Published online May 16, 2021. doi: 10.4253/wjge.v13.i5.155
Peer-review started: December 19, 2020
First decision: February 15, 2021
Revised: March 6, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: May 16, 2021
Processing time: 131 Days and 7.7 Hours
Achalasia is a primary esophageal motility disease characterized by impairment of normal esophageal peristalsis and absence of relaxation of the lower esopha
We report about a pregnant woman with a history of symptoms associated with inherited mitochondrial disease, which was confirmed by genetic tests, and who was treated via peroral endoscopic myotomy.
Peroral endoscopic myotomy is possible treatment option for a pregnant woman with achalasia caused by mitochondrial disease.
Core Tip: Achalasia is a primary esophageal motility disease. Sometimes is can be a part of some genetic disorders. One of the causes of gastrointestinal motility disorders, including achalasia, is mitochondrial defects. We report about a pregnant woman with a history of symptoms associated with inherited mitochondrial disease, which was confirmed by genetic tests, and who was successfully treated via peroral endoscopic myotomy.
- Citation: Smirnov AA, Kiriltseva MM, Lyubchenko ME, Nazarov VD, Botina AV, Burakov AN, Lapin SV. Peroral endoscopic myotomy in a pregnant woman diagnosed with mitochondrial disease: A case report. World J Gastrointest Endosc 2021; 13(5): 155-160
- URL: https://www.wjgnet.com/1948-5190/full/v13/i5/155.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i5.155
Achalasia is a primary esophageal motility disease characterized by impairment of normal esophageal peristalsis and absence of relaxation of the lower esophageal sphincter[1]. It can exist as an independent disease or part of some genetic disorders. One of the causes of gastrointestinal (GI) motility disorders, including achalasia, is mitochondrial defects[2,3]. Peroral endoscopic myotomy (POEM) is the safest and most effective method for achalasia treatment[4-7].
A 30-year-old woman presented to our hospital complaining of swallowing difficulty.
A patient had a violation of physical development and constipation from an early age. At the age of 7 years, she was diagnosed with partial bilateral symmetric ptosis. At the age of 8 years, she was referred to the hospital with diagnoses of generalized viral infection of unspecified etiology, postinfectious encephalopathy, cerebro-asthenic syndrome, neurosis, urinary bladder and gut atony, chronic pyelonephritis, mydriasis, semiptosis, and dystrophy. At the age of 9 years, she had suspected high intestinal obstruction which was followed by surgery. The obstruction was not revealed during the surgery. In the postoperative period, signs of intestinal obstruction persisted, and they were managed conservatively. After the surgery, she developed meningeal signs, gaze paresis, double vision, and reduced vision. Electrocardiogram showed an incomplete type of blockade of the right branch of the bundle of His. Esophagogastroduodenoscopy (EGD) showed gastric hypotony. Computed tomography scans of the head revealed moderate diffuse cortex atrophy. Cerebrospinal fluid was clear with 0.066. The patient was seen by a neurologist, ophthalmologist, infectious diseases specialist, and neurosurgeon. However, the diagnosis remained unclear. The following pathologies were excluded: neuro infections, intestinal infections, oncohematology, and endocrine pathologies. Further generalized pathology persisted. At the age of 10 years, a second laparotomy was performed followed by a temporary ileostomy because of signs of acute intestinal obstruction. From the ages of 11 years to 14 years, the patient was annually referred to the surgery department with signs of acute intestinal obstruction, which were managed conservatively. At the age of 11 years, she was diagnosed with intestinal pseudo-obstruction. From the age of 11 years, paradontosis began. From the age of 14 years, the patient had daily dysphagia while eating solid and liquid food. She lost 5 kg and began feeling weak and fatigued. At the age of 15 years, resection of the jejunum was performed two times with an overall resection length of 90 cm because of acute intestinal obstruction which was not managed conservatively. The patient was dystrophic, which was thought to be because of malabsorption as a consequence of the resection of the jejunum. At the age of 25 years, the patient lost all her teeth because of progressive paradontosis. From the age of 26 years, she developed amenorrhea. At the age of 29 years, esophagography showed signs of achalasia, gastroptosis, and delayed gastric and duodenum emptying time. At the age of 30 years, the patient was referred to the endoscopy department of Pavlov Medical University for achalasia treatment.
History of present illness includes the patient’s entire life. That is why we suppose that this part is irrelevant in this case.
The mother, father, and sister are healthy. There was no family history of GI or autoimmune pathologies or allergic disorders. The niece (4 years of age) had sensorineural hearing loss.
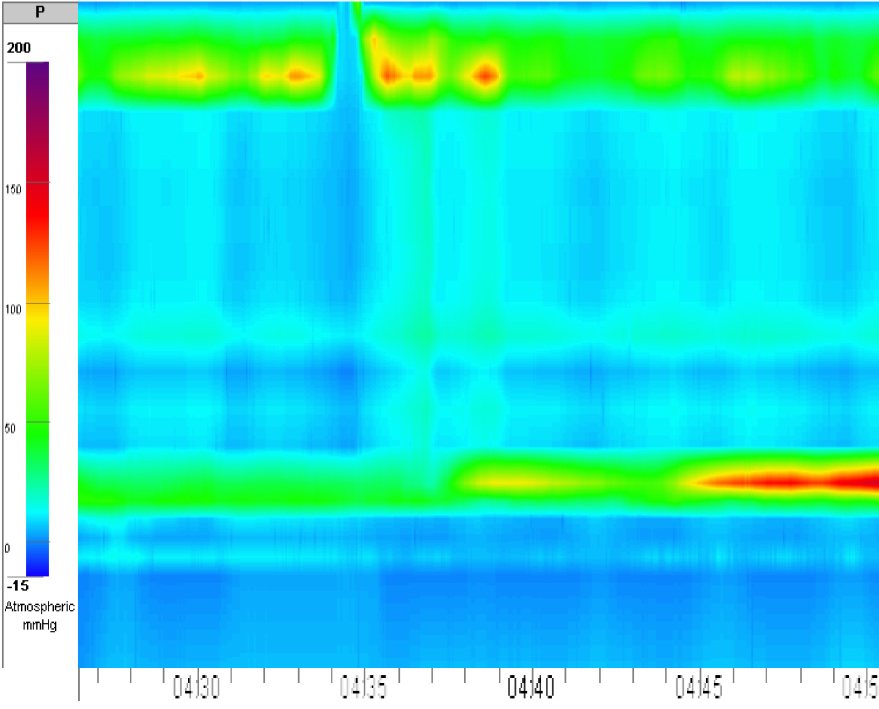
Eckardt score was 4. Her weight was 38 kg. Her body mass index was 16.9, and she had protein energy malnutrition. During preoperative preparation, the patient was revealed to be 16 wk pregnant. She was not aware of the pregnancy. In addition, intraventricular blockage was diagnosed. High-resolution esophageal manometry showed achalasia type I (Figure 1). Hemoglobin and total blood protein levels were 106 g/L and 64 g/L, respectively. Creatine phosphokinase and lactate levels were normal. Neurologic and ophthalmologic disorders were not observed. Considering all data, we suspected mitochondrial disease: incomplete Kearns-Sayre syndrome (KSS) or mitochondrial neurogastrointestinal encephalopathy (MNGIE) disease.
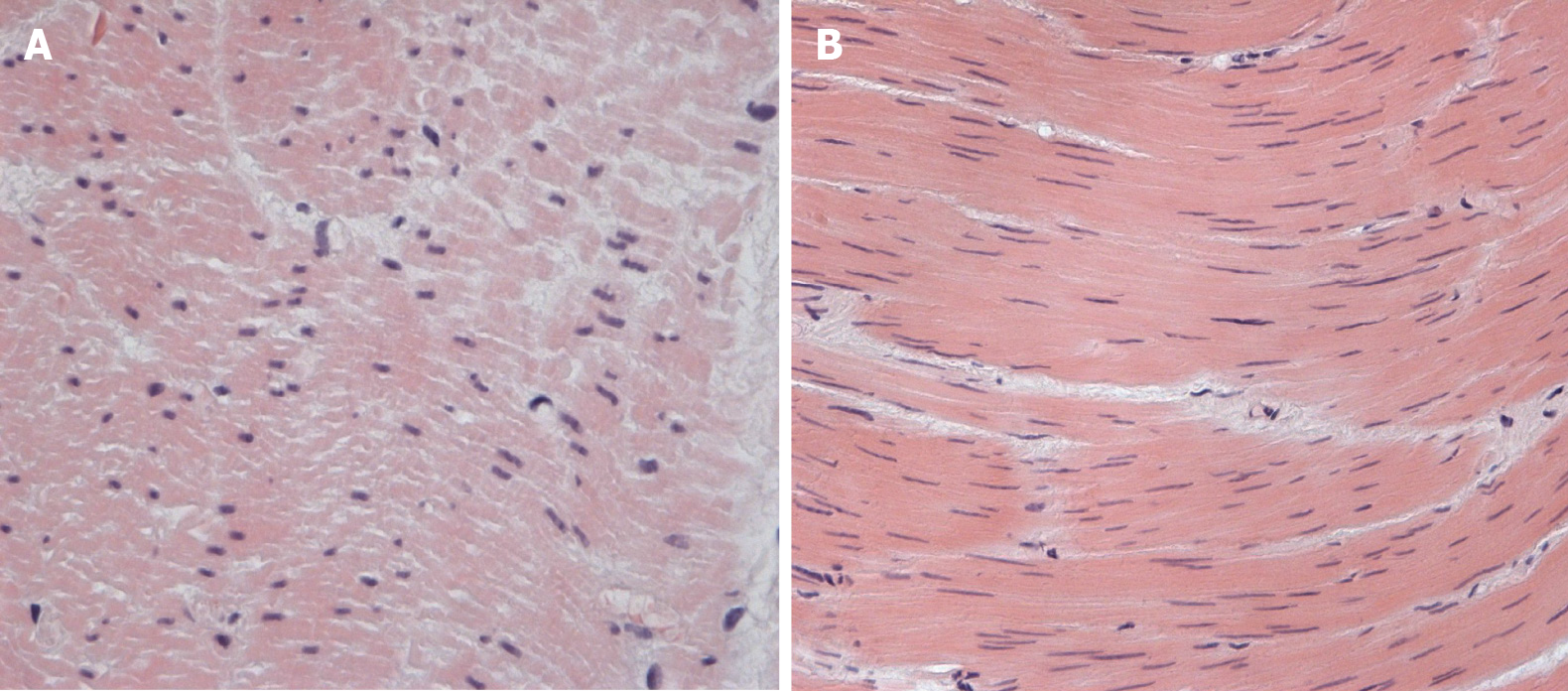
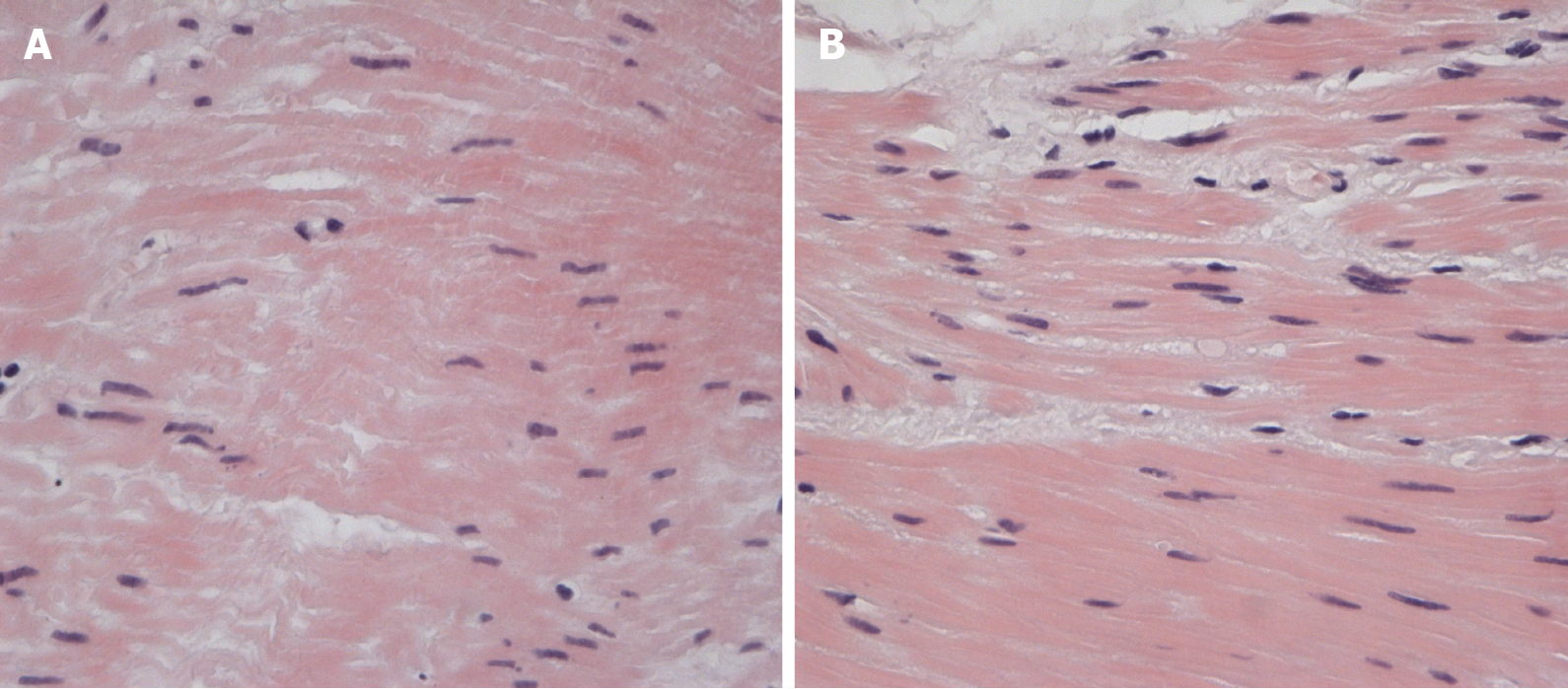
Histology of the esophageal muscular layer specimens: There were myocytes of different thicknesses with sites of wave-like deformation and dystrophic changes. There were also single myocytes with necrobiotic changes and small vessels with “edge standing” leukocytes (Figure 2 and 3).
Genetic testing of mitochondrial DNA (lymphocytic): It showed segment deletion in mitochondrial DNA (mDNA) which affected the genes RNR1 (MTRNR1) and RNR2 (MTRNR2). This aberration is considered to be pathogenic and most frequently observed in patients with KSS[8]. Unfortunately, after discharge, the patient refused further genetic testing.
Achalasia. Mitochondrial disease. KSS? MNGIE?
Considering the severe dysphagia and cachexia, a multidisciplinary team decided to perform POEM. After performing a submucosal tunnel myotomy of 8 cm in the esophageal muscular layer, a myotomy of 3 cm in the gastric muscular layer was also performed. From the region of the lower esophageal sphincter, 5 mm × 5 mm specimens of the lower and middle parts of the esophageal muscle (circular and longitudinal muscles) were obtained for further histological investigation. After the procedure, the endoscope was able to freely pass the lower esophageal sphincter.
The postoperative period was unremarkable. On postoperative day (POD) 2, liquid intake was initiated. It was later followed by eating liquid food. On POD 6, she was discharged in a satisfactory condition with a continuing pregnancy. The first follow-up was performed 3 mo after POEM: Eckardt score was 2, weight was 39 kg (+ 1 kg), EGD was normal, and pregnancy was 29 wk without any ultrasound findings of fetal pathology.
There are no guidelines on achalasia management in pregnant women. In the literature, achalasia cases in pregnant women were treated in different ways based on the duration of gestation, severity of the disease, and maternal and fetal risk. The most common are botulotoxin injections[9], balloon dilatation[10], Heller myotomy, or in some cases, treatment was delayed until childbirth, and patients received parenteral or enteral nutrition. Concerning nasojejunal feeding tube, the patient was in the beginning of second trimestr of pregnancy. Thus we decided that enteral nutrition is impractical for that long period because it can cause erosions and ulcers in stomach and esophagus. In addition to, long-term usage of nasojejunal feeding tube can also be a source of psychological stress to the patient. As far as dilatation concerned, the first course of dilatation with the use of 30 mm balloon has an efficacy of no more than 80% over the next 6 mo after surgery, resulting in an esophageal perforation rate of 1.1%[11,12]. The patient had not undergone Balloon Dilatation before, and we know from the literature that initiating dilatation is 10 times more likely to result in perforation, with a rate of up to 9.7%[13]. At the same time, the immediate clinical efficacy of POEM in some studies is more than 1.5 times higher than the efficacy of Balloon Dilatation (94% and 52%, respectively), and POEM is less likely to cause significant complications[14].
To the best of our knowledge, there are no cases of POEM in pregnant women published in the literature. A study by Vogel et al[15] showed a significant deterioration of the disease when achalasia developed and was not treated before preg
In our case, we chose POEM as the treatment method because we have extensive experience in such endoscopic procedures (more than 150 POEMs). In addition, we have a multidisciplinary team taking care of patients with achalasia.
We revealed a deletion in mDNA; however, this phenotype can as well be observed when mDNA damage is caused by a primary mutation in nuclear DNA (nDNA). These genetic disorders, unlike sporadic isolated mDNA mutations, usually have autosomal recessive inheritance, are less frequently autosomal dominant, and steadily progress[16]. Mutations in TYMP (MNGIE syndrome) and gene POLG (MNGIE–like syndrome) are the most common mutations of nDNA, which cause impairment of mDNA replication, resulting in severe GI motility disorders, cachexia, polyneuro
To the best of our knowledge, this is the first case of a pregnant woman with a mitochondrial disorder treated successfully with POEM and the first histology of the esophageal muscle layer of a patient with achalasia caused by mitochondrial disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tomizawa Y S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Ivashkin VT, Maev IV, Truhmanov AS, Lapina TL, Sheptulin AA, Storonova OA, Andreev DN. Diagnostics and treatment of gastroesophageal reflux disease: clinical guidelines of the Russian gastroenterological association. Rossijskij žurnal gastroènterologii, gepatologii, koloproktologii. 2015;84-93. |
| 2. | Finsterer J, Frank M. Gastrointestinal manifestations of mitochondrial disorders: a systematic review. Therap Adv Gastroenterol. 2017;10:142-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Suhorukov VS. Gastrointestinal dsorders in polysystemic mitochondrial insufficiency. Ross Vestn Perinatol i Pediatr. 2008;43-47. |
| 4. | Crespin OM, Liu LWC, Parmar A, Jackson TD, Hamid J, Shlomovitz E, Okrainec A. Safety and efficacy of POEM for treatment of achalasia: a systematic review of the literature. Surg Endosc. 2017;31:2187-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Schlottmann F, Luckett DJ, Fine J, Shaheen NJ, Patti MG. Laparoscopic Heller Myotomy Versus Peroral Endoscopic Myotomy (POEM) for Achalasia: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | Aiolfi A, Bona D, Riva CG, Micheletto G, Rausa E, Campanelli G, Olmo G, Bonitta G, Bonavina L. Systematic Review and Bayesian Network Meta-Analysis Comparing Laparoscopic Heller Myotomy, Pneumatic Dilatation, and Peroral Endoscopic Myotomy for Esophageal Achalasia. J Laparoendosc Adv Surg Tech A. 2020;30:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Cappell MS, Stavropoulos SN, Friedel D. Updated Systematic Review of Achalasia, with a Focus on POEM Therapy. Dig Dis Sci. 2020;65:38-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Phadke M, Lokeshwar MR, Bhutada S, Tampi C, Saxena R, Kohli S, Shah KN. Kearns Sayre Syndrome--case report with review of literature. Indian J Pediatr. 2012;79:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Hooft N, Schmidt ES, Bremner RM. Achalasia in Pregnancy: Botulinum Toxin A Injection of Lower Esophageal Sphincter. Case Rep Surg. 2015;2015:328970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Narang U, Narang L. Oesophageal achalasia diagnosed in pregnancy in a woman managed as severe hyperemesis refractory to medical management. J Obstet Gynaecol. 2019;39:1032-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Chuah SK, Hu TH, Wu KL, Kuo CM, Fong TV, Lee CM, Changchien CS. Endoscope-guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: a report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Tanaka Y, Iwakiri K, Kawami N, Sano H, Umezawa M, Kotoyori M, Hoshihara Y, Nomura T, Miyashita M, Sakamoto C. Predictors of a better outcome of pneumatic dilatation in patients with primary achalasia. J Gastroenterol. 2010;45:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Moonen A, Annese V, Belmans A, Bredenoord AJ, Bruley des Varannes S, Costantini M, Dousset B, Elizalde JI, Fumagalli U, Gaudric M, Merla A, Smout AJ, Tack J, Zaninotto G, Busch OR, Boeckxstaens GE. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation vs laparoscopic Heller myotomy. Gut. 2016;65:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 14. | Ponds FA, Fockens P, Lei A, Neuhaus H, Beyna T, Kandler J, Frieling T, Chiu PWY, Wu JCY, Wong VWY, Costamagna G, Familiari P, Kahrilas PJ, Pandolfino JE, Smout AJPM, Bredenoord AJ. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients With Achalasia: A Randomized Clinical Trial. JAMA. 2019;322:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 15. | Vogel T, Wrobel RM, Graupner O, Lobmaier S, Feussner H, Kuschel B. Esophageal achalasia and pregnancy: own observations in 43 patients and a review of the literature. Arch Gynecol Obstet. 2018;298:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Chelimsky G, Shanske S, Hirano M, Zinn AB, Cohen M, McNeeley K, Chelimsky TC. Achalasia as the harbinger of a novel mitochondrial disorder in childhood. J Pediatr Gastroenterol Nutr. 2005;40:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Teitelbaum JE, Berde CB, Nurko S, Buonomo C, Perez-Atayde AR, Fox VL. Diagnosis and management of MNGIE syndrome in children: case report and review of the literature. J Pediatr Gastroenterol Nutr. 2002;35:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tang S, Dimberg EL, Milone M, Wong LJ. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)-like phenotype: an expanded clinical spectrum of POLG1 mutations. J Neurol. 2012;259:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Shaibani A, Shchelochkov OA, Zhang S, Katsonis P, Lichtarge O, Wong LJ, Shinawi M. Mitochondrial neurogastrointestinal encephalopathy due to mutations in RRM2B. Arch Neurol. 2009;66:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Finsterer J, Zarrouk-Mahjoub S. Phenotypic and Genotypic Heterogeneity of RRM2B Variants. Neuropediatrics. 2018;49:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |