Published online Feb 16, 2022. doi: 10.4253/wjge.v14.i2.63
Peer-review started: June 30, 2021
First decision: July 29, 2021
Revised: August 25, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: February 16, 2022
Processing time: 224 Days and 15.5 Hours
Peroral cholangioscopy (POC) is an endoscopic procedure that allows direct intraductal visualization of the biliary tract. POC has emerged as a vital tool for indeterminate biliary stricture evaluation and treatment of difficult biliary stones. Over several generations of devices, POC has fulfilled additional clinical needs where other diagnostic or therapeutic modalities have been inadequate. With adverse event rates comparable to standard endoscopic retrograde cholangioscopy and unique technical attributes, the role of POC is likely to continue expand. In this frontiers article, we highlight the existing and growing clinical applications of POC as well as areas of ongoing research.
Core Tip: Cholangioscopy is an endoscopic technique that was first developed in the 1970s as a minimally-invasive modality for the evaluation of various biliopancreatic pathologies. Since the advent of the digital single-operator cholangioscopy (D-SOC) in 2015 as well as other, complementary advancements in the field, diagnostic and therapeutic applications have further expanded. Herein, we discuss the various current applications of cholangioscopy, with a focus on D-SOC, and areas of ongoing research to better understand potential future directions.
- Citation: Subhash A, Buxbaum JL, Tabibian JH. Peroral cholangioscopy: Update on the state-of-the-art. World J Gastrointest Endosc 2022; 14(2): 63-76
- URL: https://www.wjgnet.com/1948-5190/full/v14/i2/63.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i2.63
Endoscopic retrograde cholangiopancreatography (ERCP) was first reported in 1968 as a method to cannulate the major duodenal papilla[1]. It is now widely utilized as the primary interventional modality for many biliopancreatic disorders. Despite its vast utility, ERCP technique relies on indirect visualization of the biliary tree via fluoroscopy; this can be limiting for certain diagnostic and/or therapeutic applications (e.g. evaluation of biliary strictures, mapping of intraductal tumors for operative planning, tumor-directed ablative therapy, etc.).
In order to provide direct visualization of the biliopancreatic tree, peroral cholangioscopy (POC) was introduced in the 1970s[2,3]. POC was originally designed as a “mother-baby” system that required two endoscopists to operate the “mother” duodenoscope and “baby” cholangioscope[2]. In addition to the multi-operator requirement, there was a notable deficiency in this setup in the ability to acquire tissue following visualization, thus further limiting its use. Moreover, the initial scopes provided only two-way tip deflection, were fragile, and costly[4].
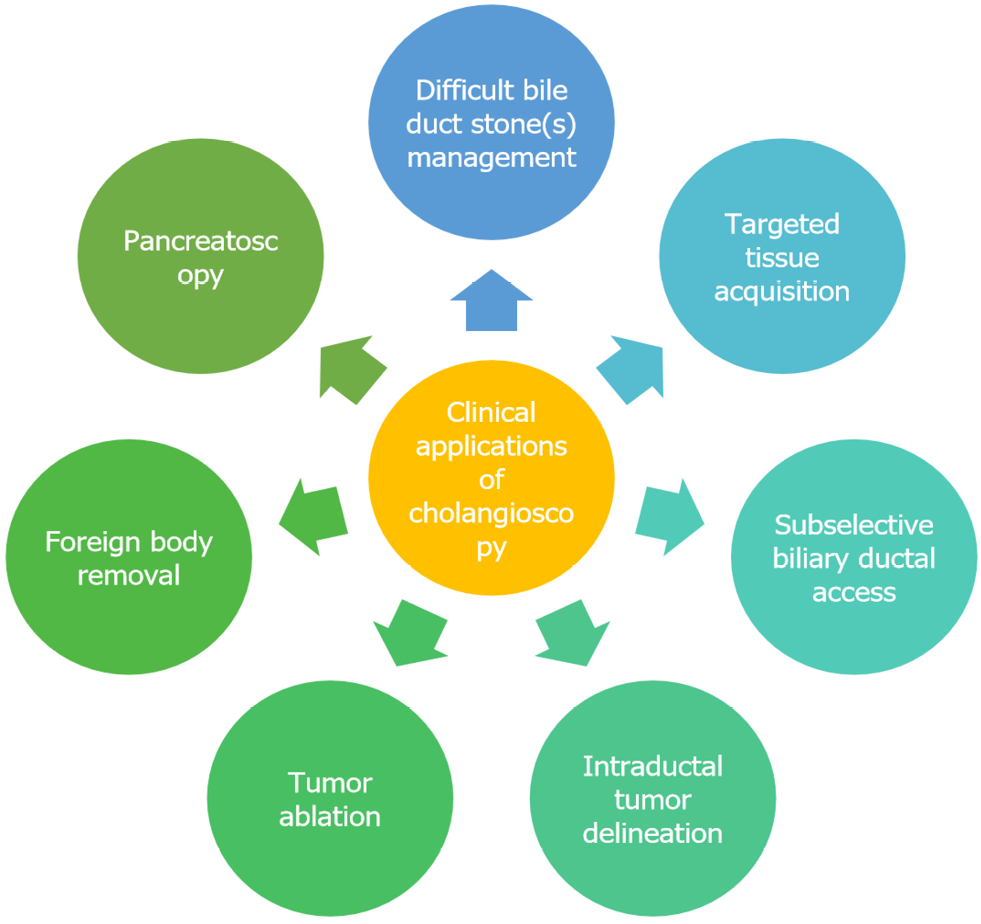
Over the past several decades, technologic improvements in the equipment utilized for POC has led to more widespread adoption and a growing number of applications (Figure 1). In the early 2000s, a new single-operator duodenoscope-assisted cholangioscopy technique utilizing a Pentax cholangioscope (FCP-8P/FCP-9P, Pentax Precision Instruments, Orangeburg, New York, United States) was introduced. However, this technique required the use of an endoscopist-worn breastplate to mount the cholangioscope, which allowed for manipulation of the duodenoscope with the left hand and the cholangioscope with the right hand[5]. In 2005, Boston Scientific released the first commercially available single-operator cholangioscopy (SOC) system (SpyGlassTM, Boston Scientific Corporation, Natick, MA, United States), a catheter-based system that utilizes an optical probe inserted through the duodenoscope working channel[6]. Ten years later, a digital SOC (D-SOC) system was introduced (SpyGlassTM DS, Boston Scientific Corporation)[6]; this updated digital system brought improvements in image size and quality, a wider field of view, and a redesigned working channel allowing for larger diameter cholangioscopic accessories, among other changes[4,7]. In 2018, a third generation SpyScopeTM DSII Catheter (Boston Scientific Corporation) featuring increased resolution and improved lighting was introduced alongside new cholangioscopic accessories. Alternatively, direct POC (DPOC) can be performed utilizing a modern ultraslim upper endoscope that can be advanced into the biliary tree following endoscopic sphincterotomy, a technique first published in a pilot study in 2006[8-10]; however, this setup is primarily used outside the United States and available in only select markets[7].
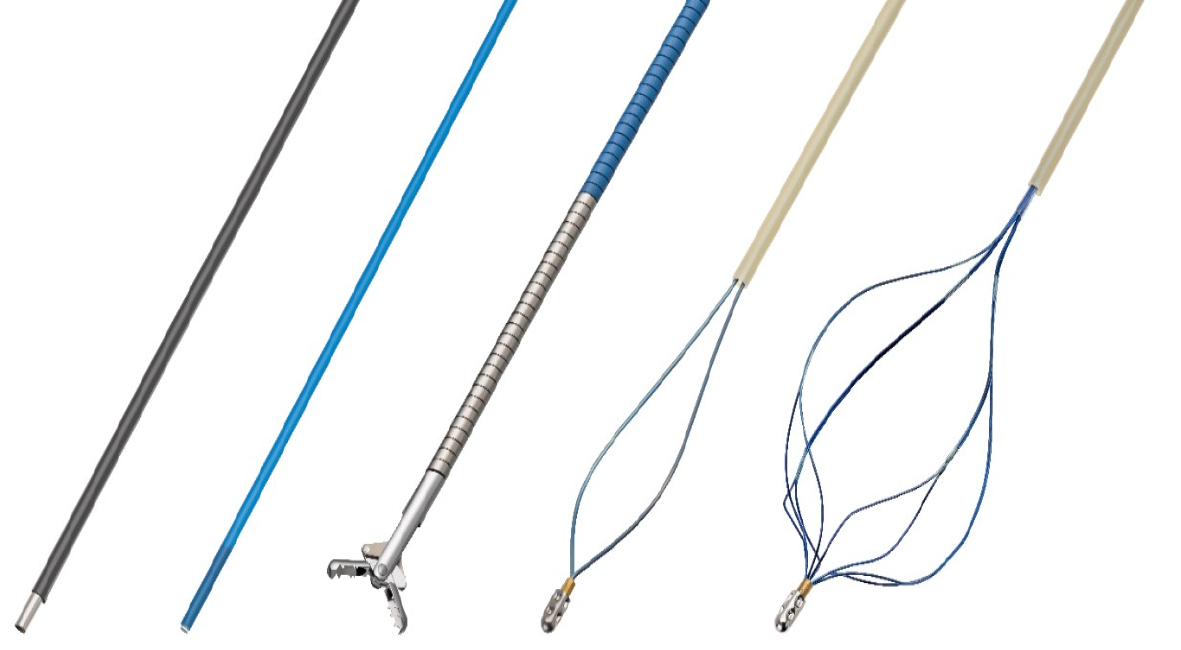
Given the recent technologic advancements in POC, its array of accessories (Figure 2), and improved training of advanced endoscopists, there has been wide propagation of this technique across most large medical centers. In this Frontiers article, we aim to underscore the major developments in the growing body of literature on POC, with particular emphasis on SOC and D-SOC, including diagnostic and therapeutic applications as well as established and investigational indications.
Approximately 10%-18% of patients with symptomatic cholelithiasis will have concomitant choledocholithiasis[11]. The standard of care for these patients is ERCP with endoscopic sphincterotomy followed by stone extraction with a balloon or basket[4,11]. In a minority of cases, bile duct stones may be more difficult to extract, requiring additional measures[12]. Difficult bile duct stones have been previously defined as large size (> 1.5 cm in diameter), impacted stones in the bile or cystic duct, intrahepatic location, hard stone consistency, stricture distal to stones, and/or anatomical variants (e.g. unusual size/shape of bile duct) posing technical challenges[12,13].
POC allows for direct visualization and decreased risk of bile duct injury and is a vital addition to the ERCP armamentarium for stone disease. Indeed, a recent meta-analysis found the estimated success rate for difficult bile duct stone clearance to be 88% [95% confidence interval (CI): 85%-91%] across 820 patients (n = 31 studies)[14]. Furthermore, POC was found to have a low adverse event (AE) rate of 7% (95%CI: 6%-95%), comparable to ERCP[14,15]. Thus, POC is a valuable modality in addition to or in lieu of conventional ERCP methods such as mechanical lithotripsy (ML) and endoscopic papillary large balloon dilation (EPLBD).
Since the time of publication of the aforementioned meta-analysis, three randomized controlled trials (RCTs) comparing POC-guided electrohydraulic lithotripsy (EHL) or holmium laser lithotripsy (LL) vs conventional therapy (i.e. ML, EPLBD, and balloon extraction) have been published. In the first study, the investigators randomized patients with bile duct stones > 1 cm in diameter in a 2:1 ratio to SOC-guided LL vs conventional therapy. Stone clearance was achieved in 39 of 42 (93%) patients treated with SOC-guided LL compared to 12 of 18 (67%) treated with conventional therapy (P = 0.009). AE rates were similar in the two treatment groups[16]. In the second study, successful stone removal did not differ in the SOC-guided EHL arm (37 of 48) vs conventional therapy arm (36 of 50) (P > 0.05); similarly, crossover yielded non-statistically significant differences in the two groups (successful stone removal in 40 of 47 patients vs 42 of 44 patients, P > 0.05)[17]. In the final study, the investigators randomized 32 patients with large CBD stones in whom sphincterotomy and/or EPLBD had failed into ML or D-SOC-guided LL treatment arms. Crossover was permitted as a rescue treatment if the primarily assigned technique failed to achieve stone clearance. Stone clearance rates for ML and D-SOC-guided LL groups were 63% and 100%, respectively (P < 0.01). In six patients, ML was considered a failure; when crossed over to LL, four of these patients achieved stone clearance in the same session, and the remaining two patients achieved stone clearance in subsequent LL sessions. AEs were reported at similar rates, 13% in the ML group and 6% in the LL group (P = 0.76). The median length of hospital stay following the respective procedures was 1 d in both groups (P = 0.27). At six months follow-up, neither group had recurrent cholangitis or evidence of recurrent CBD stones[18]. While the RCT data presented above may appear mixed or only partially in favor of POC in the management of difficult bile duct stones, it is important to note that only the last of the three studies discussed above utilized the newer generation of D-SOC. Thus, additional RCT data using the contemporary D-SOC system is needed.
POC can also be utilized to confirm stone clearance in cases of choledocholithiasis. In a retrospective study of 36 patients who underwent ERCP with EPLBD for difficult biliary stones, DPOC was performed immediately after a negative balloon-occluded cholangiography[19]. In 31 of 36 patients (86%), technical success was achieved with hepatic hilum visualization. Residual stones were found in 7 of these 31 patients (22.5%) upon DPOC, among which 4 patients underwent successful stone extraction during the same DPOC session. The remaining 3 patients underwent secondary ERCP for residual stone removal. There were no reported AEs in the study.
Visual evaluation: Another major indication for POC is the evaluation of inde
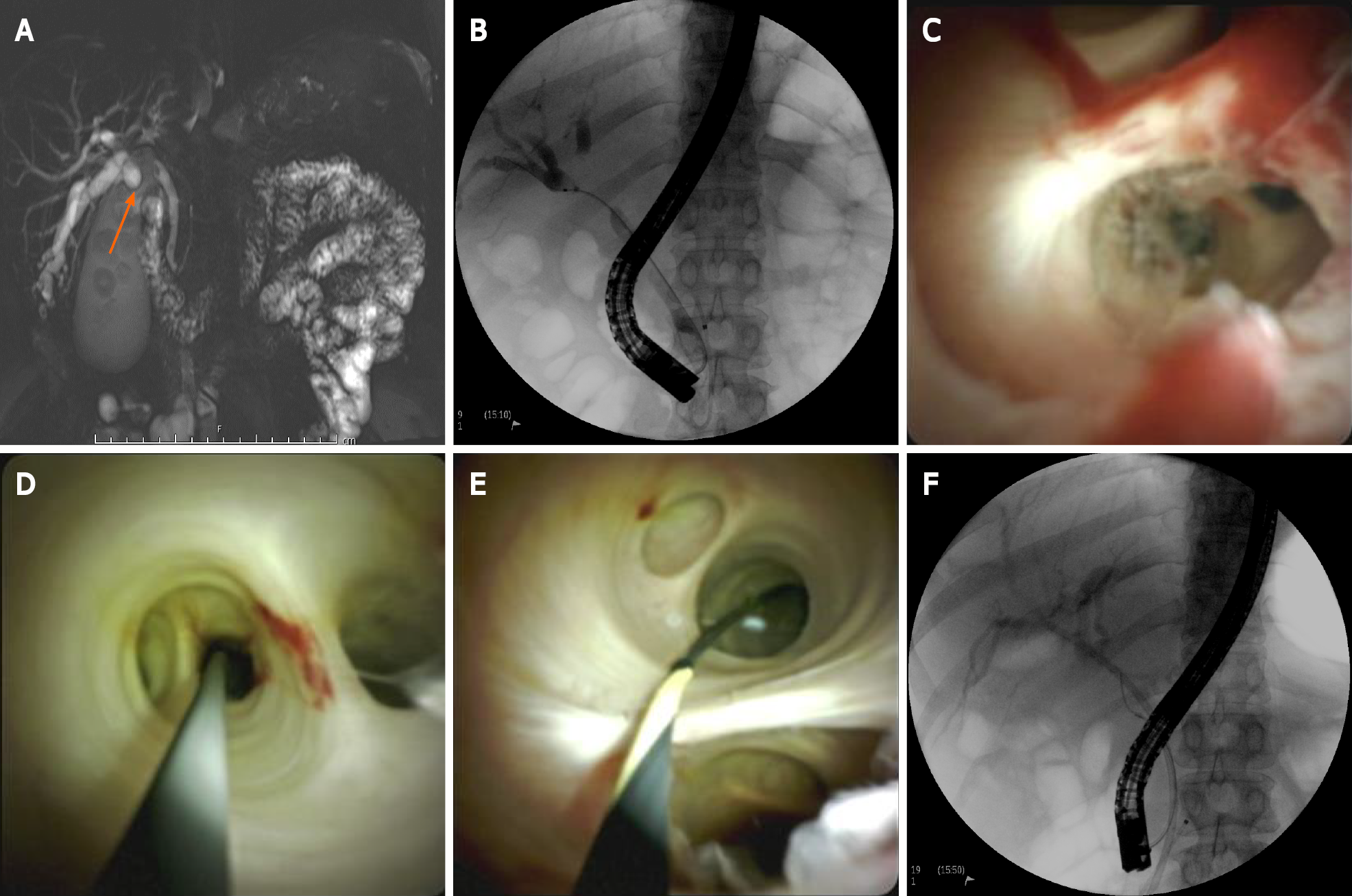
The visual diagnosis of intraductal lesions can be aided by direct visualization during POC (Figure 3). Currently, there is no widely accepted classification system for visual diagnosis; however, some cholangioscopic findings are highly suggestive of malignancy in the appropriate clinical context. These findings include the presence of neovascularization, mucosal changes and projections, and intraductal nodules, among others[22-24]. Historically, neovascularization, also termed “tumor vessels,” has had the most consensus regarding its description and malignant implications[24]. It has been described as irregularly dilated, tortuous, and abnormally proliferating vessels on the mucosa adjacent to a stricture.
In a recent systematic review and meta-analysis of 21 studies examining the diagnostic performance characteristics of POC-based visual assessments of IDBSs, the pooled sensitivity and specificity for establishing a malignancy diagnosis were 88% (95%CI: 83%-91%) and 95% (95%CI: 89-98%), respectively[25]. Subgroup analysis of studies that utilized D-SOC found a higher sensitivity for visual diagnosis [94% (95%CI: 89%-97%)] compared to D-SOC-guided biopsy [79% (95%CI: 72%-84%), P < 0.001] while also showing a higher specificity for D-SOC-guided biopsy [100% (95%CI: 97%-100%)] compared to D-SOC visual impression [86% (95%CI: 76%-92%), P < 0.001][25]. Subgroup analysis of studies that utilized DPOC did not reveal statistically significant differences in performance characteristics of visual impression vs DPOC-guided biopsy (possibly suggesting superior optical performance of DPOC compared to D-SOC), though power was limited[25]. Overall, performance characteristics of visual impression utilizing modern POC (both D-SOC and DPOC) appears promising.
A recent group of researchers have produced a new schema, the “Monaco Classification,” in order to attempt to standardize visual criteria in evaluating IDBSs as malignant vs benign. Twelve expert biliary endoscopists from around the world reviewed 40 video clips (13 benign pathology, 27 malignant) in order to consolidate visual criteria into the following: (1) Presence of stricture (symmetric or asymmetric); (2) Presence of lesion (with associated mass, nodule, or polypoid in appearance); (3) Smooth or granular mucosal features; (4) Papillary projections; (5) Ulceration; (6) Abnormal vessels; (7) Scarring (local or diffuse); and (8) Pronounced pit pattern[26]. Thereafter, 21 D-SOC video clips were reviewed by 14 interventional endoscopists utilizing these criteria, ranging from slight to moderate in interobserver agreement[26]. Diagnostic accuracy of visual interpretation of malignant vs benign pathology was 70% based on the new criteria, compared to an average accuracy less than 50% on prior attempts to establish visual criteria[26,27]. While the Monaco Classification has taken a crucial step in a forward direction, it would benefit from further refinement and validation.
Cytopathologic evaluation: In addition to the visual diagnosis of IDBSs, POC-guided biopsy can provide further histopathologic interpretation of IDBSs. In a systematic review with meta-analysis of 10 studies evaluating the use of SOC-guided biopsy for the diagnosis of malignant biliary strictures, the overall pooled sensitivity and specificity were 60.1% (95%CI: 54.9%-65.2%) and 98.0% (95%CI: 96.0%-99.0%), respectively[28]. In a subset of four studies, patients (n = 148) had previously undergone ERCP with benign or non-diagnostic brushing/biopsy results (with strong suspicion for malignancy); in this specific cohort, the pooled sensitivity and specificity of SOC-guided biopsy were 74.7% (95%CI: 63.3%-84.0%) and 93.3% (95%CI: 85.1%-97.8%), respectively[28]. More recently, a systematic review with meta-analysis of 11 studies examined the use of D-SOC-guided biopsy for evaluation of IDBSs. The pooled sensitivity and specificity were 74% (95%CI: 67%-80%) and 98% (95%CI: 95%-100%), respectively[29]. These data suggest that POC-guided biopsy, in particular D-SOC-guided biopsy, yields improved diagnostic sensitivity when evaluating IDBSs.
POC-guided biopsies can be useful in cases where prior ERCP biopsies/brushings return benign or non-diagnostic results (when a strong suspicion for malignancy nevertheless remains) (Figure 3). In addition, a retrospective study of 40 patients found that biliary lavage cytology can be combined with POC-guided biopsy to further improve diagnostic sensitivity and accuracy when compared to POC-guided biopsy alone (sensitivity 88% vs 70% and accuracy 90% vs 75%, respectively)[30]. Of note, the data presented above predates the advent of the SpyBiteTM Max biopsy forceps, which has increased tissue capacity compared to the first-generation SpyBite (legacy) forceps. This, along with other improvements, is expected to further improve the diagnostic performance of POC-guided intraductal biopsy.
One limiting factor that has been thought to potentially hamper the utility of SOC-guided biopsy is the absence of on-site cytopathology for real-time tissue processing, a concern recently addressed by the SOCRATES (single-operator cholangioscopy randomized trial evaluating specimens) trial[31]. In this RCT, patients (n = 62) with IDBSs were randomized to an off-site tissue processing cohort (n = 30) and an on-site cohort (n = 32) in order to compare diagnostic accuracy. The study found a diagnostic accuracy of 90% (95%CI: 73.5%-97.9%) versus 84.4% (95%CI: 67.2%-94.7%) when comparing off-site tissue processing vs on-site, respectively (P = 0.86). Additionally, the overall treatment costs of D-SOC based on the Medicare reimbursement fee structure (including anesthesia, hospital fees, laboratory fees, medications, supplies, and radiologic fees) was found to be $14423 for the off-site cohort compared to $13015 for the on-site cohort (P = 0.60). Thus, this RCT suggests that D-SOC is a cost-effective option for the evaluation of IDBSs, even in centers without on-site cytopathology.
Primary sclerosing cholangitis (PSC) is a chronic, progressive disease that causes inflammation and fibrosis of the biliary tract, often leading to end-stage liver disease and/or cholangiocarcinoma (CCA)[32]. Patients with PSC can develop “dominant strictures,” or focal narrowing defined at ERCP as stenosis with diameter ≤ 1.5 mm in the CBD and/or ≤ 1.0 mm in a hepatic duct within 2 cm of the ductal confluence[20,32-34]. Dominant strictures are clinically significant in light of their higher propensity for bacterial cholangitis and for underlying dysplasia or carcinoma[32,35]. A recent systematic review and meta-analysis of 21 studies found the that the pooled sensitivity and specificity of POC for diagnosis of CCA was 65% (95%CI: 35%-87%) and 97% (95%CI: 87%-99%), respectively[36]. POC-guided biopsy also had the highest diagnostic accuracy (96%), compared to bile duct brushings (87%), fluorescence in situ hybridization (FISH) (69% for polysomy and 47% for trisomy), and probe-based confocal laser endomicroscopy (75%)[36].
However, not all data to date support the use of POC in patients with PSC. For example, a prospective study of 47 patients with PSC evaluating the use of POC-guided biopsy of strictures found a significantly lower sensitivity (33%) than previously reported[37]. Additionally, a retrospective study of 92 patients, both with (n = 36) and without (n = 56) PSC, examined the performance characteristics of ERCP with brush cytology, FISH, POC-guided biopsy, transpapillary biopsy and each possible combination of the aforementioned for the detection of CCA. When com
Overall, the precise role of POC in the diagnostic evaluation of dominant strictures in PSC remains unclear. POC can potentially play an important role in studying the natural history and progression of PSC and in general facilitate better characterization and sampling of dominant strictures. For instance, with the newly proposed cholangioscopy-based “Edmonton Classification” system for phenotypic classification, dominant strictures can be classified into one of the three following phenotypes: Inflammatory, fibro-stenotic, or nodular or mass-forming. One theory is that these and other POC findings may differ by disease stage/pathobiological involvement (e.g. nodular or mass forming may be indicative of developing or nascent CCA)[39]. It is proposed that combining phenotypic data with histopathology, biochemical markers, and cholangiography scores over time could lead to improved management algorithms[40]. For now, validation of this classification system remains the initial step prior to determining its ultimate clinical utility.
POC is becoming increasingly useful in the mapping of biliopancreatic neoplasms such as CCA and intraductal papillary mucinous neoplasms (IPMNs). With improved visual delineation of neoplastic margins in the biliary tree and pancreatic ducts, staging can be more precise, and thus a better-informed therapeutic plan can be formulated (Figure 3). A multicenter prospective cohort study of 118 patients evaluated the impact of cholangiopancreatoscopy on preoperative assessment of biliopancreatic neoplasms. Following cholangiopancreatoscopy, the initial therapeutic plan was altered in 34% of patients[41]. Of these patients, more extensive surgery was required in 10%, less extensive surgery was required in 65%, and surgery was avoided in the remaining 25%[41]. Additionally, the study reported a 88% correlation in histology between the surgical specimens and cholangiopancreatoscopy specimens[41].
Cholangiopancreatoscopy is also being utilized to directly examine pancreatic duct abnormalities, such as distinguishing between pancreatic duct dilation secondary to chronic pancreatitis vs IPMNs[42]. When used in conjunction with non-invasive imaging, POC/cholangiopancreatoscopy improves diagnostic and therapeutic ability. As has been discussed in prior sections, this is mainly from direct visual tissue inspection and the ability to obtain targeted biopsies. Simultaneously, it also offers the opportunity for facilitate therapeutic intervention (e.g. management of pancreatolithiasis).
Numerous case reports, series, and a retrospective study have all demonstrated the potential benefits of POC-guided guidewire placement across strictures of varying causes (malignant, post-OLT, PSC, etc.)[43-45]. In the retrospective study, a total of 23 patients with known biliary strictures in whom endoscopic guidewire placement had previously failed underwent 30 procedures; technical success (guidewire placement) was achieved in 70%[43]. Subgroup analysis demonstrated a higher technical success rate among benign biliary strictures vs malignant strictures (88% vs 46%, P = 0.02). Of the 23 patients, 7 underwent repeat procedures, both in patients with previous failure of guidewire placement (n = 3) and prior success of guidewire placement (n = 4). A higher technical success rate was demonstrated on initial exam compared to subsequent exams (78% vs 43%, P = 0.15)[43]. While data are limited, POC-guided guidewire placement can be an effective alternative option, though traditional ERCP approaches should be attempted primarily given the significantly higher costs associated with POC and the ability to potentially troubleshoot successfully with varying guidewire diameters, tip designs, tip core materials, etc. during ERCP.
The use of POC-guided radiofrequency ablation (RFA) to provide locoregional cancer-directed therapy for the management of extrahepatic CCA or other intraductal malignancies has been presented in various case reports[46,47]. Historically, percutaneous RFA has been well studied, though this technique has demonstrated an association with various AEs[48]. ERCP-RFA (without POC) has thus been explored as a possible alternative in porcine models, yielding similar concerns for high AE rates[49]. In a review article, the pooled data from 12 studies evaluating endoscopic RFA treatment for the management of patients with unresectable malignant biliary strictures showed similarly high AE rates (16%) across 318 total patients[50]. In a retrospective study of 12 patients, POC-guided RFA was both technically (RFA probe insertion into stricture site) and clinically successful (tumor ablation with POC imaging) while demonstrating safety (1 AE in study population) and efficacy in maintaining stent patency (median of 154 d) following POC-guided RFA. Though data are limited, POC-guided RFA could be explored in further studies as a potentially viable, safer (compared to percutaneous RFA and endoscopic RFA) palliative treatment option for select patients with unresectable malignant biliary strictures.
POC-guided photodynamic therapy (PDT) has also been suggested to improve symptoms and prolong survival in cases of unresectable biliary tumors, with relatively few complications[51]. PDT begins with the administration of intravenous photosensitizer, which is preferentially retained by malignant tissue, approximately 24 h prior to POC. Subsequently, light energy can be delivered under POC guidance to the target tissue at a photoactivating wavelength, resulting in a photochemical reaction inducing ischemia and necrosis of tumor cells[52]. RCT data is limited to ERCP-based studies, in which PDT plus endoscopic stenting (n = 20) vs endoscopic stenting alone
One AE orthotopic liver transplantation (OLT) patients face is the development of biliary strictures, either anastomotic (more common) or nonanstomotic (less common). Biliary strictures affect up to nearly 40% of post-OLT patients[54]. In these cases, POC can be utilized for visual assessment of the biliary epithelium and/or targeted biopsy, if needed[55]. Additionally, some strictures are not amenable to guidewire insertion or cannulation with standard ERCP (e.g. angulated strictures)[56]; the addition of POC can facilitate guidewire insertion and possibly obviate the need for biliary drainage or surgical intervention[55,56].
In a recent observational study of 26 patients who underwent ERCP followed by POC for suspected biliary complications post-OLT, 33 biliary complications were found in 22 patients. The remaining 4 patients were found to have normal bile ducts. Of the biliary complications, anastomotic strictures were the most common (14), followed by nonastomotic strictures (7), biliary stones (6), and lastly biliary casts (3). In 12 patients (46%), POC demonstrated a clear benefit: Selective guidewire placement, identification of biliary cast and/or stones not previously found on ERCP, or epithelial changes (e.g. ulceration or inflammation) secondary to infection[44]. Additional case series have shown the potential benefits of POC-guided steroid injections for management of anastomotic strictures and POC-guided guidewire placement across strictures (previously failed under fluoroscopic guidance)[56,57]. All of these observational studies suggest low rates of AEs, even in the post-OLT population[44,56,57]. Of note, in immunocompromised post-OLT patients, it is important to provide a prophylactic course of antibiotics given the potential increased risk of bacterial translocation with POC[58].
One of the disadvantages of conventional ERCP therapy is radiation exposure to patients and medical staff from the use of fluoroscopy. In particular, there can be teratogenic risk posed to pregnant patients in the first trimester[59]. While ERCP remains the standard of care and every effort should be made to use fluoroscopy selectively and with proper safety measures, POC can be utilized as an alternative management strategy to minimize or obviate the use of radiation[60]. A recent retrospective, multicenter study demonstrated 100% success rate in achieving bile duct cannulation without the use of fluoroscopy in the study population of pregnant patients (n = 10) with a mean gestational age of 23 wk. Indications for intervention included: Choledocholithiasis (7), stent removal (1), biliary stricture (1), and combined choledocholithiasis/stent removal (1). Fifty-percent of patients were able to undergo a completely radiation-free procedure, while an additional 30% received a dose mini
Novel applications of POC continue to emerge. One area of demonstrated utility has been in the removal of migrated stents and other foreign bodies. Following failed retrieval attempts with ERCP, POC can provide better visualization and/or access for successful extraction, thereby avoiding more invasive procedures[64-67]. Additionally, POC can aid in the evaluation and management of hemobilia. After magnetic resonance cholangiopancreatography (MRCP) or ERCP demonstrates the presence of blood in the bile duct, POC can facilitate determining the source and etiology of bleeding. In one case report, POC was utilized to confirm hemobilia arising from the gallbladder, and ultimately a diagnosis of diffusely infiltrative gallbladder cancer was made[68]. Another case report describes the detection of biliary angiodysplasia during POC following an unrevealing MRCP[69]. There have also been reports of the use of POC in select cases of cholecystitis, where patients may not otherwise be surgical candidates and/or in the presence of anatomical challenges. In these instances, POC can be utilized to access and traverse the cystic duct with subsequent deployment of metal or plastic stents as a means of minimally-invasive management[70-72]. Finally, there has been a reported case of POC-guided EHL for the removal of a calcified stool bezoar in an elderly patient with chronic, severe constipation[73].
Though the clinical applications of POC continue to expand, several factors hinder further widespread use. In particular, the financial implications of POC vs conventional ERCP, owing to the high cumulative costs of the POC processor, cholangioscopes, and cholangioscopic accessories, are major hindering factors. Overall, start-up costs have been estimated to range between 50000 to $90000, though they can vary substantially by institutional contract[74]. Additionally, cholangioscopes (D-SOC) and their accessories are both single-use, and each one costs on the order of thousands and hundreds of dollars, respectively. Based on a micro-costing approach, one European study suggested that POC could be cost-effective for both treatment of difficult bile duct stones and diagnosis of IDBSs when compared to conventional ERCP[75]. However, robust economic data are lacking in the United States. Moreover, procedure times are often longer with POC when compared to conventional ERCP; thus, this may deter performance of POC due to the ability to generate more revenue with conventional ERCP per unit of time.
The overall AE rate associated with POC has been reported to be between 4% and 22%[76]. The major AEs include: Cholangitis, bacteremia, liver abscess, pancreatitis, and bleeding[77]. In a nationwide study in Sweden analyzing 36352 ERCP procedures and 408 cholangioscopy procedures between 2007 and 2012, reported post-procedural AEs were higher with POC when compared to ERCP (19.1% vs 14.0%)[78]. Pancreatitis (7.4% vs 3.9%) and cholangitis (4.4% vs 2.7%) showed similar increases, though multivariate analysis did not demonstrate a statistically significant difference when adjusted for confounders[78]. While higher rates of AEs with POC remain a concern, one group found that administration of peri-interventional antibiotics can substantially reduce rates of cholangitis[79]. With ongoing evolution of POC technology, its safety profile when directly compared to conventional ERCP will need continued assessment.
In May 2019, a next generation “mother-baby” videocholangioscope system (CHF-B290, Olympus Medical Systems Corporation, Tokyo, Japan) was introduced[80,81]. Despite being a newer iteration with notable improvements, some previously known limitations (e.g. two endoscopist operators and two equipment towers) remain, while others, such as scope fragility and accessory channel diameter, have been reported to be improved[80]. Currently, this system is only available for use in certain markets in Asia and Europe[80].
In July 2020, Ambu Inc. received FDA approval for the Ambu® aScopeTM (Ambu Inc, Columbia, MD United States) Duodeno, a single-use duodenoscope. It is anticipated that a single-use cholangioscope and additional accessories will follow in the next 1-2 years, with the potential for new clinical applications. It will be interesting to compare these developments to existing scopes and accessories.
With growing evidence to support its use, POC has evolved into an important tool in the biliopancreatic armamentarium. It is an important therapeutic option for difficult biliary stones and a core part of the evaluation of indeterminate strictures. Outcomes from the use of D-SOC for other ongoing and investigational indications (e.g. radiation-free intervention in pregnant patients, migrated stent/foreign body extraction, post-OLT biliary complication management, and selective guidewire placement) appear promising. Still, as discussed in this review, there are constraining factors and limitations to consider, e.g. device costs, paucity of standardized cholangioscopic visual classification systems, anatomical challenges, etc.[82].
In the future, further research and data are needed to solidify the evidence for POC and clarify the outcomes of its investigational applications. For now, endoscopists may continue to explore additional frontiers of clinical application, particularly with the advent of new accessories and further technologic enhancements that may be on the horizon.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maetani I S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Urakami Y, Seifert E, Butke H. Peroral direct cholangioscopy (PDCS) using routine straight-view endoscope: first report. Endoscopy. 1977;9:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Choi JH, Lee SK. Percutaneous transhepatic cholangioscopy: does its role still exist? Clin Endosc. 2013;46:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Ayoub F, Yang D, Draganov PV. Cholangioscopy in the digital era. Transl Gastroenterol Hepatol. 2018;3:82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Farrell JJ, Bounds BC, Al-Shalabi S, Jacobson BC, Brugge WR, Schapiro RH, Kelsey PB. Single-operator duodenoscope-assisted cholangioscopy is an effective alternative in the management of choledocholithiasis not removed by conventional methods, including mechanical lithotripsy. Endoscopy. 2005;37:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Hoffman A, Rey JW, Kiesslich R. Single operator choledochoscopy and its role in daily endoscopy routine. World J Gastrointest Endosc. 2013;5:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 7. | Ghersi S, Fuccio L, Bassi M, Fabbri C, Cennamo V. Current status of peroral cholangioscopy in biliary tract diseases. World J Gastrointest Endosc. 2015;7:510-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Larghi A, Waxman I. Endoscopic direct cholangioscopy by using an ultra-slim upper endoscope: a feasibility study. Gastrointest Endosc. 2006;63:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Parsi MA. Direct peroral cholangioscopy. World J Gastrointest Endosc. 2014;6:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Moon JH, Choi HJ. The role of direct peroral cholangioscopy using an ultraslim endoscope for biliary lesions: indications, limitations, and complications. Clin Endosc. 2013;46:537-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, Diamond T, Taylor MA. Surgical vs endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2013;CD003327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Carr-Locke DL. Difficult bile-duct stones: cut, dilate, or both? Gastrointest Endosc. 2008;67:1053-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | McHenry L, Lehman G. Difficult bile duct stones. Curr Treat Options Gastroenterol. 2006;9:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Korrapati P, Ciolino J, Wani S, Shah J, Watson R, Muthusamy VR, Klapman J, Komanduri S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E263-E275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 16. | Buxbaum J, Sahakian A, Ko C, Jayaram P, Lane C, Yu CY, Kankotia R, Laine L. Randomized trial of cholangioscopy-guided laser lithotripsy versus conventional therapy for large bile duct stones (with videos). Gastrointest Endosc. 2018;87:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Franzini T, Moura RN, Bonifácio P, Luz GO, de Souza TF, Dos Santos MEL, Rodela GL, Ide E, Herman P, Montagnini AL, D'Albuquerque LAC, Sakai P, de Moura EGH. Complex biliary stones management: cholangioscopy vs papillary large balloon dilation - a randomized controlled trial. Endosc Int Open. 2018;6:E131-E138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Angsuwatcharakon P, Kulpatcharapong S, Ridtitid W, Boonmee C, Piyachaturawat P, Kongkam P, Pareesri W, Rerknimitr R. Digital cholangioscopy-guided laser vs mechanical lithotripsy for large bile duct stone removal after failed papillary large-balloon dilation: a randomized study. Endoscopy. 2019;51:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Anderloni A, Auriemma F, Fugazza A, Troncone E, Maia L, Maselli R, Carrara S, D'Amico F, Belletrutti PJ, Repici A. Direct peroral cholangioscopy in the management of difficult biliary stones: a new tool to confirm common bile duct clearance. Results of a preliminary study. J Gastrointestin Liver Dis. 2019;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hilscher MB, Tabibian JH, Carey EJ, Gostout CJ, Lindor KD. Dominant strictures in primary sclerosing cholangitis: A multicenter survey of clinical definitions and practices. Hepatol Commun. 2018;2:836-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Seo DW, Lee SK, Yoo KS, Kang GH, Kim MH, Suh DJ, Min YI. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Shah RJ, Raijman I, Brauer B, Gumustop B, Pleskow DK. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy. 2017;49:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Kulpatcharapong S, Pittayanon R, Kerr SJ, Rerknimitr R. Diagnostic performance of digital and video cholangioscopes in patients with suspected malignant biliary strictures: a systematic review and meta-analysis. Surg Endosc. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Sethi A, Tyberg A, Slivka A, Adler DG, Desai AP, Sejpal DV, Pleskow DK, Bertani H, Gan SI, Shah R, Arnelo U, Tarnasky PR, Banerjee S, Itoi T, Moon JH, Kim DC, Gaidhane M, Raijman I, Peterson BT, Gress FG, Kahaleh M. Digital Single-operator Cholangioscopy (DSOC) Improves Interobserver Agreement (IOA) and Accuracy for Evaluation of Indeterminate Biliary Strictures: The Monaco Classification. J Clin Gastroenterol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 27. | Sethi A, Doukides T, Sejpal DV, Pleskow DK, Slivka A, Adler DG, Shah RJ, Edmundowicz SA, Itoi T, Petersen BT, Gress FG, Gaidhane M, Kahaleh M. Interobserver agreement for single operator choledochoscopy imaging: can we do better? Diagn Ther Endosc. 2014;2014:730731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608-14.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Wen LJ, Chen JH, Xu HJ, Yu Q, Liu K. Efficacy and Safety of Digital Single-Operator Cholangioscopy in the Diagnosis of Indeterminate Biliary Strictures by Targeted Biopsies: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Motomura Y, Akahoshi K, Kajiyama K. Utility of lavage cytology plus targeted biopsy during cholangioscopy for the diagnosis of indeterminate biliary lesions. Gastroenterol Hepatol Endosc. 2017;2:1-4. [DOI] [Full Text] |
| 31. | Bang JY, Navaneethan U, Hasan M, Sutton B, Hawes R, Varadarajulu S. Optimizing Outcomes of Single-Operator Cholangioscopy-Guided Biopsies Based on a Randomized Trial. Clin Gastroenterol Hepatol. 2020;18:441-448.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Fung BM, Tabibian JH. Biliary endoscopy in the management of primary sclerosing cholangitis and its complications. Liver Res. 2019;3:106-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Stiehl A, Rudolph G, Klöters-Plachky P, Sauer P, Walker S. Development of dominant bile duct stenoses in patients with primary sclerosing cholangitis treated with ursodeoxycholic acid: outcome after endoscopic treatment. J Hepatol. 2002;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Aabakken L, Karlsen TH, Albert J, Arvanitakis M, Chazouilleres O, Dumonceau JM, Färkkilä M, Fickert P, Hirschfield GM, Laghi A, Marzioni M, Fernandez M, Pereira SP, Pohl J, Poley JW, Ponsioen CY, Schramm C, Swahn F, Tringali A, Hassan C. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. Endoscopy. 2017;49:588-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Waldthaler A, Schramm C, Bergquist A. Present and future role of endoscopic retrograde cholangiography in primary sclerosing cholangitis. Eur J Med Genet. 2021;64:104231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Arnelo U, von Seth E, Bergquist A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy. 2015;47:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Kaura K, Sawas T, Bazerbachi F, Storm AC, Martin JA, Gores GJ, Abu Dayyeh BK, Topazian MD, Levy MJ, Petersen BT, Chandrasekhara V. Cholangioscopy Biopsies Improve Detection of Cholangiocarcinoma When Combined with Cytology and FISH, but Not in Patients with PSC. Dig Dis Sci. 2020;65:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Fujisawa T, Ushio M, Takahashi S, Yamagata W, Takasaki Y, Suzuki A, Okawa Y, Ochiai K, Tomishima K, Ishii S, Saito H, Isayama H. Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Sandha G, D'Souza P, Halloran B, Montano-Loza AJ. A Cholangioscopy-Based Novel Classification System for the Phenotypic Stratification of Dominant Bile Duct Strictures in Primary Sclerosing Cholangitis-the Edmonton Classification. J Can Assoc Gastroenterol. 2018;1:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Tyberg A, Raijman I, Siddiqui A, Arnelo U, Adler DG, Xu MM, Nassani N, Sejpal DV, Kedia P, Nah Lee Y, Gress FG, Ho S, Gaidhane M, Kahaleh M. Digital Pancreaticocholangioscopy for Mapping of Pancreaticobiliary Neoplasia: Can We Alter the Surgical Resection Margin? J Clin Gastroenterol. 2019;53:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | De Luca L, Repici A, Koçollari A, Auriemma F, Bianchetti M, Mangiavillano B. Pancreatoscopy: An update. World J Gastrointest Endosc. 2019;11:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Bokemeyer A, Gross D, Brückner M, Nowacki T, Bettenworth D, Schmidt H, Heinzow H, Kabar I, Ullerich H, Lenze F. Digital single-operator cholangioscopy: a useful tool for selective guidewire placements across complex biliary strictures. Surg Endosc. 2019;33:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Pillai S, Zhou GX, Arnaud P, Jiang H, Butler WJ, Zhang H. Antibodies to endometrial transferrin and alpha 2-Heremans Schmidt (HS) glycoprotein in patients with endometriosis. Am J Reprod Immunol. 1996;35:483-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Imanishi M, Ogura T, Kurisu Y, Onda S, Takagi W, Okuda A, Miyano A, Amano M, Nishioka N, Masuda D, Higuchi K. A feasibility study of digital single-operator cholangioscopy for diagnostic and therapeutic procedure (with videos). Medicine (Baltimore). 2017;96:e6619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Natov NS, Horton LC, Hegde SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc. 2017;9:238-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Gunasingam N, Craig PI. Cholangioscopy-directed radiofrequency ablation of complex biliary cholangiocarcinoma. VideoGIE. 2019;4:211-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 932] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 49. | Wadsworth CA, Westaby D, Khan SA. Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Larghi A, Rimbaș M, Tringali A, Boškoski I, Rizzatti G, Costamagna G. Endoscopic radiofrequency biliary ablation treatment: A comprehensive review. Dig Endosc. 2019;31:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Talreja JP, DeGaetani M, Sauer BG, Kahaleh M. Photodynamic therapy for unresectable cholangiocarcinoma: contribution of single operator cholangioscopy for targeted treatment. Photochem Photobiol Sci. 2011;10:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Chahal P, Baron TH. Endoscopic palliation of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Ortner ME, Caca K, Berr F, Liebetruth J, Mansmann U, Huster D, Voderholzer W, Schachschal G, Mössner J, Lochs H. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 54. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Boeva I, Karagyozov PI, Tishkov I. Post-liver transplant biliary complications: Current knowledge and therapeutic advances. World J Hepatol. 2021;13:66-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (5)] |
| 56. | Martins FP, Ferrari AP. Cholangioscopy-assisted guidewire placement in post-liver transplant anastomotic biliary stricture: efficient and potentially also cost-effective. Endoscopy. 2017;49:E283-E284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Franzini T, Sagae VMT, Guedes HG, Sakai P, Waisberg DR, Andraus W, D'Albuquerque LAC, Sethi A, de Moura EGH. Cholangioscopy-guided steroid injection for refractory post liver transplant anastomotic strictures: a rescue case series. Ther Adv Gastrointest Endosc. 2019;12:2631774519867786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Moy BT, Birk JW. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J Clin Transl Hepatol. 2019;7:61-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 59. | Wu W, Faigel DO, Sun G, Yang Y. Non-radiation endoscopic retrograde cholangiopancreatography in the management of choledocholithiasis during pregnancy. Dig Endosc. 2014;26:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Barakat MT, Girotra M, Choudhary A, Huang RJ, Sethi S, Banerjee S. A prospective evaluation of radiation-free direct solitary cholangioscopy for the management of choledocholithiasis. Gastrointest Endosc. 2018;87:584-589.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Brewer Gutierrez OI, Godoy Brewer G, Zulli C, Tejaswi S, Pawa R, Jamidar P, Robles-Medranda C, Pawa S, Camilion JV, Oleas R, Parsa N, Runge T, Miaw D, Ichkhanian Y, Khashab MA. Multicenter experience with digital single-operator cholangioscopy in pregnant patients. Endosc Int Open. 2021;9:E116-E121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Achanta CR. Radiation-free removal of common bile duct stones in pregnancy facilitated by endoscopic ultrasound and single-operator cholangioscopy. Endoscopy. 2021;53:E100-E101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Uradomo L, Pandolfe F, Aragon G, Borum ML. SpyGlass cholangioscopy for management of choledocholithiasis during pregnancy. Hepatobiliary Pancreat Dis Int. 2011;10:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Sanaka MR, Wadhwa V, Patel M. Retrieval of proximally migrated biliary stent with direct peroral cholangioscopy with an ultraslim endoscope. Gastrointest Endosc. 2015;81:1483-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Ogura T, Okuda A, Miyano A, Nishioka N, Higuchi K. Migrated endoclip removal after cholecystectomy under digital single-operator cholangioscopy guidance. Endoscopy. 2018;50:E74-E75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Al Lehibi A, Al Mtawa A, Almasoudi T, Al Ghamdi A, Al Otaibi N, Al Balkhi A. Removal of proximally migrated biliary stents by using single-operator cholangioscopy. VideoGIE. 2020;5:213-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Bas-Cutrina F, Garcia-Sumalla A, Velasquez J, Consiglieri CF, Lladó L, Gornals JB. Removal of a migrated biliary stent using new digital cholangioscopy retrieval devices in a transplant patient. Endoscopy. 2019;51:E323-E324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Zhang L, Craig PI. A case of hemobilia secondary to cancer of the gallbladder confirmed by cholangioscopy and treated with a fully covered self-expanding metal stent. VideoGIE. 2018;3:381-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Sum Foong K, Lee A, Kudakachira S, Ramberan H. Hemobilia from Biliary Angiodysplasia Diagnosed with Cholangioscopy. ACG Case Rep J. 2016;3:e132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Kedia P, Kuo V, Tarnasky P. Digital cholangioscopy-assisted endoscopic gallbladder drainage. Gastrointest Endosc. 2017;85:257-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Gutkin E, Hussain SA, Kim SH. The Successful Treatment of Chronic Cholecystitis with SpyGlass Cholangioscopy-Assisted Gallbladder Drainage and Irrigation through Self-Expandable Metal Stents. Gut Liver. 2012;6:136-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Tyberg A, Zerbo S, Kahaleh M, Sharaiha RZ. Digital cholangioscopy-assisted gallbladder drainage: seeing is accessing. Endoscopy. 2015;47 Suppl 1 UCTN:E417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Shi L, Lin S, Yao J, Li F. A novel endoscopic method for removal of giant calcified stool bezoar by cholangioscopy-guided electric hydraulic lithotripsy. Gastrointest Endosc. 2019;90:531-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | ASGE Technology Committee, Komanduri S, Thosani N, Abu Dayyeh BK, Aslanian HR, Enestvedt BK, Manfredi M, Maple JT, Navaneethan U, Pannala R, Parsi MA, Smith ZL, Sullivan SA, Banerjee S. Cholangiopancreatoscopy. Gastrointest Endosc. 2016;84:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Deprez PH, Garces Duran R, Moreels T, Furneri G, Demma F, Verbeke L, Van der Merwe SW, Laleman W. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy. 2018;50:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Karagyozov P, Boeva I, Tishkov I. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc. 2019;11:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 77. | Gravito-Soares M, Almeida N. Peroral Cholangiopancreatoscopy: New Advances Bring New Concerns. GE Port J Gastroenterol. 2018;25:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Lübbe J, Arnelo U, Lundell L, Swahn F, Törnqvist B, Jonas E, Löhr JM, Enochsson L. ERCP-guided cholangioscopy using a single-use system: nationwide register-based study of its use in clinical practice. Endoscopy. 2015;47:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Turowski F, Hügle U, Dormann A, Bechtler M, Jakobs R, Gottschalk U, Nötzel E, Hartmann D, Lorenz A, Kolligs F, Veltzke-Schlieker W, Adler A, Becker O, Wiedenmann B, Bürgel N, Tröger H, Schumann M, Daum S, Siegmund B, Bojarski C. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS™: results of a multicenter retrospective cohort study. Surg Endosc. 2018;32:3981-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Murabayashi T, Ogawa T, Koshita S, Kanno Y, Kusunose H, Sakai T, Masu K, Yonamine K, Miyamoto K, Kozakai F, Endo K, Noda Y, Ito K. Peroral Cholangioscopy-guided Electrohydraulic Lithotripsy with a SpyGlass DS Versus a Conventional Digital Cholangioscope for Difficult Bile Duct Stones. Intern Med. 2020;59:1925-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Xu MM, Kahaleh M. Recent developments in choledochoscopy: technical and clinical advances. Clin Exp Gastroenterol. 2016;9:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Subhash A, Abadir A, Iskander JM, Tabibian JH. Applications, Limitations, and Expansion of Cholangioscopy in Clinical Practice. Gastroenterol Hepatol (N Y). 2021;17:110-120. [PubMed] |
| 83. | Boston Scientific Corporation. An Expanding Suite of Compatible Accessories and Applications. [cited June 23, 2021]. Available from: https://www.bostonscientific.com/en-EU/products/direct-visualization-systems/spyglass-ds-direct-visualization-system/accessories-and-applications.html. |