Published online Feb 16, 2022. doi: 10.4253/wjge.v14.i2.77
Peer-review started: March 20, 2021
First decision: July 17, 2021
Revised: July 31, 2021
Accepted: January 13, 2022
Article in press: January 13, 2022
Published online: February 16, 2022
Processing time: 326 Days and 18.9 Hours
Exposed endoscopic full-thickness resection (EFTR), with or without laparoscopic assistance, is an emergent natural orifice transluminal endoscopic surgery technique with promising safety and efficacy for the management of gastro
Core Tip: Exposed endoscopic full-thickness resection (EFTR) is a promising minimally invasive alternative to surgery for the removal of gastrointestinal submucosal tumors (SMTs) originating from the muscularis propria. To date, evidence concerning duodenal exposed EFTR is lacking, mainly due to both the technical difficulty and concerns about an effective closure of the transmural defect. However, given the non-negligible morbidity and mortality associated with duodenal surgery, the recent availability of dedicated endoscopic devices able to achieve a full-thickness defect closure could help in overcoming these concerns. Our study aimed to review the current evidence regarding exposed EFTR for deep duodenal SMTs.
- Citation: Granata A, Martino A, Zito FP, Ligresti D, Amata M, Lombardi G, Traina M. Exposed endoscopic full-thickness resection for duodenal submucosal tumors: Current status and future perspectives. World J Gastrointest Endosc 2022; 14(2): 77-84
- URL: https://www.wjgnet.com/1948-5190/full/v14/i2/77.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i2.77
Though relatively infrequent, the diagnosis of duodenal submucosal tumors (D-SMTs) has increased due to the widespread use of gastrointestinal endoscopy[1,2]. D-SMTs originating from the submucosa and from the muscularis propria (MP) include lesions with malignant potential, such as gastrointestinal stromal tumors (GISTs) and neuroendocrine tumors (NETs)[3,4].
According to current guidelines, either suspected or histologically proven GISTs larger than 20 mm in diameter or with high-risk endoscopic ultrasonography (EUS) features (i.e., irregular borders, cystic spaces, ulcerations, echogenic foci and heterogeneity) should be removed with histologically negative margins. Given the limited intramural extension of GISTs and their rare lymph node involvement, surgical local resection without additional lymphadenectomy is currently regarded as the gold standard of treatment[4-6]. Furthermore, resection of gastric NETs ≥ 10 mm in diameter is recommended, while all duodenal NETs should be excised, regardless of their size[7]. However, traditional duodenal surgery, such as open pancreaticoduodenectomy (PD), carries a significantly higher risk of morbidity and mortality compared to that for other gastrointestinal (GI) sites[8]. Moreover, various types of laparoscopic limited resection of the duodenum have been reported, including laparoscopic wedge resection, laparoscopic and endoscopic cooperative surgery, and laparoscopic segmental duodenectomy[9,10]. Though less invasive, they are technically challenging due to the retroperitoneal anatomical location of the duodenum and its intimate relationship with the pancreas, ampulla of Vater, and distal common bile duct. Thus, conversion to PD may be required[11].
In this setting, endoscopy may offer the chance for a minimally invasive curative approach for D-SMTs. Safe and effective removal of small D-SMT without invol
The aim of our study was to review the current evidence concerning exposed EFTR with or without laparoscopic assistance for the treatment of MP-originating D-SMTs.
A literature search by using PubMed (MEDLINE) and EMBASE for the period January 1998 (the year EFTR was first reported) to February 2021 was undertaken in order to identify relevant studies on duodenal ESD-assisted exposed EFTR, with or without laparoscopic assistance. The search strategy usedthe following terms: "Endoscopic full-thickness resection," “EFTR,” “exposed endoscopic full-thickness resection,” “laparoscopy assisted endoscopic full-thickness resection,” and “LAEFR.” The literature search was limited to human studies and English language. Meeting abstracts were excluded. Articles reporting on both LECS procedures, in which tumor resection is mainly performed surgically, and non ESD-assisted EFTR were also excluded from the current review. The references of review articles and relevant papers were hand-searched to identify any additional studies.
Exposed EFTR is a “cut then close” technique carrying out full-thickness excision with the creation of an intentionalperforation, followed by wall defect suture. Thus, the term “exposed” is derived from the temporary peritoneal exposure to the GI contents[19].
The exposed EFTR technique was first described by Ikeda et al[20] in a porcine stomach in 2006[20], and finally translated into clinical practice by Zhou et al[21] a few years later[21]. The principal procedures of ESD-assisted exposed EFTR are as follows[4]: (1) Circumferential mucosal and submucosal incision around the lesion by means of typical ESD technique; (2) Muscular and serosal incision, pursuing an active perforation; and (3) Endoscopic closure of the resulting transmural wall defect. Alternatively, post-EFTR defect closure by means of laparoscopic hand-suturing has been reported in the laparoscopy-assisted endoscopic full-thickness resection (LAEFR)[22].
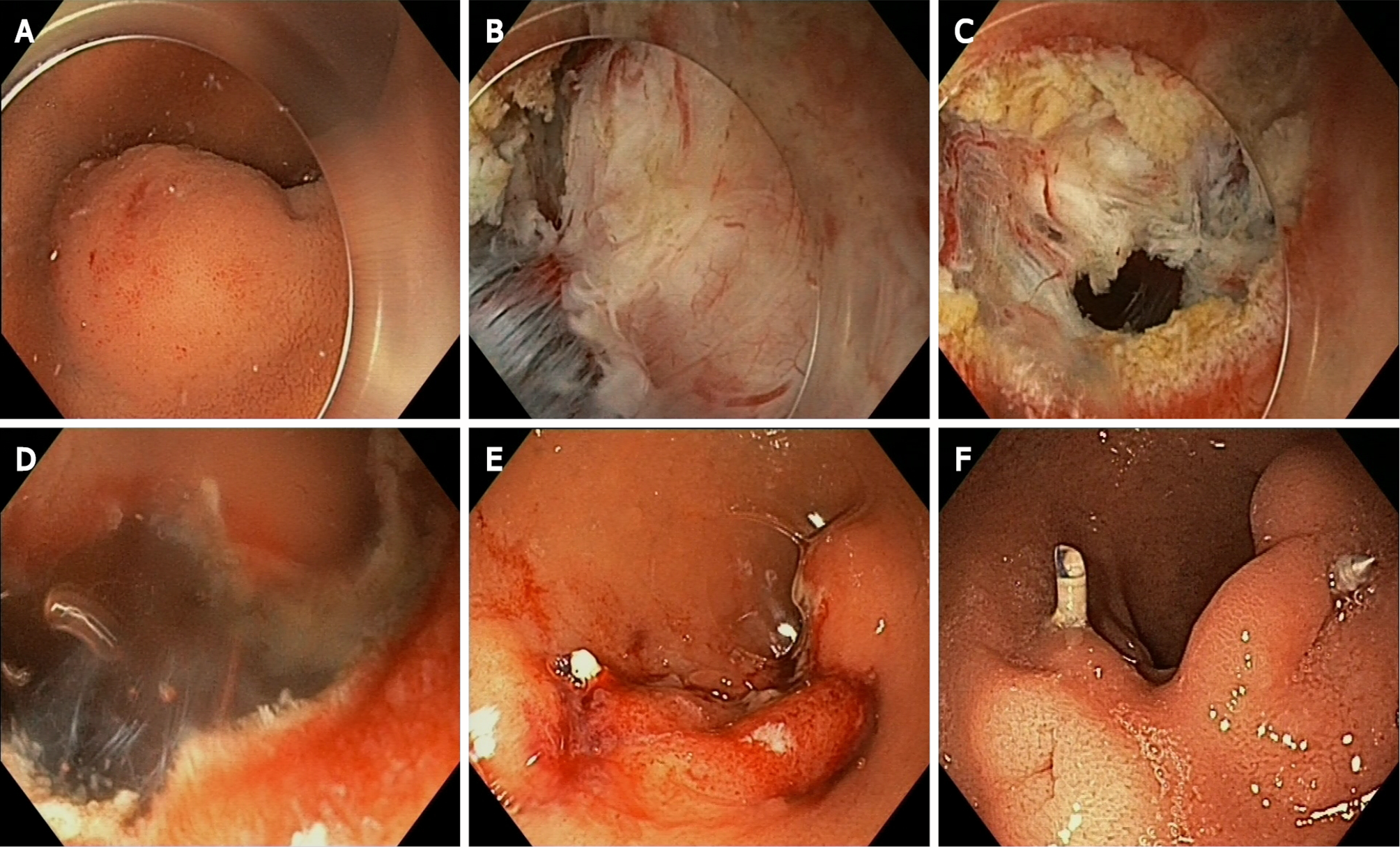
The exposed ESD-assisted EFTR without laparoscopic assistance technique is illustrated in Figure 1.
In 2012, Abe et al[22] reported the first case of LAEFR for a 10 mm carcinoid tumor of the duodenal bulb. Resection with histologically negative margins was accomplished, and the duodenal post-EFTR wall defect was sutured laparoscopically by means of an Albert anastomosis. No major adverse events were reported. Of note, during the same operative session laparoscopic lymphadenectomy was done before the EFTR, with intra-operative histological examination showing the absence of metastatic tumor cells[23].
In a multicenter prospective cohort study enrolling 42 patients undergoing gastrointestinal exposed EFTR, five procedures performed for SMTs located in the duodenal bulb were also included. The resulting post-EFTR transmural defect was effectively closed by the application of pursestring sutures with nylon loops and clips in all cases, and no major adverse events were observed[24].
A large retrospective study evaluated the efficacy and safety of exposed EFTR without laparoscopic assistance in 32 patients with non-ampullary MP-arising duodenal SMTs. With regard to post-EFTR defect closure, various endoscopic techniques were adopted (Table 1). In one case, endoscopic closure of a 2.5 cm post-EFTR defect located at the anterior wall of the bulb-descending junction appeared technically unfeasible; thus, conversion to open surgery was undertaken, with successful defect suture. Complete resection was achieved in all cases, and no recurrence was observed during a mean follow-up period of 38 mo. The occurrence of major adverse events was reported in two of 32 procedures. A case of EFTR performed for a 2.5 cm lesion in the anterior wall of the bulb-descending junction with defect closure by means of endoloops and clips was complicated by delayed perforation. Laparoscopic exploration with drainage tube placement was performed, and the patient was discharged on post-operative day 6. Finally, in a male patient aged 81, with a history of chronic obstructive pulmonary disease post-operative decline in blood oxygen saturation was observed. The patient was transferred to the intensive care unit and successfully treated conservatively[25].
| Ref. | Study design | Lesions, n | Mean size (range), cm | Site | R0 | Histology | Surgical conversion | Closure method | Mean operation time (range), min | Major AEs | Mean poLOS (range), days | Mean follow-up (range), months | Recurrence |
| Abe et al[23], 2012 | CR | 1 | 1.0 | Bulb: Anterior wall | Carcinoid | 0 | Laparoscopic hand-suturing | 200 | 0 | 7 | - | - | |
| Qiao et al[24], 2018 | R | 5 | - | Bulb | - | - | 0 | EPSS | - | 0 | 4.5 | 12 | 0 |
| Ren et al[25], 2019 | R | 32 | 1.2 (0.5–3.0) | Bulb: Anterior wall (n = 21); posterior wall via (n = 1); Bulb-D2 junction: Anterior wall (n = 8); D2 (n = 2) | 32 | GIST (n = 14); NET (n = 4); Heterotopic pancreas (n = 11); Leiomyoma (n = 2); Lipoma (n = 1) | 2 | Clips (n = 6); Clips + endoloops (n = 20). Clips + endoloops + fibrin glue (n = 4); ESS (n = 1) | - | Delayed perforation (n = 1); SO2 decline (n = 1) | 6.2 (2–19) | 38 (14–73) | 0 |
| Yuan et al[26], 2019 | CR | 1 | 2.0 | Bulb | 1 | GIST | 0 | EPSS | 55 | 0 | 4 | 3 | 0 |
| Granata et al[27], 2021 | R | 2 | 2.4 (1.8–3.0) | Bulb: Anterior wall (n = 1); inferior wall (n = 1) | 2 | GIST (n = 1); NET (n = 1) | 0 | ESS | 293 (145–148) | 0 | 3.5 (3–4) | 15 (12–18) | 0 |
In 2019, Yuan et al[26] reported a case of successful exposed EFTR without laparoscopic assistance performed for a 20 mm duodenal bulb low-grade GIST. The resulting transmural wall defect was effectively closed with endoloops and endoclips using the purse-string suture technique. R0 resection was achieved, no major adverse events were observed, and the patient was discharged home on post-operative day 4[26].
Finally, in a recent retrospective case series from Italy, two exposed EFTR procedures of the duodenal bulb were reported. Wall defect closure was successfully performed by means of the OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, Texas, United States). Histological examination showed free resection margins in both cases (1 NET, 1 GIST) and no major adverse events were encountered[27].
Results of the included studies in which duodenal ESD-assisted exposed EFTR was performed are summarized in Table 1.
To date, the optimal resection modality for the treatment of MP-originating D-SMTs has not been established. PD carries a high rate of morbidity[8,11], while pancreas-preserving limited duodenal resection techniques are technically challenging, with a non-negligible rate of conversion to PD[9,11]. Furthermore, both EMR and ESD techniques are technically unsuitable for the complete resection of D-SMTs arising from the MP and adhering to the serosa layer, being limited to mucosal and submucosal layer, respectively. Intriguingly, non-exposed EFTR have been proposed for the resection of deep D-SMTs, with promising outcomes[28]. With the use of this “close then cut” technique, the lesion is resected after the GI wall patency is secured by creation of full-thickness wall duplication. Non-exposed EFTR can be realized with the use of a dedicated full-thickness resection device (FTRD; Ovesco Endoscopy, Tuebingen, Germany), consisting of an over-the-scope clip (OTSC) preloaded into a cap with an integrated snare. Alternatively, the application of an OTSC (OTSC, Ovesco Endoscopy GmbH, Tuebingen, Germany; Padlock Clip, Aponos Medical, Kingston, NH, United States) is followed by excision of the created pseudopolyp by the use of a snare or a needle knife. Non-exposed EFTR provides the potential avoidance of both peritoneal dissemination of tumor cells and extraluminal spillage of gastrointestinal content. In addition, this approach is technically much easier and faster to perform. However, this technique has a lower R0 resection rate than exposed EFTR. This is probably due to the technical unfeasibility of a “real-time” and direct visualization of the circumferential cutting margins. Furthermore, OTSC cannot be repositioned after its deployment, and non-exposed EFTR is reserved for smaller lesions (< 25 mm)[19,28].
In this scenario, ESD-assisted exposed EFTR with or without laparoscopic assistance could replace traditional surgery for the radical treatment of select cases of deep D-SMTs. However, evidence concerning the use of this NOTES procedure for D-SMTs is lacking. Traditionally, the duodenum has been considered a “forbidden” zone for exposed EFTR mainly due to technical difficulties related to complex anatomic relationships with surrounding organs and vessels, a narrow lumen, and a “C-loop,” resulting in troublesome maintenance of the desired endoscope position. Hence, concerns about an effective and reliable post-EFTR transmural defect closure must be raised.
Delayed perforation of the duodenum is associated with higher morbidity and mortality than other GI sites[8]. However, the recent development of dedicated endoscopic devices for tissue-approximation capable of achieving a full-thickness “surgical-quality” defect closure, such as the OverStitch Endoscopic Suturing System and OTSC systems, could help in overcoming these concerns[29,30].
In our opinion, a step-up approach with exposed EFTR as the first-line of treatment for selected deep D-SMTs appears particularly intriguing. Its adoption should be reserved for non-periampullary MP-originating D-SMTs up to 30 mm in diameter and without predominant extraluminal growth pattern, and limited to highly experienced centers. Full-thickness closure of the post-EFTR wall defect is strongly advised.
High morbidity and mortality associated with duodenal surgery justify active research in this field. Further large prospective studies in high-volume referral centers are needed to better clarify the role of exposed EFTR with or without laparoscopic assistance for the treatment of MP-arising D-SMTs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy (ESGE); Italian Society for Digestive Endoscopy (SIED); Italian Association of Hospital Gastroenterologists and Digestive Endoscopists (AIGO).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Libânio D S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Caletti G, Fusaroli P, Bocus P. Endoscopic ultrasonography. Digestion. 1998;59:509-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Rösch T, Lorenz R, Dancygier H, von Wickert A, Classen M. Endosonographic diagnosis of submucosal upper gastrointestinal tract tumors. Scand J Gastroenterol. 1992;27:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 864] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 4. | Standards of Practice Committee, Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry SV, Meyer C, Morris ZS, O'Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:536-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 6. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (1)] |
| 7. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 8. | Tien YW, Lee CY, Huang CC, Hu RH, Lee PH. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. 2010;17:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hashimoto D, Arima K, Chikamoto A, Taki K, Inoue R, Kaida T, Higashi T, Imai K, Beppu T, Baba H. Limited Resection of the Duodenum for Nonampullary Duodenal Tumors, with Review of the Literature. Am Surg. 2016;82:1126-1132. [PubMed] |
| 10. | Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, Yamaue H. Laparoscopic Limited Resection for Duodenal Gastrointestinal Stromal Tumors. J Gastrointest Surg. 2020;24:2404-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Yang F, Jin C, Du Z, Subedi S, Jiang Y, Li J, Di Y, Zhou Z, Tang F, Fu D. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg. 2013;206:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 926] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 13. | Vanbiervliet G, Moss A, Arvanitakis M, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Napoleon B, Nalankilli K, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Strijker M, Barthet M, van Hooft JE. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 14. | Pérez-Cuadrado-Robles E, Quénéhervé L, Margos W, Moreels TG, Yeung R, Piessevaux H, Coron E, Jouret-Mourin A, Deprez PH. ESD vs EMR in non-ampullary superficial duodenal tumors: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E998-E1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Granata A, Martino A, Amata M, Ligresti D, Tuzzolino F, Traina M. Erratum: Efficacy and safety of gastric exposed endoscopic full-thickness resection without laparoscopic assistance: a systematic review. Endosc Int Open. 2020;8:C4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Wang C, Gao Z, Shen K, Cao J, Shen Z, Jiang K, Wang S, Ye Y. Safety and efficiency of endoscopic resection vs laparoscopic resection in gastric gastrointestinal stromal tumours: A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22:600-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 18. | Paspatis GA, Arvanitakis M, Dumonceau JM, Barthet M, Saunders B, Turino SY, Dhillon A, Fragaki M, Gonzalez JM, Repici A, van Wanrooij RLJ, van Hooft JE. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement - Update 2020. Endoscopy. 2020;52:792-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | ASGE Technology Committee, Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 20. | Ikeda K, Mosse CA, Park PO, Fritscher-Ravens A, Bergström M, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection: circumferential cutting method. Gastrointest Endosc. 2006;64:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Abe N, Takeuchi H, Shibuya M, Ohki A, Yanagida O, Masaki T, Mori T, Sugiyama M. Successful treatment of duodenal carcinoid tumor by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Asian J Endosc Surg. 2012;5:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Qiao Z, Ling X, Zhu J, Ying G, Xu L, Zhu H, Tang J. Therapeutic application of purse-string sutures with nylon loops and metal clips under single-channel endoscopy for repair of gastrointestinal wall defects. Exp Ther Med. 2018;15:4356-4360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Ren Z, Lin SL, Zhou PH, Cai SL, Qi ZP, Li J, Yao LQ. Endoscopic full-thickness resection (EFTR) without laparoscopic assistance for nonampullary duodenal subepithelial lesions: our clinical experience of 32 cases. Surg Endosc. 2019;33:3605-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Yuan XL, Liu XW, Hu B. Endoscopic full-thickness resection for a duodenal gastrointestinal stromal tumour. Arab J Gastroenterol. 2019;20:211-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Granata A, Martino A, Amata M, Ligresti D, Traina M. Gastrointestinal exposed endoscopic full-thickness resection in the era of endoscopic suturing: a retrospective single-center case series. Wideochir Inne Tech Maloinwazyjne. 2021;16:321-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Brewer Gutierrez OI, Akshintala VS, Ichkhanian Y, Brewer GG, Hanada Y, Truskey MP, Agarwal A, Hajiyeva G, Kumbhari V, Kalloo AN, Khashab MA, Ngamruengphong S. Endoscopic full-thickness resection using a clip non-exposed method for gastrointestinal tract lesions: a meta-analysis. Endosc Int Open. 2020;8:E313-E325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am. 2020;30:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Weiland T, Rohrer S, Schmidt A, Wedi E, Bauerfeind P, Caca K, Khashab MA, Hochberger J, Baur F, Gottwald T, Schurr MO. Efficacy of the OTSC System in the treatment of GI bleeding and wall defects: a PMCF meta-analysis. Minim Invasive Ther Allied Technol. 2020;29:121-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |