Published online Nov 16, 2022. doi: 10.4253/wjge.v14.i11.718
Peer-review started: May 27, 2022
First decision: June 9, 2022
Revised: June 22, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 16, 2022
Processing time: 170 Days and 16.6 Hours
The prophylactic use of antibiotics in endoscopic retrograde cholangiopancreatography (ERCP) is still controversial.
To assess whether antibiotic prophylaxis reduces the rates of complications in patients undergoing elective ERCP.
This systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. A comprehensive search of multiple electronic databases was performed. Only randomized controlled trials were included. The outcomes analyzed included bacteremia, cholangitis, sepsis, pancreatitis, and mortality. The risk of bias was assessed by the Cochrane revised Risk-of-Bias tool for randomized controlled trials. The quality of evidence was assessed by the Grading of Recommendation Assessment, Deve
Ten randomized controlled trials with a total of 1757 patients that compared the use of antibiotic and non-antibiotic prophylaxis in patients undergoing elective ERCP were included. There was no significant difference between groups regarding incidence of cholangitis after ERCP [risk difference (RD) = -0.02, 95% confidence interval (CI): -0.05, 0.02, P = 0.32], cholangitis in patients with suspected biliary obstruction (RD = 0.02, 95%CI: -0.08 to 0.13, P = 0.66), cholangitis on intravenous antibiotic prophylaxis (RD = -0.02, 95%CI: -0.05 to 0.01, P = 0.25), septicemia (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.25), pancreatitis (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.19), and all-cause mortality (RD = 0.00, 95%CI: -0.01 to 0.01, P = 0.71]. However, the antibiotic prophylaxis group presented a 7% risk reduction in the incidence of bacteremia (RD= -0.07, 95%CI: -0.14 to -0.01, P = 0.03).
The prophylactic use of antibiotics in patients undergoing elective ERCP reduces the risk of bacteremia but does not appear to have an impact on the rates of cholangitis, septicemia, pancreatitis, and mortality.
Core Tip: There is controversy about antibiotic prophylaxis in patients undergoing elective endoscopic retrograde cholangiopancreatography. This is a systematic review and meta-analysis based on randomized controlled trials that analyzed whether the use of antibiotic prophylaxis is beneficial in preventing complications after this procedure. Outcomes evaluated include the rate of cholangitis, bacteremia, sepsis, pancreatitis, and mortality. Based on this meta-analysis, antibiotic prophylaxis reduces the risk of bacteremia but does not impact the rate of cholangitis, septicemia, pancreatitis, and mortality.
- Citation: Merchan MFS, de Moura DTH, de Oliveira GHP, Proença IM, do Monte Junior ES, Ide E, Moll C, Sánchez-Luna SA, Bernardo WM, de Moura EGH. Antibiotic prophylaxis to prevent complications in endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2022; 14(11): 718-730
- URL: https://www.wjgnet.com/1948-5190/full/v14/i11/718.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i11.718
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most technically challenging procedures in digestive endoscopy, associated with high rates of adverse events (AEs), reported in up to 18.9% of cases[1-3]. The most common adverse events include bacteremia, cholangitis, and pancreatitis occurring in about 6.5% to 18.0%[4], 3.0%[5,6], and 5.5%[7] respectively.
Prophylactic antibiotics are used with the intent to prevent complications of ERCP. Their use is controversial and is currently being recommended in patients with incomplete biliary drainage, such as hilar tumors and primary sclerosing cholangitis[8] due to the potential risk of septic complications from the manipulation of obstructed bile ducts that could serve as a source of bacterial colonization, thus increasing the risk of bacteremia[4] and cholangitis.
The European Society for Gastrointestinal Endoscopy[9] and the American Society for Gastr
Although both guidelines regarding antibiotic prophylaxis for ERCP do not recommend its routine use, the data to support this recommendation is not robust. Therefore, we performed a systematic review and meta-analysis to evaluate whether the use of antibiotic prophylaxis has an impact on the rate of complications related to elective ERCP.
The study protocol was registered in the International Prospective Register of Systematic Reviews under the file number CRD42022289127 and was approved by the Ethics Committee of Hospital das Clínicas, Faculty of Medicine at The University of São Paulo. This systematic review and meta-analysis were performed in conformity with the recommendations from the Cochrane Handbook of Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines[14].
Individualized searches of multiple electronic databases (MEDLINE, Embase, Cochrane, LILACS, clincaltrials.gov, and gray literature) were performed based upon a standardized protocol from their inception through February 2022. The search included the following Medical Subject Headings: “(Endoscopy OR Endoscopic) AND (Anti-Bacterial Agents OR Antibacterial Agents OR Antibacterial Agent OR Antibiotics OR Antibiotic) AND [prophylaxis OR preventive OR (prevention and control)].” A further literature search was conducted with the Reference Citation Analysis engine, an artificial intelligence technology-based open multidisciplinary citation analysis database (https://www.referencecitationanalysis.com). Following a search within the Reference Citation Analysis database no further studies were identified that fit our inclusion criteria.
Two researchers independently conducted the eligibility screening. From the initial search results, duplicate articles were excluded, and the titles and abstracts of all potentially relevant studies were screened for eligibility. Any disagreements were settled by consensus or by consulting a third reviewer.
Only randomized controlled trials (RCTs) comparing antibiotic prophylaxis vs no use of prophylactic antibiotics in patients undergoing elective ERCP regardless of publication date or language were considered.
Patients with cholangitis or other types of active infection, history of antibiotic allergy, and immun
Items included in data extraction were first author, year of publication, study design, and outcomes of interest such as cholangitis, bacteremia, septicemia, pancreatitis, and mortality. We defined cholangitis as the presence of fever (> 38.5 °C), abdominal pain, leukocytosis, and elevated C-reactive protein. Blood cultures and bile samples were taken to evaluate for bacteremia. Bacteremia was defined as a positive culture with no evidence of systemic inflammatory response. Blood culture samples were taken before and after the ERCP procedure and in the presence of fever. In one of the studies a blood culture was obtained only if the patients presented signs of cholangitis. Septicemia was defined as a positive blood culture with systemic inflammatory response (fever, hypotension, tachycardia, leukocytosis > 10 g/dL, leukopenia < 3 g/L, and chills). The diagnosis of pancreatitis was based on clinical findings, increased serum amylase or lipase three-fold or more over the normal upper range. Antibiotic prophylaxis is defined as administering antibiotics to patients who underwent invasive procedures without evidence of infection at the time of the procedure. The goal of such prophylaxis was to reduce the risk of infection.
We assessed the risk of bias using the Cochrane Risk of Bias tool version 2[14].
The quality of evidence was assessed utilizing the objective criteria from Grading Recommendations Assessment, Development, and Evaluation for each of the prespecified results and outcomes using the GRADEpro-Guideline Development Tool software (McMaster University, 2015; Evidence Prime, Inc., Ontario, Canada)[15].
Continuous variables were analyzed using mean difference and standard deviation with a 95% confidence interval. For categorical variables, the risk difference (RD) was used, with a 95% confidence interval. The RD and mean difference were considered statistically significant at a value of P ≤ 0.05. If a study provided medians and interquartiles or ranges, they were attributed to means, and standard deviation was estimated as described by the McGrath et al[16] method.
The inconsistency index was evaluated using the Higgins I2 method[17], in which the presence of heterogeneity can be observed. The random effect was used for all analyses. The meta-analysis was performed using the RevMan software (Review Manager Software version 5.4-Cochrane Collaboration Copyright© 2020).
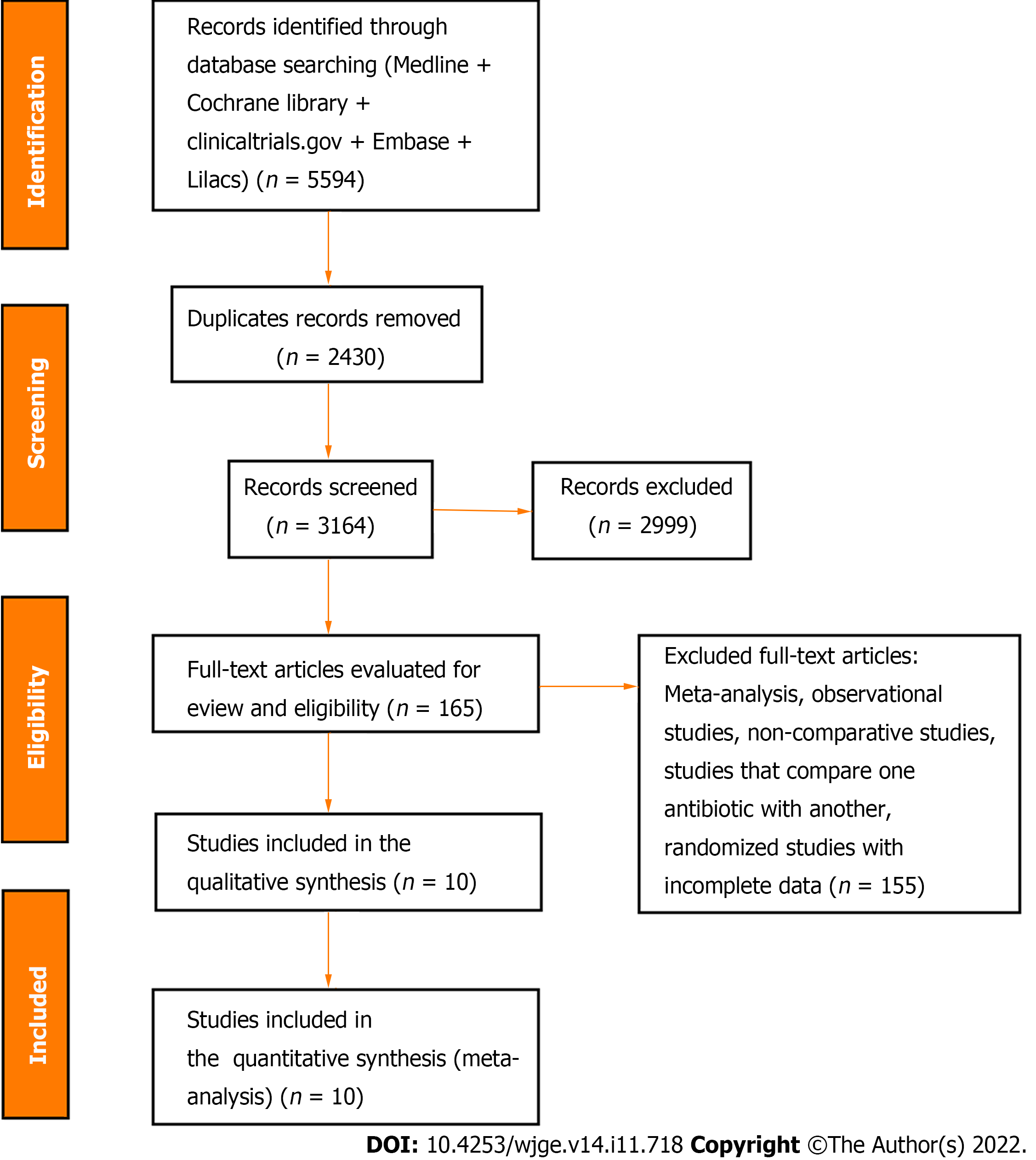
The initial search strategy identified 5594 articles. Through the evaluation by title and abstract, 2999 articles were excluded, yielding 165 studies. Of these, 10 RCTs, including 1757 patients (843 in the control group and 914 in the intervention group) met the eligibility criteria and were included in this systematic review and meta-analysis (Figure 1). The characteristics and results of the included studies are summarized in Table 1.
| Ref. | Year | Type of study | Intervention | Participants | Bacteremia | Cholangitis | Pancreatitis | Septicemia | Mortality |
| Brandes et al[23] | 1981 | RCT | Minocycline 300 mg orally | Total: 118 | N/A | Intervention: 0/39 | Intervention: 1/39 | N/A | N/A |
| Antibiotics: 39 | Control: 1/79 | Control: 2/79 | |||||||
| Control: 79 | |||||||||
| Sauter et al[22] | 1990 | RCT | Cefotaxime 2 g IV, 15 min before ERCP | Total: 100 | Intervention: 1/50 | Intervention: 1/50 | Intervention: 0/50 | Intervention: 0/50 | Intervention: 0/50 |
| Antibiotics: 50 | Control: 8/50 | Control: 2/50 | Control: 0/50 | Control: 0/50 | Control: 0/50 | ||||
| Control: 50 | |||||||||
| Niederau et al[21] | 1994 | RCT | Cefotaxime 2 g IV. 15 min before ERCP | Total: 100 | Intervention: 0/50 | Intervention: 0/50 | Intervention: 2/50 | Intervention: 0/50 | Intervention: 0/50 |
| Antibiotics: 50 | Control: 4/50 | Control: 4/50 | Control: 3/50 | Control: 8/50 | Control: 0/50 | ||||
| Control: 50 | |||||||||
| Byl et al[20] | 1995 | RCT | Piperacillin, 4 g IV, 3/d | Total: 68 | Intervention: 0/30 | Intervention: 2/34 | N/A | Intervention: 0/30 | Intervention: 0/34 |
| Antibiotics: 34 | Control: 7/32 | Control: 10/34 | Control: 5/32 | Control: 5/34 | |||||
| Control: 34 | |||||||||
| Finkelstein et al[27] | 1996 | RCT | Cefonicid 1 g IV, 1 h before ERCP | Total: 179 | Intervention: 3/88 | Intervention: 7/88 | N/A | Intervention: 0/88 | Intervention: 0/88 |
| Antibiotics: 88 | Control: 2/91 | Control: 2/91 | Control: 0/91 | Control: 0/91 | |||||
| Control: 91 | |||||||||
| Lorenz et al[24] | 1996 | RCT | Cefuroxime 1.5 g IV, 30 min before ERCP | Total: 99 | Intervention: 3/49 | N/A | N/A | Intervention: 3/49 | Intervention: 0/49 |
| Antibiotics: 49 | Control: 8/50 | Control: 5/50 | Control: 0/50 | ||||||
| Control: 50 | |||||||||
| van den Hazel et al[19] | 1996 | RCT | Piperacillin 4 g IV, 30 min before ERCP | Total: 551 | N/A | Intervention: 12/170 | N/A | Intervention: 2/170 | Intervention: 3/170 |
| Antibiotics: 270 | Control: 17/281 | Control: 3/281 | Control: 2/281 | ||||||
| Control: 281 | |||||||||
| Räty et al[18] | 2001 | RCT | 2g of ceftazidime IV, 30 min before ERCP | Total: 315 | N/A | Intervention: 0/155 | Intervention: 4/155 | N/A | Intervention: 1/155 |
| Antibiotics: 155 | Control: 7/160 | Control: 15/160 | Control: 0/160 | ||||||
| Control: 160 | |||||||||
| Spicak et al[26] | 2002 | RCT | Amoxicillin – clavulanic acid 2.4 g IV | Total 165 | Intervention: 18/73 | Intervention: 4/77 | Intervention: 6/77 | N/A | Intervention: 2/77 |
| Antibiotics: 77 | Control: 24/84 | Control: 3/88 | Control: 10/88 | Control: 2/88 | |||||
| Control: 88 | |||||||||
| Llach et al[25] | 2006 | RCT | Clindamycin 600 mg and gentamicin 80 mg IM, 1 h before ERCP | Total: 62 | Intervention: 2/31 | Intervention: 1/31 | N/A | Intervention: 0/31 | Intervention: 0/31 |
| Antibiotics: 31 | Control: 2/30 | Control: 1/31 | Control: 0/30 | Control: 0/30 | |||||
| Control: 31 |
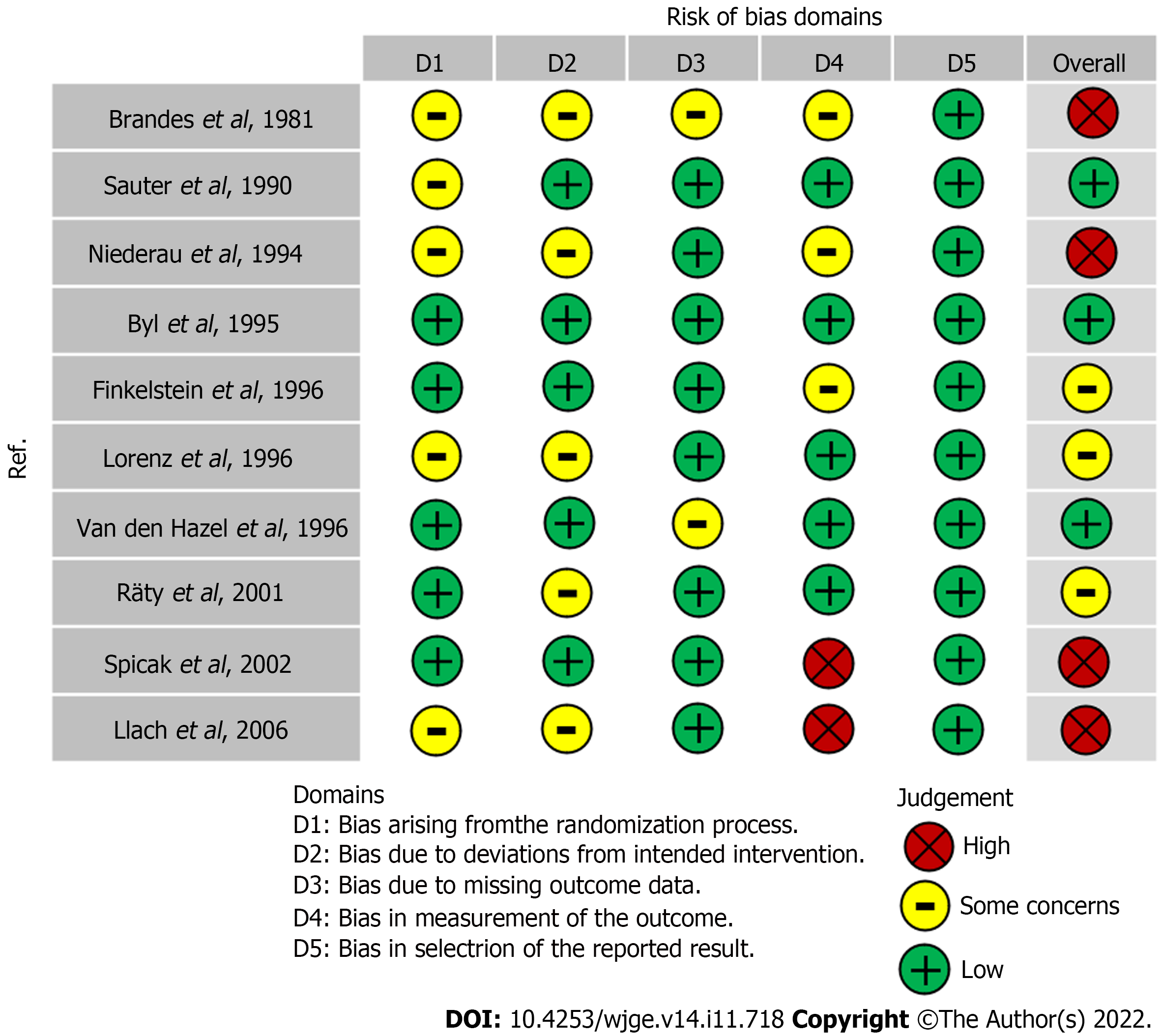
All 10 studies[18-27] were RCTs. Three studies presented a low risk bias[19,20,22]. Three studies presented a moderate risk of bias[18,24,27]. Four studies presented a serious risk of bias[21,23,25,26]. Detailed information concerning the risk of bias for each outcome is described in Figure 2.
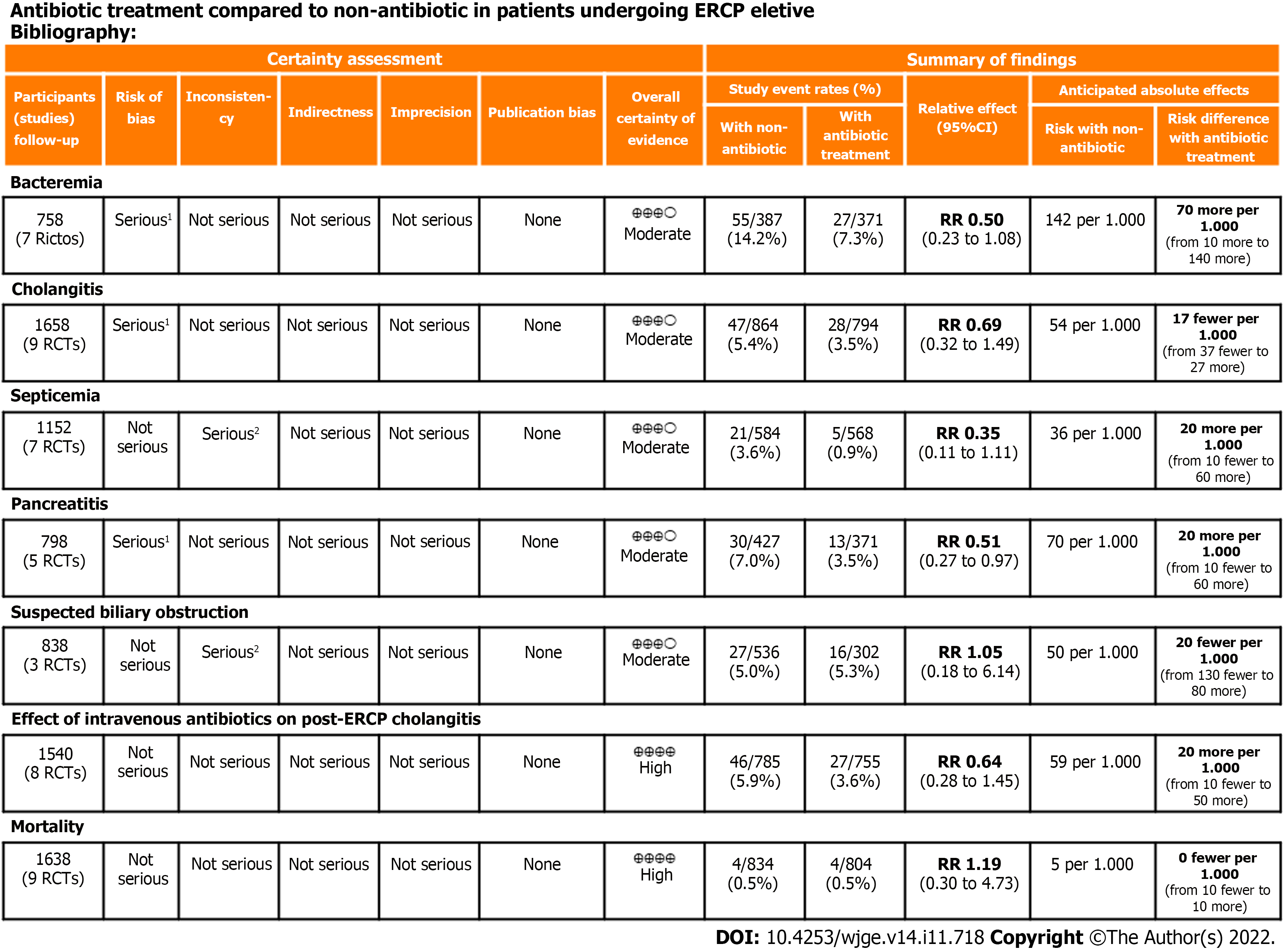
The overall quality of evidence was moderate for the outcomes of bacteremia, cholangitis, septicemia, pancreatitis, and cholangitis in patients with suspected biliary obstruction. The quality of evidence was high for the outcomes of cholangitis in patients on intravenous antibiotic prophylaxis and mortality. Detailed information on the quality of evidence (Grading Recommendations Assessment, Development, and Evaluation) is described in Figure 3.
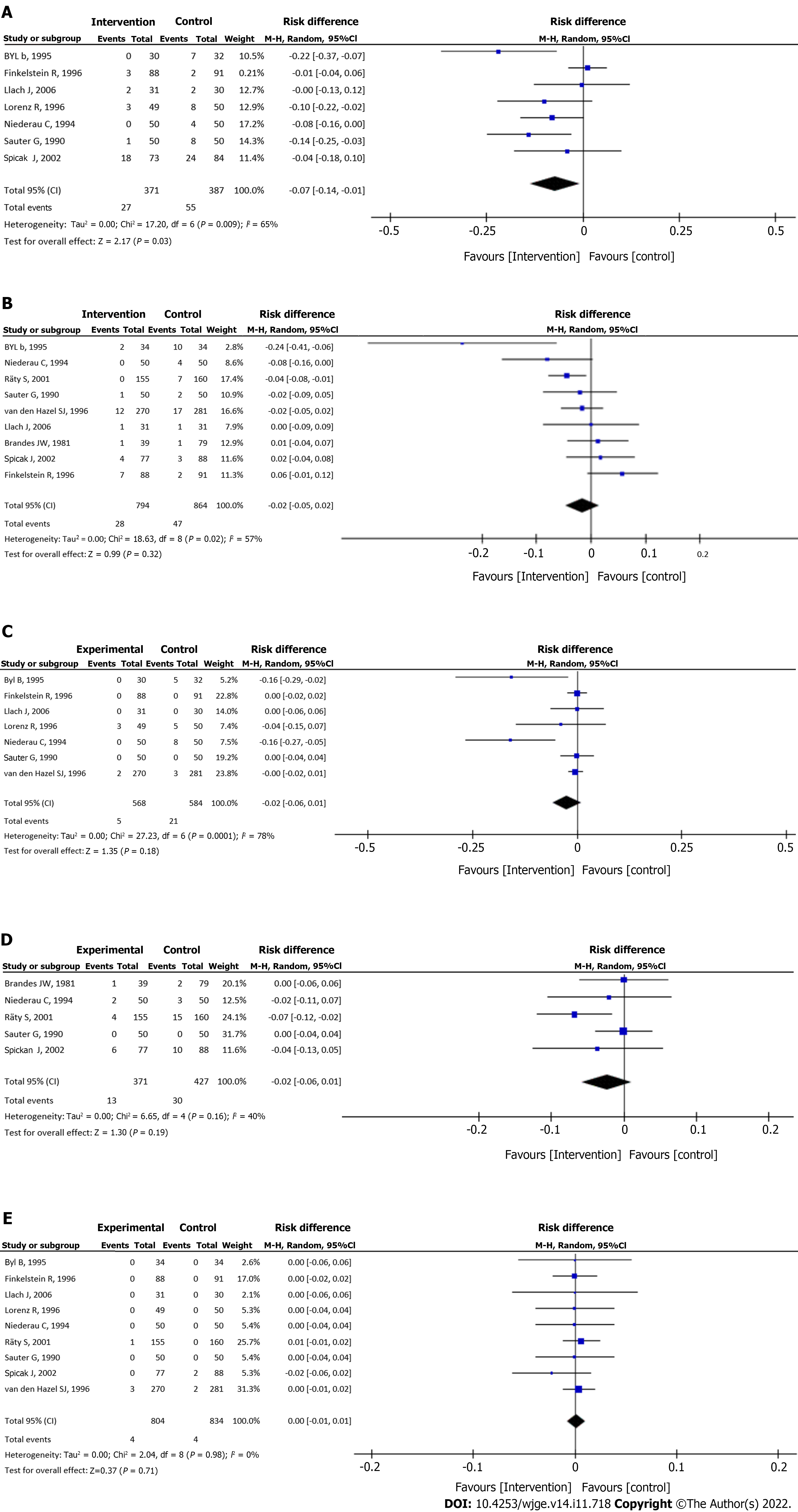
Bacteremia: Data from seven studies[20-22,24-27] were evaluated in a total of 758 patients: 371 in the intervention group and 378 in the control group. The intervention group presented a bacteremia rate of less than 7% with a statistical difference compared to the control group (RD = -0.07, 95%CI: -0.14 to -0.01, P = 0.03) (Figure 4A).
Cholangitis: Analysis of nine studies[18-23,25-27], totaling 1658 patients (794 in the intervention group and 864 in the control group) showed no significant differences between the groups (RD = -0.02, 95%CI: -0.05 to 0.02, P = 0.32) (Figure 4B).
Septicemia: Septicemia was evaluated in seven studies[19-22,24,25,27], totaling 1152 patients (568 assigned to the intervention group and 584 to the control group) and showed no significant differences between the groups (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.18) (Figure 4C).
Pancreatitis: Pancreatitis was evaluated in five studies[18,21-23,26], totaling 798 patients (371 assigned to the intervention group and 427 to the control group) and showed no significant differences between the groups (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.19) (Figure 4D).
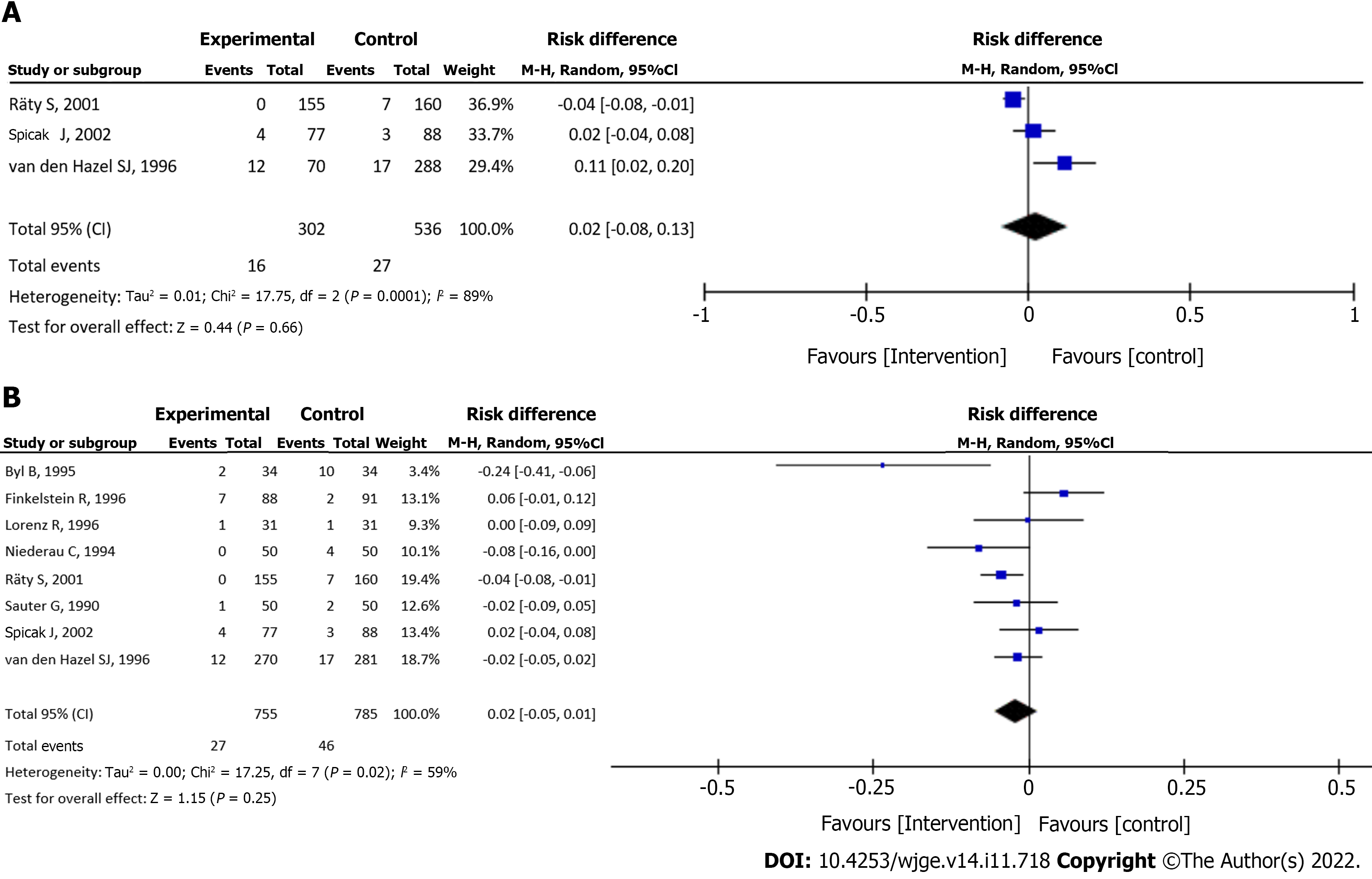
Cholangitis in patients with suspected biliary obstruction: Data from three studies[18,19,26] were evaluated in a total of 838 patients (302 assigned to the intervention group and 536 to the control group) and showed no significant difference between the groups (RD = 0.02, 95%CI: -0.08 to 0.13, P = 0.66) (Figure 5A).
Cholangitis in patients on intravenous antibiotic prophylaxis: Analysis of eight studies[18-22,24,26,27], totaling 1540 patients (755 assigned to the intervention group and 785 to the control group) showed no significant difference between the groups (RD = -0.02, 95%CI: -0.05 to 0.01, P = 0.25) (Figure 5B).
Mortality: Mortality rate was evaluated in nine studies[18-22,24-27], totaling 1638 patients (804 of the intervention group and 834 of the control group) and showed no significant difference between the groups (RD = 0.00, 95%CI: -0.01 to 0.01, P = 0.71) (Figure 4E).
We analyzed 10 RCTs to assess whether antibiotic prophylaxis positively impacts patients undergoing elective ERCP, thus preventing complications after the procedure. Including a total of 1757 patients, this meta-analysis showed no statistical difference in the rates of cholangitis, septicemia, pancreatitis, and mortality. However, our study showed a lower bacteremia rate in the antibiotic group.
Although our systematic review and meta-analysis revealed less risk of bacteremia in the group that underwent antibiotic prophylaxis, there are doubts about whether this finding has any clinical relevance. Antibiotics are highly prescribed drugs in clinical practice. It is estimated that about 50% of antibiotic use in hospitals (both outpatient and inpatient) is not appropriately prescribed[28]. A meta-analysis published in 2009, which evaluated ERCP-induced cholangitis as an outcome, showed that antibiotics do not prevent cholangitis[29]. However, another meta-analysis from 2010 showed that prophylactic antibiotics could reduce bacteremia rates and may prevent cholangitis in patients undergoing elective ERCP[30]. Nonetheless, due to conflicting findings in the literature, it is not possible to state that reducing bacteremia rates leads to less cholangitis. Another critical point is that the indiscriminate use of antibiotics has the potential to increase bacterial resistance and lead to the emergence of multiresistant germs[31]. Antimicrobial drug resistance is a global health problem that causes a high impact and inflicts an enormous economic burden worldwide. The World Health Organization reported that the ratio of morbidity and mortality rate of diseases due to the spreading of multidrug resistant strains will lead to a substantial economic loss of approximately 100 trillion US Dollars by 2050[32].
Post-ERCP cholangitis, although infrequent, is a significant concern due to its 3% mortality rate. It is mainly associated with incomplete drainage of the bile ducts, equipment contamination[8], or an immunosuppressed state[4]. Many studies[18-23,25-27] demonstrated that prophylactic antibiotics administered in patients undergoing elective ERCP do not reduce the risk of cholangitis. A prospective study that analyzed antibiotic prophylaxis in patients undergoing elective ERCP published[33] in 2014 with 138 patients who underwent this procedure showed that cholangitis was greater when incomplete biliary drainage was present. They concluded there was no benefit in using prophylactic antibiotics to reduce cholangitis and sepsis in patients with satisfactory biliary drainage. Another retrospective study published in 2008[11], with 11484 patients over 11 years to identify post-ERCP infections, was per
Sepsis is a significant cause of morbidity and mortality worldwide[34]. Antibacterial therapy is the cornerstone treatment for infection[35], reducing the risk of septic complications and the length of stay. However, prophylactic use of antibiotic agents is not a consensus in terms of minimizing infection risk after some procedures. In ERCP, the main factor for developing clinically relevant sepsis appears to be biliary obstruction. The presumed mechanism by which obstruction leads to sepsis is increased biliary pressure leading to bile-venous reflux. The manner this manifests clinically depends on the content of the bile: whether it contains a contrast medium during ERCP or percutaneous transhepatic cholangiography[36]. The use of prophylactic antibiotics to prevent bacterial colonization in an unobstructed biliary system is not recommended because bacteria in the bile (bacterobilia) are clinically silent. On the other hand, using prophylactic antibiotics appears to be beneficial for patients with biliary obstruction and known or suspected bacterobilia. Antibiotics should be continued until the obstruction is relieved. In addition, antibiotic prophylaxis to prevent biliary colonization that can lead to systemic sepsis is warranted in particular circumstances of an immunocompromised patient or a patient with primary sclerosing cholangitis[37]. When analyzing specific trials of patients with suspected biliary obstruction[18,19,26], they also showed no significant effect in antibiotic prophylaxis to prevent cholangitis, especially when drainage was effective. The study published in 2007 by Thawee et al[38], including patients who underwent complete biliary drainage, showed that antibiotic prophylaxis did not reduce the rate of cholangitis.
Studies[18-22,24,26,27] that used the intravenous route of administration of prophylactic antibiotics found no significant differences in the incidence of cholangitis. The type of antibiotic also did not influence the prevention of infectious complications. It should be noted that many classes of antibiotics were used, so it is not possible to determine which of them may be indicated for antibiotic prophylaxis. Besides, it is important to study the best antibiotic regimen and dosage when indicated, which is still not clear in the current literature.
In the present study, there was no significant difference in the incidence of pancreatitis in patients undergoing ERCP. The most recent study[39] from 2015 demonstrated that antibiotic prophylaxis did not influence the rate of pancreatitis in patients with risk factors such as choledocholithiasis, primary sclerosing cholangitis, and incomplete biliary drainage.
Also, there was no significant difference between the intervention and control groups regarding mortality. In general, mortality rates in the analyzed studies were low. The deaths were related to bleeding from percutaneous transhepatic drainage, cholangitis, severe sepsis, and pancreatic cancer.
Despite this being the largest study on the subject and included only RCTs, our study was not exempt from limitations. Some of the included studies[21,23,25,26] presented a high risk of bias. Also, in some studies[27], some high-risk groups (patients with incomplete biliary drainage) were not excluded when analyzing the results of cholangitis and sepsis. The absence of a homogeneous antibiotic regimen protocol and standardized methods to assess bacterial resistance may also limit the interpretation of the results. Also, the studies included in this meta-analysis are not recent, but this could be explained because during our literature search we found randomized studies that did not reach the estimated sample size of patients and thus were not included for this reason. Others are still under development. However, for our systematic review and meta-analysis, we relied on current clinical guidelines with recommendations on the use of antibiotic prophylaxis as well as references from recent prospective clinical studies that also analyzed its use.
Overall, antibiotic prophylaxis for ERCP reduces the rate of bacteremia without affecting other complications. Bacteremia is defined as the presence of bacteria in the bloodstream[40]. Among hospitalized patients, the incidence of bacteremia is highest within a few days of admission and varies according to clinical and patient characteristics[41]. Bacteremia related to endoscopic procedures can result in local infections due to contamination of “sterile” bile ducts by an endoscopic accessory and contrast material[42]. Patients undergoing ERCP may develop infectious complications depending on their comorbidities, especially in those in whom immunity is compromised and in patients with incomplete biliary drainage. In these patients, the use of prophylactic antibiotics is recommended. Appropriate use of antibiotics will reduce hospitalization time, health care costs, and the risk of mortality. On the other hand, the indiscriminate and inappropriate use of antibiotics is of concern, and bacterial resistance has become an increasing challenge. Also, the profile of procedure-related pathogens has evolved in recent years and multidrug resistant organisms have been reported[42]. Therefore, appropriate and timely selection of empiric antimicrobial treatment has become difficult. The clinical relevance and bacterial resistance should be weighed before routinely using antibiotic prophylaxis for ERCP. Considering the findings of our meta-analysis and in agreement with previous studies[29,30], the recommendation to not use antibiotic prophylaxis is maintained.
Prophylactic antibiotics reduce the rate of bacteremia in patients undergoing elective ERCP. However, its use does not have an impact on other associated complications such as cholangitis, septicemia, pancreatitis, and mortality.
The prophylactic use of antibiotics in endoscopic retrograde cholangiopancreatography (ERCP) is controversial. The most common adverse events include bacteremia, cholangitis, and pancreatitis. Although recent guidelines regarding antibiotic prophylaxis for ERCP do not recommend its routine use, the data to support this recommendation is not robust.
Antimicrobial drug resistance is a global health problem that causes a high impact and inflicts an enormous economic burden worldwide. The World Health Organization reported that the ratio of morbidity and the mortality rate of diseases due to the spreading of multidrug resistant strains will lead to a substantial economic loss by 2050. Due to the lack of data in the literature, we performed a systematic review and meta-analysis to evaluate whether antibiotic prophylaxis impacts the rate of complications related to elective ERCP.
This systematic review and meta-analysis aimed to assess whether antibiotic prophylaxis reduced the rates of complications such as bacteremia, cholangitis, sepsis, pancreatitis, and mortality in patients undergoing elective ERCP.
This systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. A comprehensive search of multiple electronic databases was performed only including randomized controlled trials.
Ten randomized clinical trials with a total of 1757 patients that compared the use of antibiotic and non-antibiotic prophylaxis in patients undergoing elective ERCP were included. There was no significant difference between groups regarding the incidence of cholangitis [risk difference (RD) = -0.02, 95% confidence interval (CI): -0.05 to 0.02, P = 0.32], cholangitis in patients with suspected biliary obstruction (RD = 0.02, 95%CI: -0.08 to 0.13, P = 0.66), cholangitis on intravenous antibiotic prophylaxis (RD = -0.02, 95%CI: -0.05 to 0.01, P = 0.25), septicemia (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.25), pancreatitis (RD = -0.02, 95%CI: -0.06 to 0.01, P = 0.19), and all-cause mortality (RD = 0.00, 95%CI: -0.01 to 0.01, P = 0.71). However, the antibiotic prophylaxis group presented a 7% risk reduction in the incidence of bacteremia (RD= -0.07, 95%CI: -0.14 to -0.01, P = 0.03).
Considering our findings, antibiotic prophylaxis in patients undergoing elective ERCP reduces the risk of bacteremia. Still, it does not appear to impact the rate of other adverse events.
Antibiotics are highly prescribed drugs in clinical practice, but they can have adverse effects. Larger randomized controlled trials regarding the use of prophylactic antibiotics on ERCP in specific populations of patients are still warranted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chang A, Thailand; Ding J, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Cai YX
| 1. | Han S, Attwell AR, Tatman P, Edmundowicz SA, Hammad HT, Wagh MS, Wani S, Shah RJ. Adverse Events Associated With Therapeutic Endoscopic Retrograde Pancreatography. Pancreas. 2021;50:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Fujita K, Yazumi S, Matsumoto H, Asada M, Nebiki H, Matsumoto K, Maruo T, Takenaka M, Tomoda T, Onoyama T, Kurita A, Ueki T, Katayama T, Kawamura T, Kawamoto H; Bilio-pancreatic Study Group of West Japan. Multicenter prospective cohort study of adverse events associated with biliary endoscopic retrograde cholangiopancreatography: Incidence of adverse events and preventive measures for post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2022;34:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP Jr, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 4. | Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 5. | Chen M, Wang L, Wang Y, Wei W, Yao YL, Ling TS, Shen YH, Zou XP. Risk factor analysis of post-ERCP cholangitis: A single-center experience. Hepatobiliary Pancreat Dis Int. 2018;17:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Bilbao MK, Dotter CT, Lee TG, Katon RM. Complications of endoscopic retrograde cholangiopancreatography (ERCP). A study of 10,000 cases. Gastroenterology. 1976;70:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 352] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | García-Cano Lizcano J, González Martín JA, Morillas Ariño J, Pérez Sola A. Complications of endoscopic retrograde cholangiopancreatography. A study in a small ERCP unit. Rev Esp Enferm Dig. 2004;96:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Motte S, Deviere J, Dumonceau JM, Serruys E, Thys JP, Cremer M. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology. 1991;101:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 496] [Article Influence: 99.2] [Reference Citation Analysis (1)] |
| 10. | ASGE Standards of Practice Committee; Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 11. | Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: a sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Othman MO, Guerrero R, Elhanafi S, Davis B, Hernandez J, Houle J, Mallawaarachchi I, Dwivedi AK, Zuckerman MJ. A prospective study of the risk of bacteremia in directed cholangioscopic examination of the common bile duct. Gastrointest Endosc. 2016;83:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Bangarulingam SY, Gossard AA, Petersen BT, Ott BJ, Lindor KD. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15191] [Article Influence: 2531.8] [Reference Citation Analysis (0)] |
| 15. | Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11058] [Cited by in RCA: 14897] [Article Influence: 876.3] [Reference Citation Analysis (0)] |
| 16. | McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A; DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020;29:2520-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 485] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 18. | Räty S, Sand J, Pulkkinen M, Matikainen M, Nordback I. Post-ERCP pancreatitis: reduction by routine antibiotics. J Gastrointest Surg. 2001;5:339-45; discussion 345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | van den Hazel SJ, Speelman P, Dankert J, Huibregtse K, Tytgat GN, van Leeuwen DJ. Piperacillin to prevent cholangitis after endoscopic retrograde cholangiopancreatography. A randomized, controlled trial. Ann Intern Med. 1996;125:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Byl B, Devière J, Struelens MJ, Roucloux I, De Coninck A, Thys JP, Cremer M. Antibiotic prophylaxis for infectious complications after therapeutic endoscopic retrograde cholangiopancreatography: a randomized, double-blind, placebo-controlled study. Clin Infect Dis. 1995;20:1236-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Niederau C, Pohlmann U, Lübke H, Thomas L. Prophylactic antibiotic treatment in therapeutic or complicated diagnostic ERCP: results of a randomized controlled clinical study. Gastrointest Endosc. 1994;40:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sauter G, Grabein B, Huber G, Mannes GA, Ruckdeschel G, Sauerbruch T. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography. A randomized controlled study. Endoscopy. 1990;22:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Brandes JW, Scheffer B, Lorenz-Meyer H, Körst HA, Littmann KP. ERCP: Complications and prophylaxis a controlled study. Endoscopy. 1981;13:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Lorenz R, Lehn N, Born P, Herrmann M, Neuhaus H. [Antibiotic prophylaxis using cefuroxime in bile duct endoscopy]. Dtsch Med Wochenschr. 1996;121:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 25. | Llach J, Bordas JM, Almela M, Pellisé M, Mata A, Soria M, Fernández-Esparrach G, Ginès A, Elizalde JI, Feu F, Piqué JM. Prospective assessment of the role of antibiotic prophylaxis in ERCP. Hepatogastroenterology. 2006;53:540-542. [PubMed] |
| 26. | Spicak J, Stirand P, Zavoral M, Keil R, Zavada F, Drabek J. Antibiotic prophylaxis of cholangitis complicating endoscopic management of biliary obstruction (*T1753). Gastrointest Endosc. 2002;55:AB150-AB157. [DOI] [Full Text] |
| 27. | Finkelstein R, Yassin K, Suissa A, Lavy A, Eidelman S. Failure of cefonicid prophylaxis for infectious complications related to endoscopic retrograde cholangiopancreatography. Clin Infect Dis. 1996;23:378-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Botelho J, Grosso F, Peixe L. Antibiotic resistance in Pseudomonas aeruginosa - Mechanisms, epidemiology and evolution. Drug Resist Updat. 2019;44:100640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 29. | Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: a meta-analysis. Pancreas. 2009;38:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Brand M, Bizos D, O'Farrell P Jr. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010;CD007345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Minami T, Sasaki T, Serikawa M, Ishigaki T, Murakami Y, Chayama K. Antibiotic prophylaxis for endoscopic retrograde chlangiopancreatography increases the detection rate of drug-resistant bacteria in bile. J Hepatobiliary Pancreat Sci. 2014;21:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Indraningrat AA, Smidt H, Sipkema D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Mar Drugs. 2016;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Voiosu TA, Bengus A, Haidar A, Rimbas M, Zlate A, Balanescu P, Voiosu A, Voiosu R, Mateescu B. Antibiotic Prophylaxis Prior to Elective ERCP Does Not Alter Cholangitis Rates or Shorten Hospital Stay: Results of an Observational Prospective Study of 138 Consecutive ERCPS. Maedica (Bucur). 2014;9:328-332. [PubMed] |
| 34. | Salomão R, Ferreira BL, Salomão MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: evolving concepts and challenges. Braz J Med Biol Res. 2019;52:e8595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 35. | Minasyan H. Sepsis: mechanisms of bacterial injury to the patient. Scand J Trauma Resusc Emerg Med. 2019;27:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 36. | Yoshimoto H, Ikeda S, Tanaka M, Matsumoto S. Relationship of biliary pressure to cholangiovenous reflux during endoscopic retrograde balloon catheter cholangiography. Dig Dis Sci. 1989;34:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Subhani, Kibbler, Dooley. Review article: antibiotic prophylaxis for endoscopic retrograde cholangiopancreatography (ERCP): REVIEW: ANTIBIOTIC PROPHYLAXIS FOR ERCP. Aliment Pharmacol Ther. 1999;13:103-16.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Ratanachu-ek T, Prajanphanit P, Leelawat K, Chantawibul S, Panpimanmas S, Subwongcharoen S, Wannaprasert J. Role of ciprofloxacin in patients with cholestasis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2007;13:276-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Ishigaki T, Sasaki T, Serikawa M, Kobayashi K, Kamigaki M, Minami T, Okazaki A, Yukutake M, Ishii Y, Kosaka K, Mouri T, Yoshimi S, Chayama K. Evaluation of antibiotic use to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis and cholangitis. Hepatogastroenterology. 2015;62:417-424. [PubMed] |
| 40. | Yamashiro Y, Nomoto K. Bacteremia and Probiotics: A Novel Culture-Independent Analytical Method Evolves Disease Concepts. Ann Nutr Metab. 2017;71 Suppl 1:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Nielsen SL. The incidence and prognosis of patients with bacteremia. Dan Med J. 2015;62. [PubMed] |
| 42. | Deb A, Perisetti A, Goyal H, Aloysius MM, Sachdeva S, Dahiya D, Sharma N, Thosani N. Gastrointestinal Endoscopy-Associated Infections: Update on an Emerging Issue. Dig Dis Sci. 2022;67:1718-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |