Published online Nov 16, 2022. doi: 10.4253/wjge.v14.i11.694
Peer-review started: September 5, 2022
First decision: October 12, 2022
Revised: October 20, 2022
Accepted: November 6, 2022
Article in press: November 6, 2022
Published online: November 16, 2022
Processing time: 68 Days and 21.8 Hours
The presence of premalignant polyps on colonoscopy is an indicator of meta
To assess the effects of looping on premalignant polyp detection using logistic regression analyses.
We retrospectively investigated patients who underwent colonoscopy at Toy
We assessed 12259 patients (mean age, 53.6 years; men, 50.7%). Looping occurred in 54.3% of the patients. Mild and severe looping were noted in 4399 and 2253 patients, respectively. The detection rates of adenomas, advanced adenomas, high-risk adenomas, clinically significant serrated polyps (CSSPs), and sessile serrated lesions (SSLs) were 44.7%, 2.0%, 9.9%, 8.9% and 3.5%, respectively. The mean numbers of adenomas and SSLs were 0.82 and 0.04, respectively. The detection rates of adenomas, high-risk adenomas, and CSSPs increased with looping severity (all P < 0.001). The number of adenomas increased with looping severity (P < 0.001). Multivariate analyses found that detection of adenomas, high-risk adenomas, and CSSPs was associated with severe looping (P < 0.001, P < 0.001, and P = 0.007, respectively) regardless of age, sex, time required for colonoscope insertion and withdrawal, and endoscopist experience.
Looping severity was independently associated with high detection rates of premalignant polyps. Therefore, looping may predict the risk of metachronous colorectal cancer. Endoscopists should carefully examine the colorectum of patients with looping.
Core Tip: This study aimed to clarify the effect of colonic looping on colorectal premalignant polyp detection during colonoscopy. We retrospectively investigated 12259 patients who underwent colonoscopies. Looping occurred in 54.3% (35.9% and 18.4% with mild and severe looping, respectively) of the cases. The detection rates of adenomas (44.7%), high-risk adenomas (9.9%), and clinically significant serrated polyps (CSSPs) (8.9%) increased with the looping severity. The number of adenomas per colonoscopy (0.82) increased with the looping severity. Multivariate analyses found that detection of adenomas, high-risk adenomas, and CSSPs was associated with severe looping regardless of age, sex, time required for colonoscope insertion and withdrawal, and endoscopist experience.
- Citation: Toyoshima O, Nishizawa T, Yoshida S, Matsuno T, Arano T, Kondo R, Kinoshita K, Yasumi Y, Tsuji Y, Fujishiro M. Impact of looping on premalignant polyp detection during colonoscopy. World J Gastrointest Endosc 2022; 14(11): 694-703
- URL: https://www.wjgnet.com/1948-5190/full/v14/i11/694.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i11.694
Colorectal cancer mainly occurs because of adenomas or serrated polyps[1-3]. Colonoscopy is the gold standard for cancer screening and detection of premalignant polyps. The prevalence of metachronous colorectal cancer is high in patients with adenomas, especially high-risk adenomas, removed during colonoscopy[4]. Similarly, individuals with colonoscopically resected clinically significant serrated polyps (CSSPs) have a long-term risk of colorectal cancer[5-7]. Thus, the detection of adenomas and CSSPs on colonoscopy is a surrogate marker for the risk of metachronous colorectal cancer. Factors related to premalignant polyp detection include patient characteristics, such as age and sex[8,9], endoscopic procedure-related factors, such as cecal intubation time[10] and withdrawal time[11-14], and endoscopist experience[8].
Colonic looping is a common obstacle during routine colonoscopy[15,16]. Looping is associated with a redundant colon, older age, female sex, and cecal intubation time[17-20]. However, the clinical significance of looping is poorly understood. Therefore, this study aimed to clarify the effect of looping on colorectal premalignant polyp detection by using multivariate analysis to control for potential confounding factors.
This retrospective study was conducted at a single institute, Toyoshima Endoscopy Clinic, a representative outpatient endoscopy-specialized clinic located in an urban area of Japan. Toyoshima Endoscopy Clinic performs 10000 endoscopies annually. The study design was described in a protocol prepared at Toyoshima Endoscopy Clinic and approved by the Certified Institutional Review Board of Yoyogi Mental Clinic on July 16, 2021 (Approval no. RKK227). We published this study’s protocol on our institute’s website (www.ichou.com). Thus, patients could opt out of the study if desired. All the authors approved the final manuscript. No funding was received for this study.
Patients who underwent colonoscopy at Toyoshima Endoscopy Clinic between May, 2017 and October, 2020 were enrolled in this study. The indications for colonoscopy included the examination of symptoms and abnormal findings, screening, and surveillance for colorectal diseases. Patients under
Common colonic looping patterns observed during colonoscopy have been described previously. Loops occur in the transverse and sigmoid colons, and sigmoid loops include alpha and N shapes[19,24]. When forming a loop, there is no one-to-one relationship between the transmission of the colonoscope shaft movement and colonoscope tip motion. In the case of looping, further insertion of the scope results in a larger loop size without de-looping the scope[24,25].
Cecal insertion without loop formation was defined as the absence of looping. Cecal insertion that required straightening of the colonic loop once was defined as mild looping. Cecal insertion that re
Small and gentle shaking and jiggling of the colonoscope shaft were performed. Right-turn shortening maneuvers for straightening the shaft were used for colonoscope insertion. Water-assisted, carbon dioxide-assisted, and cap-assisted chromoendoscopies with sedation were performed[26]. Position changes and rectal retroflexion were performed[8,27]. When looping was formed, we usually controlled the colonoscope by changing the patient’s position to supine or right lateral, and manual abdominal compression was performed by the assistant[15].
Thirty endoscopists with various levels of experience performed the colonoscopies[28,29]. This study defined experienced endoscopists as those with > 15 years of experience in performing endoscopy. We used a combination of the Elite system and CF-HQ290ZI, CF-HQ290I, or PCF-H290ZI colonoscopes (Olympus Corporation, Tokyo, Japan). Poor bowel preparation was defined as at least one colon segment that could not be examined because of the presence of remnant solid stool[9,16,27].
All polyps suspected to be cancerous, adenomatous, or CSSP were removed or biopsied. All polyps were histologically diagnosed by an experienced gastrointestinal pathologist using the resected specimens and biopsy samples. Advanced adenomas included adenomas ≥ 10 mm in size, villous ad
We extracted data from the endoscopy database of Toyoshima Endoscopy Clinic, including patient age, sex, endoscopist-assessed looping, colonoscope insertion time, withdrawal time, endoscopists, detection rates of adenomas, advanced adenomas, high-risk adenomas, CSSPs, and SSLs, and numbers of adenomas and SSLs. Withdrawal time was defined as the time required to examine the colorectal mucosa and remove the polyps. The polyp detection rate was defined as the rate of colonoscopies that detected at least one polyp.
The significance of any orderly increase or decrease along the three stratifications (i.e., no, mild, and severe looping) was assessed using Cochran-Armitage trend test or Jonckheere-Terpstra trend test for categorical and continuous variables, respectively. Because of the significant association between looping severity and polyp detection in the trend test, the effect of subject characteristics on polyp detection was analyzed using a multivariate analysis. Furthermore, a subgroup analysis, limited to experienced endoscopists, was performed. Multivariate analysis was performed using a binomial logistic regression model, with no, mild, and severe looping scores of 0, 1, and 2, respectively. Statistical significance was defined as a P-value < 0.05. The calculations were performed using Bell Curve for Excel version 3.22 (Social Survey Research Information Co., Ltd., Tokyo, Japan) and R version 4.1.2 (R Core Team 2021, R Foundation for Statistical Computing, Vienna, Austria).
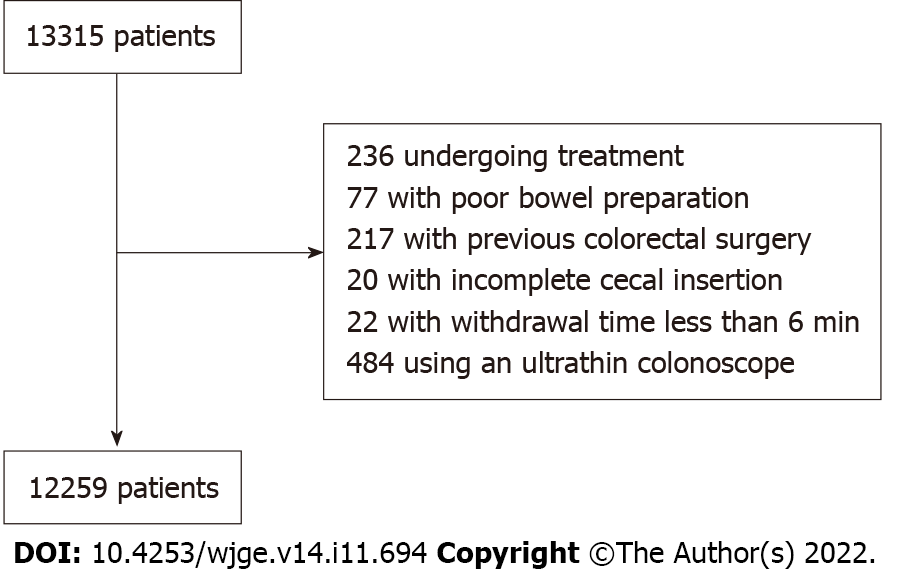
During the study period, colonoscopies were performed on 13315 patients. We excluded 236 patients undergoing treatment, such as polypectomy and hemostasis, 77 with poor bowel preparation, 217 with previous colorectal surgery, 20 with incomplete cecal insertion (including 8 with stenosis caused by colorectal tumor and 6 with colonic looping), 22 with withdrawal time < 6 min, and 484 who were examined using an ultrathin colonoscope. Ultimately, 12259 patients were enrolled in this study. A patient flowchart is shown in Figure 1.
The mean patient age was 53.6 years. Men accounted for 50.7% of the participants. Looping occurred in 54.3% of the patients. There were 4399 and 2253 patients with mild and severe looping, respectively. The mean insertion and withdrawal times were 4.6 and 13.9 min, respectively. Experienced endoscopists performed 70.4% of the colonoscopies. The polyp detection rates for adenomas, advanced adenomas, high-risk adenomas, CSSPs, and SSLs were 44.7%, 2.0%, 9.9%, 8.9%, and 3.5%, respectively. The mean number of adenomas and SSLs was 0.82 and 0.04, respectively (Table 1).
| Characteristics | |
| n | 12259 |
| Age, mean (SD), yr | 53.6 (12.2) |
| Male sex, % | 50.7 |
| Looping, none/mild/severe, n | 5532/4399/2253 |
| Insertion time, mean (SD), min | 4.57 (2.66) |
| Withdrawal time, mean (SD), min | 13.87 (4.19) |
| Experienced endoscopist, % | 70.4 |
| Polyp detection | |
| Adenoma DR, % | 44.7 |
| Advanced adenoma DR, % | 2.0 |
| High-risk adenoma DR, % | 9.9 |
| CSSP DR, % | 8.9 |
| SSL DR, % | 3.5 |
| Number of adenomas, mean (SD), n | 0.82 (1.25) |
| Number of SSLs, mean (SD), n | 0.04 (0.24) |
Patients with severe looping tended to be older and more likely to be female (both P < 0.001). Cecal insertion and withdrawal times tended to be longer in severe looping (both P < 0.001). Experienced endoscopists performed cases with severe looping more often. The polyp detection rates of adenomas (P < 0.001), advanced adenomas, high-risk adenomas (P < 0.001), CSSPs (P < 0.001), and SSLs tended to increase with looping severity. However, the tendency of advanced adenoma and SSL detection rates were not statistically significant (P = 0.166 and P = 0.064, respectively). The number of adenomas increased with looping severity (P < 0.001, Table 2).
| No looping | Mild looping | Severe looping | P value | |
| n | 5532 | 4399 | 2253 | |
| Age, mean (SD), yr | 51.5 (11.5) | 54.2 (12.2) | 56.7 (13.0) | < 0.001 |
| Male sex, % | 62.8 | 44.6 | 33.4 | < 0.001 |
| Insertion time, mean (SD), min | 3.53 (1.89) | 4.95 (2.41) | 6.38 (3.44) | < 0.001 |
| Withdrawal time, mean (SD), min | 13.70 (4.30) | 14.17 (4.29) | 13.74 (3.66) | < 0.0011 |
| Experienced endoscopist, % | 61.1 | 73.7 | 87.6 | < 0.001 |
| Polyp detection | ||||
| Adenoma DR, % | 42.2 | 45.0 | 50.2 | < 0.001 |
| Advanced adenoma DR, % | 1.8 | 2.1 | 2.3 | 0.166 |
| High-risk adenoma DR, % | 8.4 | 9.8 | 13.5 | < 0.001 |
| CSSP DR, % | 7.8 | 9.5 | 10.3 | < 0.001 |
| SSL DR, % | 3.2 | 3.7 | 3.9 | 0.064 |
| Number of adenomas, mean (SD), n | 0.74 (1.16) | 0.81 (1.25) | 1.03 (1.44) | < 0.001 |
| Number of SSLs, mean (SD), n | 0.04 (0.22) | 0.05 (0.26) | 0.05 (0.26) | 0.553 |
We investigated the effect of subject characteristics on the detection of adenomas, high-risk adenomas, and CSSPs using multivariate analyses. The detection of adenomas and high-risk adenomas was independently associated with severe looping (both P < 0.001), old age, male sex, short insertion time, long withdrawal time, and endoscopist experience. CSSP detection was independently associated with severe looping (P = 0.007), female sex, short insertion time, long withdrawal time, and endoscopist experience (Table 3).
| Odds ratio | 95% confidence interval | DOF | P value | |
| Adenoma | ||||
| Looping1 | 1.13 | 1.06-1.20 | 1 | < 0.001 |
| Age | 1.05 | 1.04-1.05 | 1 | < 0.001 |
| Male sex | 1.39 | 1.28-1.50 | 1 | < 0.001 |
| Insertion time | 0.94 | 0.92-0.96 | 1 | < 0.001 |
| Withdrawal time | 1.14 | 1.13-1.15 | 1 | < 0.001 |
| Endoscopist experience | 1.68 | 1.53-1.85 | 1 | < 0.001 |
| High-risk adenoma | ||||
| Looping1 | 1.25 | 1.13-1.38 | 1 | < 0.001 |
| Age | 1.05 | 1.05-1.06 | 1 | < 0.001 |
| Male sex | 1.527 | 1.33-1.74 | 1 | < 0.001 |
| Insertion time | 0.90 | 0.87-0.93 | 1 | < 0.001 |
| Withdrawal time | 1.20 | 1.18-1.21 | 1 | < 0.001 |
| Endoscopist experience | 3.91 | 3.17-4.82 | 1 | < 0.001 |
| Clinically significant serrated polyp | ||||
| Looping1 | 1.14 | 1.04-1.26 | 1 | 0.007 |
| Age | 1.00 | 0.99-1.01 | 1 | 0.999 |
| Male sex | 0.60 | 0.52-0.68 | 1 | < 0.001 |
| Insertion time | 0.92 | 0.88-0.95 | 1 | < 0.001 |
| Withdrawal time | 1.16 | 1.14-1.17 | 1 | < 0.001 |
| Endoscopist experience | 2.04 | 1.71-2.43 | 1 | < 0.001 |
We performed a subgroup analysis that was limited to experienced endoscopists. Multivariate analyses showed similar results to the all-case analyses, that is, severe looping was independently associated with high detection rates of adenomas, high-risk adenomas, and CSSPs (P < 0.001, P < 0.001, and P = 0.008, respectively; Table 4).
| Odds ratio | 95% confidence interval | DOF | P value | |
| Adenoma | ||||
| Looping1 | 1.14 | 1.07-1.23 | 1 | < 0.001 |
| Age | 1.05 | 1.05-1.05 | 1 | < 0.001 |
| Male sex | 1.42 | 1.29-1.56 | 1 | < 0.001 |
| Insertion time | 0.93 | 0.91-0.95 | 1 | < 0.001 |
| Withdrawal time | 1.13 | 1.11-1.14 | 1 | < 0.001 |
| High-risk adenoma | ||||
| Looping1 | 1.27 | 1.14-1.41 | 1 | < 0.001 |
| Age | 1.05 | 1.05-1.06 | 1 | < 0.001 |
| Male sex | 1.56 | 1.35-1.81 | 1 | < 0.001 |
| Insertion time | 0.89 | 0.85-0.92 | 1 | < 0.001 |
| Withdrawal time | 1.18 | 1.16-1.20 | 1 | < 0.001 |
| Clinically significant serrated polyp | ||||
| Looping1 | 1.15 | 1.04-1.28 | 1 | 0.008 |
| Age | 1.00 | 1.00-1.01 | 1 | 0.627 |
| Male sex | 0.66 | 0.57-0.77 | 1 | < 0.001 |
| Insertion time | 0.92 | 0.89-0.96 | 1 | < 0.001 |
| Withdrawal time | 1.13 | 1.11-1.15 | 1 | < 0.001 |
In this study, we found that the severity of looping during colonoscopy was positively associated with high detection rates of adenomas, high-risk adenomas, and CSSPs, independent of other confounding factors, such as patient age, sex, colonoscope insertion and withdrawal times, and endoscopist experience. To the best of our knowledge, this is the first study to demonstrate a relationship between looping and polyp detection. Adenomas, high-risk adenomas, and CSSPs are precancerous lesions[2]. Recent studies have also shown that adenoma, high-risk adenoma, and CSSP detection rates are associated with a high risk of metachronous colorectal cancer[4,6]. Therefore, looping may predict a high frequency of metachronous colorectal cancer; however, further analysis is needed. Colonoscopists should carefully examine the colorectal region of patients with looping considering the high premalignant polyp detection rate.
Magnetic endoscopic imaging, computed tomographic colonoscopy, and autopsy revealed that looping was more common in older adults and women. Loop formation is also associated with prolonged cecal insertion time[17-20]. In our study, looping severity was associated with older age, female sex, and longer insertion time. Our results were consistent with those of previous studies. Looping during colonoscopy mainly occurs in the intraperitoneal segments of the colon, such as the transverse and sigmoid colon[15,17,19,20,34,35]. Barium enema and computed tomographic co
Colonic redundancy is a major cause of looping during colonoscopy[39]. Colonic elongation and tortuosity appear to be related to redundancy of the colon, such as in the transverse and sigmoid colon[40,41]. Older adults and women often present with colonic redundancy and looping[41]. Raahave et al[42] reported that colonic transit time is associated with redundant colonic loops. Constipation increases the risk of colorectal cancer[43]. This causes prolonged contact between the colonic mucosa and carcinogens in the stool.
Our study showed that adenoma detection was associated with old age, male sex, short insertion time, long withdrawal time, and endoscopist experience. These results are consistent with those of previous studies[8,10-12]. Female sex and longer withdrawal time, but not older age, were associated with CSSPs in our study. These findings are also concordant with those of previous studies[44-46]. The consistency of these results strengthens the credibility of this study.
This study had several limitations. First, this study was retrospectively conducted at a single institution; however, medical data were well-controlled. Second, although patients’ body mass index, family history of colorectal cancer, and gynecological surgery are associated with the presence of premalignant polyps and looping[25,47], they were not examined. Third, since mucosal exposure can affect adenoma detection rate[48], the shape of looping, de-looping method, and successful de-looping after cecal intubation should be evaluated, not only the degree of looping during insertion. However, our data do not contain this information. Further verification is required in the future.
In conclusion, the severity of looping during colonoscopy was strongly associated with high detection rates of premalignant polyps, such as adenomas, high-risk adenomas, and CSSPs. Therefore, looping may predict the risk of metachronous colorectal cancer; however, further investigation is needed. Endoscopists should be more careful when examining for colorectal polyps in patients with looping.
Colonic looping is a common obstacle during routine colonoscopy.
Looping is associated with a redundant colon, older age, female sex, and cecal intubation time. However, the clinical significance of looping is not fully understood.
We aimed to clarify the effect of looping on colorectal premalignant polyp detection.
We extracted data from the clinic’s endoscopy database on patient age, sex, endoscopist-assessed looping, colonoscopy duration, endoscopist experience, and premalignant polyp detection. The effects of looping on premalignant polyp detection were assessed using logistic regression analyses.
The detection rates of adenomas, high-risk adenomas, and clinically significant serrated polyps (CSSPs) increased with the severity of looping (all P < 0.001). The number of adenomas increased with looping severity (P < 0.001). Multivariate analyses found that detection of adenoma, high-risk adenoma, and CSSP was associated with severe looping (P < 0.001, P < 0.001, and P = 0.007, respectively) regardless of age, sex, and the time required for colonoscope insertion and withdrawal, and endoscopist experience.
Looping severity was independently associated with high detection rates of premalignant polyps.
Looping may predict the risk of metachronous colorectal cancer; however, further investigation is needed.
We would like to thank Dr. Hidenobu Watanabe for conducting histological diagnosis. We would like to thank Shido Inc. (www.Shido.co.jp) for the statistical analysis. We would like to thank clinical laboratory engineer Tadahiro Yamakawa for managing the endoscopy database of Toyoshima Endoscopy Clinic.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu B, China; Ko J, South Korea; Mohamed SY, Egypt; Sharaf MM, Syria S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Bevan R, Rutter MD. Colorectal Cancer Screening-Who, How, and When? Clin Endosc. 2018;51:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 2. | Nagtegaal I, Arends, MJ, Odeze, RD, Lam, AK. Tumours of the colon and rectum. In: WHO Classification of Tumours Editorial Board. Digestive System Tumours: WHO Classification of Tumours (Medicine) 5th Edition. Lyon: World Health Organization, 2019: 157-191. |
| 3. | Sehgal A, Aggarwal S, Mandaliya R, Loughney T, Mattar MC. Improving sessile serrated adenoma detection rates with high definition colonoscopy: A retrospective study. World J Gastrointest Endosc. 2022;14:226-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 4. | Duvvuri A, Chandrasekar VT, Srinivasan S, Narimiti A, Dasari C, Nutalapati V, Kennedy KF, Spadaccini M, Antonelli G, Desai M, Vennalaganti P, Kohli D, Kaminski MF, Repici A, Hassan C, Sharma P. Risk of Colorectal Cancer and Cancer Related Mortality After Detection of Low-risk or High-risk Adenomas, Compared With No Adenoma, at Index Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2021;160:1986-1996.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Kahi CJ. Screening Relevance of Sessile Serrated Polyps. Clin Endosc. 2019;52:235-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL, Ogino S, Chan AT, Song M. Long-term Risk of Colorectal Cancer After Removal of Conventional Adenomas and Serrated Polyps. Gastroenterology. 2020;158:852-861.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 7. | Tan YY, Tay GSK, Wong YJ, Li JW, Kwek ABE, Ang TL, Wang LM, Tan MTK. Clinical Features and Predictors of Dysplasia in Proximal Sessile Serrated Lesions. Clin Endosc. 2021;54:578-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Toyoshima O, Nishizawa T, Yoshida S, Sekiba K, Kataoka Y, Hata K, Watanabe H, Tsuji Y, Koike K. Expert endoscopists with high adenoma detection rates frequently detect diminutive adenomas in proximal colon. Endosc Int Open. 2020;8:E775-E782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 9. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Arano T, Isomura Y, Kanazawa T, Ando H, Tsuji Y, Koike K. Simple feedback of colonoscopy performance improved the number of adenomas per colonoscopy and serrated polyp detection rate. Endosc Int Open. 2021;9:E1032-E1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | von Renteln D, Robertson DJ, Bensen S, Pohl H. Prolonged cecal insertion time is associated with decreased adenoma detection. Gastrointest Endosc. 2017;85:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 951] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 12. | Kashiwagi K, Inoue N, Yoshida T, Bessyo R, Yoneno K, Imaeda H, Ogata H, Kanai T, Sugino Y, Iwao Y. Polyp detection rate in transverse and sigmoid colon significantly increases with longer withdrawal time during screening colonoscopy. PLoS One. 2017;12:e0174155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Cavicchi M, Tharsis G, Burtin P, Cattan P, Venezia F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Karsenti D. Difference in Physician- and Patient-Dependent Factors Contributing to Adenoma Detection Rate and Serrated Polyp Detection Rate. Dig Dis Sci. 2019;64:3579-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 14. | Kim SH, Kim JH. When should we perform colonoscopy to increase the adenoma detection rate? World J Gastrointest Endosc. 2021;13:619-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 15. | Shah SG, Saunders BP, Brooker JC, Williams CB. Magnetic imaging of colonoscopy: an audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc. 2000;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Toyoshima O, Nishizawa T, Sakitani K, Yamakawa T, Yoshida S, Fukagawa K, Hata K, Ishihara S, Suzuki H. Colonoscopy using back brace support belt: A randomized, prospective trial. JGH Open. 2020;4:441-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Shah SG, Brooker JC, Thapar C, Williams CB, Saunders BP. Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging. Endoscopy. 2002;34:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Eickhoff A, Pickhardt PJ, Hartmann D, Riemann JF. Colon anatomy based on CT colonography and fluoroscopy: impact on looping, straightening and ancillary manoeuvres in colonoscopy. Dig Liver Dis. 2010;42:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Lam J, Wilkinson J, Brassett C, Brown J. Difference in real-time magnetic image analysis of colonic looping patterns between males and females undergoing diagnostic colonoscopy. Endosc Int Open. 2018;6:E575-E581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Lam J, Wilkinson J, Brown J, Spear M, Brassett C. Exploration of colonic looping patterns in undisturbed cadaveric specimens. Clin Anat. 2021;34:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, Schneider T, Kachel G, Grüger J, Köllinger M, Nagell W, Goerg KJ, Wanitschke R, Gruss HJ. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Hong SN, Sung IK, Kim JH, Choe WH, Kim BK, Ko SY, Lee JH, Seol DC, Ahn SY, Lee SY, Park HS, Shim CS. The Effect of the Bowel Preparation Status on the Risk of Missing Polyp and Adenoma during Screening Colonoscopy: A Tandem Colonoscopic Study. Clin Endosc. 2012;45:404-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Sofi AA, Nawras A, Khan MA, Howden CW, Lee WM. Meta-analysis of the performance of ultrathin vs. standard colonoscopes. Endoscopy. 2017;49:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Cheng WB, Moser MA, Kanagaratnam S, Zhang WJ. Overview of upcoming advances in colonoscopy. Dig Endosc. 2012;24:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Cheng WB, Moser MA, Kanagaratnam S, Zhang WJ. Analysis of and mathematical model insight into loop formation in colonoscopy. Proc Inst Mech Eng H. 2012;226:858-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Passi M, Rahman F, Gurram S, Kumar S, Koh C. Identifying who best tolerates moderate sedation: Results from a national database of gastrointestinal endoscopic outcomes. World J Gastrointest Endosc. 2021;13:97-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 27. | Toyoshima O, Yoshida S, Nishizawa T, Yamakawa T, Sakitani K, Hata K, Takahashi Y, Fujishiro M, Watanabe H, Koike K. CF290 for pancolonic chromoendoscopy improved sessile serrated polyp detection and procedure time: a propensity score-matching study. Endosc Int Open. 2019;7:E987-E993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Qayed E, Vora R, Levy S, Bostick RM. Colonoscopy procedural volume increases adenoma and polyp detection rates in gastroenterologytrainees. World J Gastrointest Endosc. 2017;9:540-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Lee AHH, Lojanapiwat N, Balakrishnan V, Chandra R. Is there a difference in adenoma detection rates between gastroenterologists and surgeons? World J Gastrointest Endosc. 2018;10:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O'Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315-29; quiz 1314, 1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 830] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 31. | Anderson JC, Butterly LF, Weiss JE, Robinson CM. Providing data for serrated polyp detection rate benchmarks: an analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2017;85:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 32. | Li D, Woolfrey J, Jiang SF, Jensen CD, Zhao WK, Kakar S, Santamaria M, Rumore G, Armstrong MA, Postlethwaite D, Corley DA, Levin TR. Diagnosis and predictors of sessile serrated adenoma after educational training in a large, community-based, integrated healthcare setting. Gastrointest Endosc. 2018;87:755-765.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Klair JS, Ashat M, Johnson D, Arora S, Onteddu N, Machain Palacio JG, Samuel R, Bilal M, Buddam A, Gupta A, Gunderson A, Guturu P, Soota K, Chandra S, Murali AR. Serrated polyp detection rate and advanced adenoma detection rate from a US multicenter cohort. Endoscopy. 2020;52:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Asai S, Fujimoto N, Tanoue K, Akamine E, Nakao E, Hashimoto K, Ichinona T, Nambara M, Sassa S, Yanagi H, Hirooka N, Mori T, Ogawa M, Ogawa A. Water immersion colonoscopy facilitates straight passage of the colonoscope through the sigmoid colon without loop formation: randomized controlled trial. Dig Endosc. 2015;27:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Bruce M, Choi J. Detection of endoscopic looping during colonoscopy procedure by using embedded bending sensors. Med Devices (Auckl). 2018;11:171-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Sadahiro S, Ohmura T, Yamada Y, Saito T, Taki Y. Analysis of length and surface area of each segment of the large intestine according to age, sex and physique. Surg Radiol Anat. 1992;14:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Saunders BP, Fukumoto M, Halligan S, Jobling C, Moussa ME, Bartram CI, Williams CB. Why is colonoscopy more difficult in women? Gastrointest Endosc. 1996;43:124-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Khashab MA, Pickhardt PJ, Kim DH, Rex DK. Colorectal anatomy in adults at computed tomography colonography: normal distribution and the effect of age, sex, and body mass index. Endoscopy. 2009;41:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Ritter EM, Cox TC, Trinca KD, Pearl JP. Simulated Colonoscopy Objective Performance Evaluation (SCOPE): a non-computer-based tool for assessment of endoscopic skills. Surg Endosc. 2013;27:4073-4080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Cuda T, Gunnarsson R, de Costa A. The correlation between diverticulosis and redundant colon. Int J Colorectal Dis. 2017;32:1603-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Raahave D, Christensen E, Loud FB, Knudsen LL. Correlation of bowel symptoms with colonic transit, length, and faecal load in functional faecal retention. Dan Med Bull. 2009;56:83-88. [PubMed] |
| 43. | Sundbøll J, Thygesen SK, Veres K, Liao D, Zhao J, Gregersen H, Sørensen HT. Risk of cancer in patients with constipation. Clin Epidemiol. 2019;11:299-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, Li X, Guo L, Zheng Z, Zou S, Zhang Y, Li J, Zhang K, Chen W, Dai M, He J; group of Cancer Screening Program in Urban China (CanSPUC). Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68:1450-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 45. | Meester RGS, van Herk MMAGC, Lansdorp-Vogelaar I, Ladabaum U. Prevalence and Clinical Features of Sessile Serrated Polyps: A Systematic Review. Gastroenterology. 2020;159:105-118.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 46. | Anwar S, Cock C, Young J, Young GP, Meng R, Simpson K, Coats M, Huang J, Bampton P, Fraser R, Symonds EL. Features associated with high-risk sessile serrated polyps at index and follow-up colonoscopy. J Gastroenterol Hepatol. 2021;36:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Adams C, Cardwell C, Cook C, Edwards R, Atkin WS, Morton DG. Effect of hysterectomy status on polyp detection rates at screening flexible sigmoidoscopy. Gastrointest Endosc. 2003;57:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | McGill SK, Rosenman J, Wang R, Ma R, Frahm JM, Pizer S. Artificial intelligence identifies and quantifies colonoscopy blind spots. Endoscopy. 2021;53:1284-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |