Published online Oct 16, 2022. doi: 10.4253/wjge.v14.i10.636
Peer-review started: May 4, 2022
First decision: June 16, 2022
Revised: June 24, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: October 16, 2022
Processing time: 160 Days and 17.9 Hours
Esophageal cancer is a common type of cancer and serious bleeding from esop
A 36-years old male patient with advanced esophageal cancer developed bleeding from the tumor following endoscopic stenting with a self-expanding metal stent. Due to the ineffectiveness of standard approaches, after a medical conference, the patient was treated with a novel method based on the use of a two-balloon catheter creating an isolated area in esophagus and locally dispersing hemostatic polysaccharide powder inside the isolated interior. Hemostasis was successful and subsequent endoscopic examination revealed the presence of organized clot and localized defect, which was coagulated in a planned manner.
The authors present a new catheter-based method of hemostasis of esophageal tumor bleeding.
Core Tip: We describe a novel method of managing difficult-to-treat condition using an original device/ catheter that we developed. Our experience of managing gastrointestinal and, in particular, esophageal bleeding suggests that treatment of such conditions is a major challenge with no readily available and reliably working solutions. Success depends on multiple factors, all subject to limitation of time available for decision-making and application of treatment methods. A major advantage of our method is its ease of use and ability to be deployed by physicians of all levels and in all hospital settings. We believe that our method can help save many lives.
- Citation: Kashintsev AA, Rusanov DS, Antipova MV, Anisimov SV, Granstrem OK, Kokhanenko NY, Medvedev KV, Kutumov EB, Nadeeva AA, Proutski V. Hemostasis of massive bleeding from esophageal tumor: A case report. World J Gastrointest Endosc 2022; 14(10): 636-641
- URL: https://www.wjgnet.com/1948-5190/full/v14/i10/636.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i10.636
Various stages of dysphagia are common complications of esophageal cancer. Stenting of esophageal tumors is a standard method of treatment and palliative care. Placement of a self-expandable metal stent is required, on the one hand to facilitate oral nutrition and on the other hand as the first standard step of treatment pre-empting neoadjuvant chemotherapy with brachytherapy[1,2]. At the same time, placement of a stent can lead to the development of various complications, the frequency of which can reach up to 50%[3]. The most common are esophageal perforation, fistula, stent migration, and bleeding[4,5]. The incidence of bleeding after stenting is not high and varies from 1% to 12%[6,7]. However, the volume of bleeding if it occurs is often massive and is associated with high mortality[6,7]. Due to the fact that this complication is rare, and its course is extremely aggressive, the experience of managing this group of patients is limited. The recommendations are nonsystematic in nature and one should be prepared for various scenarios, from the application of various hemostatic remedies and transfusion of blood components to angiographic methods to stop the bleeding. The unfavorable outcome of this complication can be caused by a stent itself that interferes with verification of the source of bleeding, by pathological hypervascularization of a tumor, rich blood supply of the esophagus, including from esophageal arteries stemming from the descending aorta, and by a limited amount of time available to help a patient[6-9].
Analysis of the literature suggests that time is the main factor in the unsatisfactory result of trying to achieve hemostasis during the first wave of bleeding. The time spent on patient admission and delays in identifying the source of bleeding, trying various options of endoscopic hemostasis, switching to endovascular methods, all negatively affect the outcome of treatment. To counter this, a method has been developed that consists of isolating the source of bleeding, in this case the part of the esophagus with a tumor, from other parts of the gastrointestinal tract, with the possibility of delivering hemostatic agents into it while maintaining the connectivity between the parts of esophagus proximal and distal to the isolated region. The latter feature enables concurrent and continuous drainage of the proximal part and administration of solutions and enteral nutrition. This approach achieves several important effects. First, it allows one to mechanically create an isolated area with high pressure in which blood, clots, and coagulation factors facilitate hemostasis. Second, it enables localized delivery of hemostatic agents such as polysaccharide hemostatic powders. Third, by maintaining functional connectivity of the gastrointestinal tract, the method allows both for essential nutritional support and provision of fluids, and for sufficient exposure time to achieve hemostasis.
Vomiting with blood, melena, weakness, an episode of loss of consciousness.
A 36-year-old male patient was admitted on an emergency basis on November 14, 2021, with manifestation of gastrointestinal bleeding. At the time of admission, the degree of blood loss, according to the changes in the level of hemoglobin, erythrocytes and hematocrit, was assessed as moderate.
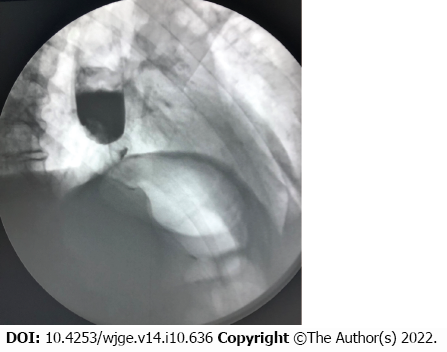
When collecting an anamnesis, it was established that for the first time the dysphagia was observed in September 2021. An X-ray investigation performed at the time revealed changes characteristic of a tumor of the gastroesophageal junction (Figure 1). The patient categorically refused further examination and treatment and was discharged. Later he was followed up at the oncology clinic, and on October 29 diagnosed with cancer of gastroesophageal junction, type II according to Siewert classification, stage IVB, Grade 2, dMMR/MSI-h-negative, HER2-negative adenocarcinoma. Concomitant diseases: obesity class III, essential hypertension. On November 10, endoscopic stenting of esophagus was performed to resolve dysphagia. The patient was discharged on November 13, 2021.
There was no personal and family history of cancer.
At the time of admission, blood pressure was 80/40 mmHg and heart rate was 114 beats/min.
Blood analysis demonstrated high volume of loss, with erythrocyte count 2.1 mln cells/uL, hemoglobin 79 g/L, and hematocrit 31.0%.
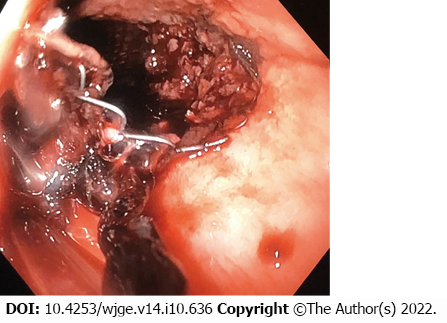
Endoscopic examination revealed that there was ongoing bleeding from under the partially covered esophageal stent (Figure 2). It was however not possible to clearly establish the localization of the source of bleeding.
Given the severity and urgency of situation, a multidisciplinary meeting was held, which included surgeons, endoscopists and anesthesiologists.
Cancer of the gastroesophageal junction, type II according to Siewert classification, stage IVB, Grade 2, dMMR/MSI-h-negative, HER2-negative adenocarcinoma. Complications: severe esophageal bleeding. Concomitant diseases: obesity class III and essential hypertension.
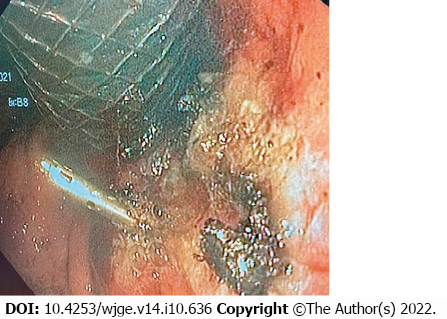
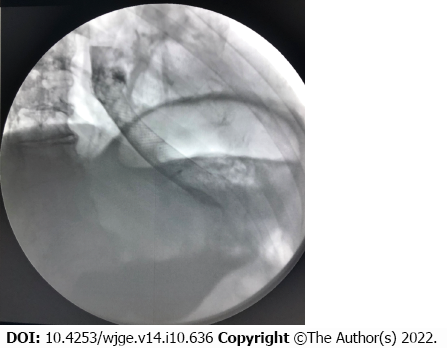
Both standard intravenous hemostatic therapy and blood component transfusion were administered. An attempt to perform endoscopic hemostasis by electrocoagulation of the tumor failed to achieve positive results. It was decided that due to the impossibility of achieving hemostasis using standard methods and further deteriorating condition of the patient, it was advisable, according to vital indications, to use the isolation method and locally introduce a polysaccharide powdered hemostatic agent. The two-balloon catheter was inserted endoscopically into the stomach past the stent, so that the tumor site with the source of bleeding were located between the balloons. Balloons were inflated isolating the area of bleeding, and hemostatic powder was injected though the catheter opening located between the balloons and dispersed inside the isolated interior. The procedure stopped the bleeding, as demonstrated by normalization of hemodynamic parameters and absence of retrograde flow of blood through the main channel of the catheter. Over the next day, there was no sign of bleeding recurrence, which was supported by stable levels of hemoglobin and erythrocyte count. On November 15, the day after hemostasis, the catheter was removed, and repeated endoscopic procedure was performed in order to identify the source of bleeding and to reposition the esophageal stent. A 1.5-cm long defect with an organized clot was detected in the gastroesophageal junction (Figure 3). Argon plasma coagulation was performed after which the same stent was repositioned and fixed. Fluoroscopy performed on November 18 showed that stent’s position was adequate, the contrast medium freely entered the stomach, and there were no streaks or signs of stent migration (Figure 4). No recurrence of bleeding was observed, and the patient was discharged on November 18 in adequate condition to continue treatment at the oncology clinic.
After 4 mo of follow-up on March 9, 2022, patient was hospitalized with recurrent dysphagia. End
Bleeding after stenting of esophageal cancer is a severe complication with a high rate of mortality. Most often it develops in the first 2 wk after manipulation[8,9]. The main reasons include mucosal trauma caused by the free uncovered part of the stent during active esophageal peristalsis and increased pressure on the wall of the organ at the time of its expansion by the stent, leading to necrotic changes[10]. Since the esophagus is well supplied with blood, the bleeding is often massive. The presence of a stent hampers identification of the source of bleeding, and prevents application of argon plasma coagulation, injection of adrenaline or clipping. Large number of collateral blood vessels and segmental type of blood supply of the esophagus are the reason why many authors recommend supplementing endoscopic approaches with endovascular methods of hemostasis, which nevertheless often fail to achieve the desired effect[8-10]. It is important to have a wide range of methods available for both identification and tackling of the source of bleeding. In clinical practice however, resources are often limited and implementation of extensive care is associated with loss of time, which in this case is critical. Presence of disseminated tumor and poor somatic status of a patient can also play an important role, limiting the surgeon’s options.
The method of hemostatic treatment described here allows for localization of the source of bleeding by isolating it from other parts of the gastrointestinal tract. At the same time, it does not require identification of precise location of the site of bleeding. The method implements four hemostatic approaches: (1) Applying pressure on the submucosal vessels by the inflated balloons; (2) tamponade of the source of bleeding by blood clots; (3) targeted delivery of hemostatic agents to the bleeding site; and (4) prevention of migration of hemostatic agents and blood clots to other parts of the gastrointestinal tract due to peristalsis. The latter prolongs exposure to hemostatic agents, which is enhanced by the ability of the two-balloon catheter used in the procedure to preserve connectivity of the gastrointestinal tract and to remain in place long enough to achieve the desired hemostatic effect.
Availability of a fast and simple method for stopping bleeding from a tumor in the esophageal lumen, which does not require a high level of specialist training, is easy to perform and that provides long-term hemostasis and ability to administer enteral nutrition and drain the upper part of the esophagus, will help save time and improve the quality of care for this group of patients. While the present case is focused on esophageal bleeding, the method proposed could be applied to treating bleeding in other parts of the gastrointestinal tract.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for cancer research, No. 461256.
Specialty type: Emergency medicine
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Destek S, Turkey; Hu B, China S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wu YXJ
| 1. | Talreja JP, Eloubeidi MA, Sauer BG, Al-Awabdy BS, Lopes T, Kahaleh M, Shami VM. Fully covered removable nitinol self-expandable metal stents (SEMS) in malignant strictures of the esophagus: a multicenter analysis. Surg Endosc. 2012;26:1664-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | NCCN Clinical practice guidelines in oncology (NCCN Guidelines). Esophageal and esophagogastric junction cancers. 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal_blocks.pdf. |
| 3. | Iwasaki H, Mizushima T, Suzuki Y, Fukusada S, Kachi K, Ozeki T, Anbe K, Tsukamoto H, Okumura F, Joh T, Sano H. Factors That Affect Stent-Related Complications in Patients with Malignant Obstruction of the Esophagus or Gastric Cardia. Gut Liver. 2017;11:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Oh SJ, Song HY, Nam DH, Ko HK, Park JH, Na HK, Lee JJ, Kang MK. Bleeding after expandable nitinol stent placement in patients with esophageal and upper gastrointestinal obstruction: incidence, management, and predictors. Acta Radiol. 2014;55:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Shan M, Lu Z, Guo Q, Liu Z, Zhang J, Wen F. Self-expanding metal stents for palliative treatment of esophageal carcinoma: risk factors for fatal massive bleeding. J Clin Gastroenterol. 2012;46:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Homann N, Noftz MR, Klingenberg-Noftz RD, Ludwig D. Delayed complications after placement of self-expanding stents in malignant esophageal obstruction: treatment strategies and survival rate. Dig Dis Sci. 2008;53:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Kos X, Trotteur G, Dondelinger RF. Delayed esophageal hemorrhage caused by a metal stent: treatment with embolization. Cardiovasc Intervent Radiol. 1998;21:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Turkyilmaz A, Eroglu A, Aydin Y, Kurt A, Bilen Y, Karaoglanoglu N. Complications of metallic stent placement in malignant esophageal stricture and their management. Surg Laparosc Endosc Percutan Tech. 2010;20:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Liu SY, Xiao P, Li TX, Cao HC, Mao AW, Jiang HS, Cao GS, Liu J, Wang YD, Zhang XS. Predictor of massive bleeding following stent placement for malignant oesophageal stricture/fistulae: a multicentre study. Clin Radiol. 2016;71:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Sarper A, Oz N, Cihangir C, Demircan A, Isin E. The efficacy of self-expanding metal stents for palliation of malignant esophageal strictures and fistulas. Eur J Cardiothorac Surg. 2003;23:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |