin needs fully guaranteed | 94.1% R0 resection 8.8% intraprocedural bleeding[58] |
| Dual-channel endoscope (EMR-D) | One channel delivers the snare and the other delivers the forceps to lift the lesion | Simple, easy-to-learn, and effective; ensuring the vertical depth of the resection by lifting | Requires dual-instrument channel endoscopy | 86.3% R0 resection, minor bleeding (1/44)[36] |
| EMR with a ligation device (EMR-L) | Injection and rubber ring to form a pseudo-polyp, retracting the snare under it and resect the tumor | More fully ensures the vertical depth of tumor resection; resects without destroying or deforming the tumor | Inadequacy for large tumors | 89.5%[37], 99.4%[59], 86.2%[55] R0 resection, 0.6% perforation and 6.1% delayed bleeding[59] |
| Underwater EMR (UEMR) | To float the tumor by the buoyancy of the water without submucosal injection before electro-coagulating resection | Ensures a clean resection margin and safe removal of rNETs | Electrocoagulation damages the edge of the specimen | 83% R0 resection, no complication[60] |
| ESD | Submucosal dissection | Lower recurrence rate | Perforation and bleeding; lasts for a long time and requires highly experienced surgeons | 94.7%[37], 100%[47], 92%[50], 97%[55], 88.4%[36], 86.1%[32], 11.5% minor bleeding[36], 2.5% adverse events[32] |
Combined with preoperative evaluation, some scholars recommend that rNETs less than 5 mm in diameter and without irregular characteristics should be treated with modified EMR or ESD. EUS and MRI should be completed prior to ESD or surgery in cases of rNETs with irregular characteristics or measuring 5 mm to 2 cm in diameter to assess whether the lesion invades the muscularis propria or regional lymph nodes. MRI and CT or functional imaging should be completed to evaluate the presence of distant metastasis in cases with infiltration of the muscularis propria or local lymph node metastasis[51]. Hepatic or systemic treatment should be performed if the lesion has metastasized to a distant location. Surgical treatment should be performed if the lesion has no distant metastasis. The 2016 updated guidelines of ENETS recommended endoscopic resection of T1-stage (G1/G2) rNETs less than 10 mm in diameter. Pathological assessment of G1 Lesions should be re-examined 6 mo after incomplete resection of rNETs. Localized resection should be performed if necessary. A G2-stage tumor identified as such by pathological assessment should be completely resected locally again. For T2-stage (G1/G2) lesions, complete localized resection is recommended; TEMS should be considered if complete resection cannot be achieved. G3 Lesions with a tumor diameter of less than 10 mm are extremely rare and should be accessed by MRI/CT/positron emission tomography (PET) to confirm the presence of distant metastasis; those without metastasis should be subjected to rectal resection or TEMS and those with metastasis complicated by intestinal obstruction or bleeding that is difficult to control should be subjected to TEMS.
The operator can select the treatment method according to the conditions and characteristics of the center under the premise of fully evaluating the rNETs before the operation in accordance with the recommendations of the guidelines. More research and comparisons of different endoscopic treatment methods are necessary to select the best approach and to explore more innovative surgical methods.
In clinical practice, pathological examinations accidentally discover NETs in the lesion sometimes after routine polypectomy, and the selection of surgical procedures is often affected by the experience of surgeons and the conditions of surgical equipment[31]. No strong literature support is available for the requirement of a second salvage endoscopic treatment or surgical treatment for unexpectedly discovered rNETs, especially very small rNETs (≤ 5 mm in diameter)[52].
Existing ENETS guidelines propose different management approaches based on three parameters[16]: Tumor size, EUS stage (T and N), and the World Health Organization classification (G1/2 or G3). Eighty to ninety percent of rNETs are less than 10 mm in diameter and confined to the submucosa. Small rNETs are usually difficult to distinguish from hyperplastic polyps or adenomas and are easily removed by cryotherapy, even during a biopsy that may easily cause incomplete resection. In this circumstance, EMR may be a feasible approach for the removal of a single rectal lesion less than 5 mm in diameter and without high-risk manifestation. A previous study observed a residual rate of 22.6% in patients with incidentally removed rectal neuroendocrine neoplasms followed by locally remedial ESD; the residual rate of patients with rectal neuroendocrine neoplasms more than 3 mm in diameter was as high as 60% to 90%[31]. These data indicate that, even for very small rNETs, ordinary polypectomy still carries a higher risk of incomplete resection. It is recommended that patients with incomplete initial resection of rNETs undergo EMR or ESD for complete resection of the scar in the resection site[34].
Patients with postoperative pathology of rNETs showing positive margins should undergo EUS evaluation of the scar area before the second remedial operation, especially those with tumors measuring greater than 5 mm in diameter. EUS assesses the remaining submucosal tissues and lymph nodes, and pelvic MRI can be used as an aid for the evaluation. Remedial operations include EMR-C[53], ESD, or TEMS. However, the therapeutic outcome of ESD is affected by the scar tissue. Scarring changed the normal stratification of the intestinal wall, affecting the accuracy of EUS in evaluating the residual lesions in the operation site[31]. Thus, it has been suggested that remedial ESD should be performed when rNETs are greater than 3 mm in diameter, regardless of the tumor classification or the EUS manifestations on the scar[31]. Patients with incompletely resected rNETs less than 10 mm in diameter and without obvious evidence of residual disease are recommended to undergo monitoring by EUS every 6 mo for two years[15].
In a previous study, pathological evaluations revealed LVI in more than 25% of small rNET specimens, and the evaluation indicator for LVI is required to be more accurate. The incidence of postoperative LVI in rNET cases might be even higher, but it did not affect the short-term prognosis so far[54]. The Guidelines of the North American Neuroendocrine Tumor Society indicate that the rate of lymph node metastasis of rNETs less than 10 mm in diameter is very low, while this review article discussed a certain probability of lymph node metastasis even in these small rNETs. These differences may be linked to the frequent additional remedial operations performed in Japan and the implementation of CT alone for the evaluation of lymph node conditions in Western countries. The latter approach lowers the sensitivity of the evaluation of lymph node metastasis. Another study has shown that patients with rNETs less than 6 mm in diameter have a 0% lymph node metastasis rate[55], which may also be related to the insufficient sample size of the study. Further discussion is needed for the risk assessment and follow-up of postoperative lymph node metastasis in patients with small rNETs. Distant metastasis of rNETs often occurs in the liver and requires systemic assessment and multidisciplinary collaboration. A reduction in local bleeding during rNET resection to improve the symptoms of intestinal obstruction should be performed in the case of distant metastasis.
Postoperative follow-up strategies for patients with rNETs are mainly chosen based on the tumor size, pathological classification, overall tumor stage, and lymphatic metastasis[15]. Patients with complete resection of rNET are still recommended to undergo colonoscopy and CT within one year after surgery. rNET patients with positive lateral or vertical margins are required to undergo additional surgery and local lymph node dissection. For those who refuse to receive additional surgery, colonoscopy, chest imaging, and abdominal CT findings must be reviewed every year. An endoscopic biopsy is required in those patients with residual tumors revealed on the postoperative scar during the colonoscopy.
The guidelines further clarify that patients with complete resection of G1/G2 rNETs less than 10 mm in diameter and without lymph node metastasis or invasion of the muscularis propria should be considered to be at low risk of recurrence and recommend no routine follow-up[5]. Patients with G3 rNETs less than 10 mm in diameter have an increased risk of recurrence and should be reviewed by colonoscopy at least once a year for five years[15] and followed for adenomatous polyps. EUS, colonoscopy, and MRI should also be included in the follow-up plan. Patients with incomplete resection of rNETs less than 10 mm in diameter are subjected to pathological examinations, and those who have no obvious residual lesions should be reviewed and evaluated by EUS every 6 mo for two years. The scar area after EMR-C or ESD is recommended to be resected in an extensive manner, and histological evaluation should be repeated[15].
Endoscopic resection is recommended for rNETs less than 10 mm with no risk of recurrence. While surgery is suggested for tumors larger than 20 mm or with depression appearing in the tumor center regardless of tumor size. For rNETs with a diameter between 10 mm to 20 mm, options should be made according to the risk of metastasis and the patient's personal choice[7,10].
Although rNETs less than 10 mm in diameter have a low risk of metastasis, complete resection and adequate prognostic evaluation are required for the development of follow-up plans. Endoscopic and surgical procedures for these cases can achieve relatively good curative effects. The application of endoscopic treatment for patients with small rNETs also achieves more beneficial outcomes. The curative rate is high by the effort of the experts. With the continuous innovation and development of endoscopic technology, we look forward to more surgical procedures to perfect the treatment. Multicenter, large-sample studies should be carried out to provide sufficient evidence for the selection of the best surgical procedure. The follow-up of patients based on disease prognosis and postoperative evaluation helps to detect disease recurrence in time and improve their quality of life.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Okasha H, Egypt; Pattarajierapan S, Thailand S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Kim J, Kim JH, Lee JY, Chun J, Im JP, Kim JS. Clinical outcomes of endoscopic mucosal resection for rectal neuroendocrine tumor. BMC Gastroenterol. 2018;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Scherübl H, Cadiot G. Early Gastroenteropancreatic Neuroendocrine Tumors: Endoscopic Therapy and Surveillance. Visc Med. 2017;33:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1851] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 4. | Gonzalez RS. Diagnosis and Management of Gastrointestinal Neuroendocrine Neoplasms. Surg Pathol Clin. 2020;13:377-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Xu G, Wang P, Xiao Y, Wu X, Lin G. Local resection of rectal neuroendocrine tumor with first clinical manifestation of giant liver metastasis by transanal endoscopic microsurgery: A case report. Medicine (Baltimore). 2017;96:e9153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Tsang ES, McConnell YJ, Schaeffer DF, Yin Y, Speers CH, Kennecke HF. Prognostic Factors for Locoregional Recurrence in Neuroendocrine Tumors of the Rectum. Dis Colon Rectum. 2018;61:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kwaan MR, Goldberg JE, Bleday R. Rectal carcinoid tumors: review of results after endoscopic and surgical therapy. Arch Surg. 2008;143:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Onozato Y, Kakizaki S, Iizuka H, Sohara N, Mori M, Itoh H. Endoscopic treatment of rectal carcinoid tumors. Dis Colon Rectum. 2010;53:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Shebani KO, Souba WW, Finkelstein DM, Stark PC, Elgadi KM, Tanabe KK, Ott MJ. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815-21; discussion 822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Fahy BN, Tang LH, Klimstra D, Wong WD, Guillem JG, Paty PB, Temple LK, Shia J, Weiser MR. Carcinoid of the rectum risk stratification (CaRRs): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:1735-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 2013;98:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 16. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Shimizu T, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Growth characteristics of rectal carcinoid tumors. Oncology. 2000;59:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Jann H, Roll S, Couvelard A, Hentic O, Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski P, Wiedenmann B, Pape UF. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 20. | Park CH, Cheon JH, Kim JO, Shin JE, Jang BI, Shin SJ, Jeen YT, Lee SH, Ji JS, Han DS, Jung SA, Park DI, Baek IH, Kim SH, Chang DK. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Chida K, Watanabe J, Hirasawa K, Inayama Y, Misumi T, Kunisaki C, Endo I. A novel risk-scoring system for predicting lymph node metastasis of rectal neuroendocrine tumors. Ann Gastroenterol Surg. 2020;4:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Wang XY, Chai NL, Linghu EQ, Qiu ST, Li LS, Zou JL, Xiang JY, Li XX. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol. 2020;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Shim KN, Yang SK, Myung SJ, Chang HS, Jung SA, Choe JW, Lee YJ, Byeon JS, Lee JH, Jung HY, Hong WS, Kim JH, Min YI, Kim JC, Kim JS. Atypical endoscopic features of rectal carcinoids. Endoscopy. 2004;36:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Sohn B, Kwon Y, Ryoo SB, Song I, Kwon YH, Lee DW, Moon SH, Park JW, Jeong SY, Park KJ. Predictive Factors for Lymph Node Metastasis and Prognostic Factors for Survival in Rectal Neuroendocrine Tumors. J Gastrointest Surg. 2017;21:2066-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Zhao ZF, Zhang N, Ma SR, Yang Z, Han X, Zhao YF, Gao F, Gong ZJ, Yang L. A comparative study on endoscopy treatment in rectal carcinoid tumors. Surg Laparosc Endosc Percutan Tech. 2012;22:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, Pagés M, Cuatrecasas M, Pellisé M, Maurel J, Ayuso-Colella C, González-Suárez B, Llach J, Castells A, Ginès A. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G; MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 28. | McDermott FD, Heeney A, Courtney D, Mohan H, Winter D. Rectal carcinoids: a systematic review. Surg Endosc. 2014;28:2020-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Son HJ, Sohn DK, Hong CW, Han KS, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis. 2013;28:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Takatsu Y, Fukunaga Y, Nagasaki T, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Ueno M. Short- and Long-term Outcomes of Laparoscopic Total Mesenteric Excision for Neuroendocrine Tumors of the Rectum. Dis Colon Rectum. 2017;60:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Pagano N, Ricci C, Brighi N, Ingaldi C, Pugliese F, Santini D, Campana D, Mosconi C, Ambrosini V, Casadei R. Incidental diagnosis of very small rectal neuroendocrine neoplasms: when should endoscopic submucosal dissection be performed? Endocrine. 2019;65:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Park SS, Han KS, Kim B, Chang Kim B, Hong CW, Sohn DK, Chang HJ. Comparison of underwater endoscopic mucosal resection and endoscopic submucosal dissection of rectal neuroendocrine tumors (with videos). Gastrointest Endosc. 2020;91:1164-1171.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Lim HK, Lee SJ, Baek DH, Park DY, Lee BE, Park EY, Park JW, Kim GH, Song GA. Resectability of Rectal Neuroendocrine Tumors Using Endoscopic Mucosal Resection with a Ligation Band Device and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2019;2019:8425157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Stier MW, Chapman CG, Shamah S, Donboli K, Yassan L, Waxman I, Siddiqui UD. Endoscopic resection is more effective than biopsy or EUS to detect residual rectal neuroendocrine tumor. Endosc Int Open. 2021;9:E4-E8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Iishi H, Tatsuta M, Yano H, Narahara H, Iseki K, Ishiguro S. More effective endoscopic resection with a two-channel colonoscope for carcinoid tumors of the rectum. Dis Colon Rectum. 1996;39:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Lee WH, Kim SW, Lim CH, Kim JS, Cho YK, Lee IS, Choi MG, Choi KY. Efficacy of endoscopic mucosal resection using a dual-channel endoscope compared with endoscopic submucosal dissection in the treatment of rectal neuroendocrine tumors. Surg Endosc. 2013;27:4313-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Ebi M, Nakagawa S, Yamaguchi Y, Tamura Y, Izawa S, Hijikata Y, Shimura T, Funaki Y, Ogasawara N, Sasaki M, Joh T, Kasugai K. Endoscopic submucosal resection with an endoscopic variceal ligation device for the treatment of rectal neuroendocrine tumors. Int J Colorectal Dis. 2018;33:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Jeon JH, Cheung DY, Lee SJ, Kim HJ, Kim HK, Cho HJ, Lee IK, Kim JI, Park SH, Kim JK. Endoscopic resection yields reliable outcomes for small rectal neuroendocrine tumors. Dig Endosc. 2014;26:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Berkelhammer C, Jasper I, Kirvaitis E, Schreiber S, Hamilton J, Walloch J. "Band-snare" resection of small rectal carcinoid tumors. Gastrointest Endosc. 1999;50:582-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Mashimo Y, Matsuda T, Uraoka T, Saito Y, Sano Y, Fu K, Kozu T, Ono A, Fujii T, Saito D. Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol. 2008;23:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Liu S, Chai N, Linghu E. Underwater EMR or endoscopic submucosal dissection for rectal neuroendocrine tumors: What are the advantages? Gastrointest Endosc. 2020;92:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Yoshii S, Hayashi Y, Matsui T, Aoi K, Tsujii Y, Iijima H, Takehara T. "Underwater" endoscopic submucosal dissection: a novel technique for complete resection of a rectal neuroendocrine tumor. Endoscopy. 2016;48 Suppl 1:E67-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Nakamura F, Saito Y, Haruyama S, Sekiguchi M, Yamada M, Sakamoto T, Nakajima T, Yamamoto S, Murakami Y, Ishikawa H, Matsuda T. Short-term Prospective Questionnaire Study of Early Postoperative Quality of Life After Colorectal Endoscopic Submucosal Dissection. Dig Dis Sci. 2017;62:3325-3335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Chen X, Li B, Wang S, Yang B, Zhu L, Ma S, Wu J, He Q, Zhao J, Zheng Z, Li S, Wang T, Liang L. Efficacy and safety of endoscopic submucosal dissection for gastrointestinal neuroendocrine tumors: a 10-year data analysis of Northern China. Scand J Gastroenterol. 2019;54:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013;47:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Sun XT, Min M, Bi YL, Xu Y, Liu Y. Endoscopic Submucosal Dissection of a Rectal Neuroendocrine Tumor Using Linked Color Imaging Technique. Chin Med J (Engl). 2017;130:1127-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Hamada Y, Tanaka K, Hattori A, Umeda Y, Yukimoto H, Yamada R, Nakamura M, Miura H, Tsuboi J, Katsurahara M, Horiki N, Takei Y. Clinical utility of endoscopic submucosal dissection using the pocket-creation method with a HookKnife and preoperative evaluation by endoscopic ultrasonography for the treatment of rectal neuroendocrine tumors. Surg Endosc. 2022;36:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | He L, Deng T, Luo H. Efficacy and safety of endoscopic resection therapies for rectal carcinoid tumors: a meta-analysis. Yonsei Med J. 2015;56:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Pan J, Zhang X, Shi Y, Pei Q. Endoscopic mucosal resection with suction vs. endoscopic submucosal dissection for small rectal neuroendocrine tumors: a meta-analysis. Scand J Gastroenterol. 2018;53:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Matsuno K, Miyamoto H, Kitada H, Yoshimatsu S, Tamura F, Sakurai K, Fukubayashi K, Shono T, Setoyama H, Matsuyama T, Suko S, Narita R, Honda M, Tateyama M, Naoe H, Morinaga J, Tanaka Y, Gushima R. Comparison of endoscopic submucosal resection with ligation and endoscopic submucosal dissection for small rectal neuroendocrine tumors: A multicenter retrospective study. DEN Open. 2023;3:e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab. 2007;21:43-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Wu J, Srirajaskanthan R, Ramage J. Rectal neuroendocrine tumor. Dig Endosc. 2014;26:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Jeon SM, Lee JH, Hong SP, Kim TI, Kim WH, Cheon JH. Feasibility of salvage endoscopic mucosal resection by using a cap for remnant rectal carcinoids after primary EMR. Gastrointest Endosc. 2011;73:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Kwon MJ, Kang HS, Soh JS, Lim H, Kim JH, Park CK, Park HR, Nam ES. Lymphovascular invasion in more than one-quarter of small rectal neuroendocrine tumors. World J Gastroenterol. 2016;22:9400-9410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Inada Y, Yoshida N, Fukumoto K, Hirose R, Inoue K, Dohi O, Murakami T, Ogiso K, Tomie A, Kugai M, Yoriki H, Inagaki Y, Hasegawa D, Okuda K, Okuda T, Morinaga Y, Kishimoto M, Itoh Y. Risk of lymph node metastasis after endoscopic treatment for rectal NETs 10 mm or less. Int J Colorectal Dis. 2021;36:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Quaresima S, Balla A, Franceschilli L, La Torre M, Iafrate C, Shalaby M, Di Lorenzo N, Sileri P. Transanal Minimally Invasive Surgery for Rectal Lesions. JSLS. 2016;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Brand M, Reimer S, Reibetanz J, Flemming S, Kornmann M, Meining A. Endoscopic full thickness resection vs. transanal endoscopic microsurgery for local treatment of rectal neuroendocrine tumors - a retrospective analysis. Int J Colorectal Dis. 2021;36:971-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-1022; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Takita M, Sakai E, Nakao T, Kimoto Y, Ishii R, Konishi T, Ueno S, Kanda K, Negishi R, Muramoto T, Hashimoto H, Morikawa T, Matsuhashi N, Ohata K. Clinical Outcomes of Patients with Small Rectal Neuroendocrine Tumors Treated Using Endoscopic Submucosal Resection with a Ligation Device. Digestion. 2019;99:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Yamashina T, Tumura T, Maruo T, Matsumae T, Yoshida H, Tanke G, Taki M, Fukuhara M, Kimura Y, Sakamoto A, Henmi S, Sawai Y, Saito S, Nishijima N, Nasu A, Komekado H, Asada M, Kita R, Kimura T, Osaki Y. Underwater endoscopic mucosal resection: a new endoscopic method for resection of rectal neuroendocrine tumor grade 1 (carcinoid) ≤ 10 mm in diameter. Endosc Int Open. 2018;6:E111-E114. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |