Published online Oct 16, 2020. doi: 10.4253/wjge.v12.i10.378
Peer-review started: April 17, 2020
First decision: June 8, 2020
Revised: June 18, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 16, 2020
Processing time: 179 Days and 22.4 Hours
Sedation is commonly performed for the endoscopic submucosal dissection (ESD) of early gastric cancer. Severe hypoxemia occasionally occurs due to the respiratory depression during sedation.
To establish predictive models for respiratory depression during sedation for ESD.
Thirty-five adult patients undergoing sedation using propofol and pentazocine for gastric ESDs participated in this prospective observational study. Preoperatively, a portable sleep monitor and STOP questionnaires, which are the established screening tools for sleep apnea syndrome, were utilized. Respiration during sedation was assessed by a standard polysomnography technique including the pulse oximeter, nasal pressure sensor, nasal thermistor sensor, and chest and abdominal respiratory motion sensors. The apnea-hypopnea index (AHI) was obtained using a preoperative portable sleep monitor and polysomnography during ESD. A predictive model for the AHI during sedation was developed using either the preoperative AHI or STOP questionnaire score.
All ESDs were completed successfully and without complications. Seventeen patients (49%) had a preoperative AHI greater than 5/h. The intraoperative AHI was significantly greater than the preoperative AHI (12.8 ± 7.6 events/h vs 9.35 ± 11.0 events/h, P = 0.049). Among the potential predictive variables, age, body mass index, STOP questionnaire score, and preoperative AHI were significantly correlated with AHI during sedation. Multiple linear regression analysis determined either STOP questionnaire score or preoperative AHI as independent predictors for intraoperative AHI ≥ 30/h (area under the curve [AUC]: 0.707 and 0.833, respectively) and AHI between 15 and 30/h (AUC: 0.761 and 0.778, respectively).
The cost-effective STOP questionnaire shows performance for predicting abnormal breathing during sedation for ESD that was equivalent to that of preoperative portable sleep monitoring.
Core Tip: Risk factors for sedation during endoscopic submucosal dissection (ESD) have not been systematically explored. Our study demonstrated that the preoperative portable sleep monitor and STOP questionnaire scores accurately predict abnormal breathing during sedation and the cost-effective questionnaire can be clinically used for risk stratification of respiratory depression during ESD, leading to a safe ESD procedure.
- Citation: Aikawa M, Uesato M, Urahama R, Hayano K, Kunii R, Kawasaki Y, Isono S, Matsubara H. Predictor of respiratory disturbances during gastric endoscopic submucosal dissection under deep sedation. World J Gastrointest Endosc 2020; 12(10): 378-387
- URL: https://www.wjgnet.com/1948-5190/full/v12/i10/378.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i10.378
Recently, endoscopic mucosal dissection (ESD) has been widely used to treat early gastric cancer. ESD is a highly difficult and often lengthy surgical procedure[1]. Appropriate sedation improves the quality of treatment and increases patient satisfaction[2-6]. The levels of sedation are divided into several stages, from minimal to deep[7]. The dangers of respiratory and circulatory system failures increase with moderate and deep sedation[8]. Moreover, the occurrence of sedation-related complications in gastrointestinal endoscopy can lead to significant morbidity and occasional mortality in patients[8]. Further, the risks can be high especially for procedures performed outside the operating room, such as the endoscopic laboratory[9]. When administering sedatives, careful attention must be paid to the respiratory status during sedation. Continuous respiratory and oxygen monitoring is critical, which is clearly stated in the guidelines on gastrointestinal endoscopy[10]. However, the required components of monitoring have not been defined yet. Additionally, respiratory monitoring often comprises only oxygen administration and pulse oximetry measurements[11,12]. In our previous study, apnea or hypopnea was not well detected by the pulse oximeter alone; it was proven that polysomnography (PSG), which is usually used for sleep apnea diagnosis, was useful for the accurate detection of abnormal breathing during sedation for ESD[13]. However, the PSG technique is a laborious and costly procedure to be used as a clinical tool for monitoring during sedation. Therefore, the preoperative identification of patients at high risk of intraoperative respiratory depression can help to ensure the optimal environment for practical sedation.
Sleep apnea syndrome (SAS) is an independent factor for postoperative respiratory complications following general anesthesia[14-17]. We previously demonstrated the occurrence of SAS-like respiration disorders during the sedation for ESDs[13].
In this study, we examined whether abnormal breathing is more frequent during sedation than during sleep and aimed to develop a clinically useful prediction model for abnormal breathing during the sedation for ESD.
We performed this prospective observational study after obtaining approval from the Institutional Ethics Committee (No. 1902-2014; Graduate School of Medicine, Chiba University, Chiba, Japan). Written informed consent was obtained from each patient. Inclusion criteria was the adult patient during ESD for early gastric cancer under propofol sedation with expected < 2 h. Exclusion criteria were patients with severe heart disease and renal failure, including high aspiration risks and drug allergy to propofol. In total, 35 patients (24 men and 11 women; mean age, 73.2 years) were enrolled between 2014 and 2016.
Preoperatively, the patients underwent a portable sleep study during natural sleep and answered the STOP questionnaire. The portable sleep study (PS) was performed using a portable sleep monitor (PSS, SAS-2100; Nihon Kohden, Tokyo, Japan), which measures the airflow via a nasal pressure cannula and oxygen saturation (SaO2). PS data were analyzed using dedicated computer software (QP-021 W; Nihon Kohden). Apnea and hypopnea were determined by absence of airflow for 10 s or more and more than 50% decrease of the nasal pressure signal for 10 s or more independently of SaO2 change, respectively. The apnea-hypopnea index (AHI) was determined as the frequencies of apneas and hypopneas/hour of monitoring period and the AHI measured by the PS before ESD was considered to be preoperative AHI. The STOP questionnaire was originally designed to preoperatively screen obstructive sleep apnea patients using four yes/no questions including habitual snoring, daytime fatigue/tiredness, observed apnea during sleep, and high blood pressure. The score is based on the number of “yes” answers and ranges from 0 to 4[19]. When two or more questions are answered with “yes,” the result is considered positive.
Prior to sedation for the ESD procedure, standard PSG electrodes were attached (PSG-1100; Nihon Kohden), in addition to routine monitors used during gastrointestinal endoscopy including those for pulse oximetry, electrocardiogram, and intermittent blood pressure measurements. Airflow measurements were obtained via a nasal pressure cannula and oronasal thermistors. Thoraco-abdominal wall motions with piezo-respiratory effort sensors, SaO2, and snoring monitored by a microphone were recorded and stored.
Oxygen at the rate of 2 L/min was administered via the nasal cannula while the patients were on their left side. Propofol (1-2 mg/kg) was carefully administered until patients lost consciousness and continuously infused at a rate of 1-4 mg/kg per hour to maintain Ramsey scores of 5-6 (loss of responses to verbal commands and light tapping on the shoulder, but arousable by painful stimulation)[18]. Pentazocine (7.5 mg) was intravenously administered for analgesia about every 30 min. Unstable cardiorespiratory abnormalities detected by patient monitors were used for decision making regarding the propofol infusion rates and airway maneuvers to restore breathing.
After the completion of measurements, a certified sleep technician (RK) and investigators manually analyzed the PSG data with using dedicated computer software (Polysmith; Nihon Kohden, Tokyo, Japan). We focused on two sensors: Nasal cannula and oro-nasal thermistor for airflow measurement; and Piezo-respiratory effort sensors (RIP-chest and/or RIP-abdomen) for thoraco-abdominal wall motion assessment. Apneic and hypopneic events were systematically classified based on the presence or absence of thoraco-abdominal respiratory movements and divided into obstructive and central.
The values are presented as means and standard deviations for continuous data and numbers of cases and proportions for categorical data. Univariate correlations between the intraoperative AHI and other variables (STOP questionnaire score, dosage of propofol, age, sex, and body mass index [BMI]) were performed using Pearson’s correlation analysis; additionally, the results were presented using coefficients and P values. Two multiple linear regression analysis models: Model 1, multiple linear regression analysis explaining the intraoperative AHI (objective variable) with all potential explanatory variables except for the STOP questionnaire score; model 2, multiple linear regression analysis explaining the intraoperative AHI (objective variable) with all explanatory variables except for preoperative AHI. We calculated the cutoff values for the pre-AHI and STOP questionnaire scores. By setting the threshold values for intraoperative AHI ≥ 30/h (severe SAS) and AHI ≥ 15 and < 30/h (moderate SAS), we converted each variable into a binary outcome. We performed logistic regression analysis using binary data as objective variables and preoperative AHI and STOP questionnaire scores as exploratory variables, deriving cutoff values for each. We plotted receiver operating characteristic curves, calculated sensitivities and specificities, and determined Youden’s Indexes. We calculated areas under the curves (AUCs) for preoperative AHI and STOP questionnaire scores to evaluate the predictive abilities of the cutoff values. We also calculated P values for the difference between the AUCs. P value < 5% was considered statistically significant. SAS Version 9.4 (SAS Institute; Cary, NC, United States) was used for all statistical analyses.
The patient background data are shown in Table 1. All procedures were performed successfully and no patient required treatment discontinuation. The average age of the patients was 73.2 years (24 men and 11 women). There were 3 patients with mild respiratory comorbidity, none of which had subjective symptoms. Fourteen patients (45.1%) were suspected to have SAS (total scores ≥ 2) using the STOP questionnaire. Seventeen patients (48.6%) were diagnosed preoperatively with SAS (preoperative AHI ≥ 5) with the aid of PS. Among those, 6 patients (35.3%) had moderate SAS (preoperative AHI ≥ 15 and < 30) and 2 patients (11.8%) had severe SAS (preoperative AHI ≥ 30). The mean preoperative AHI was 9.25 ± 11.03/h. The average intraoperative AHI was 12.76 ± 7.59/h (central: 3.2 ± 2.8/h, obstructive: 9.6 ± 6.5/h), which was significantly higher than the preoperative AHI (P = 0.049). The mean intraoperative AHI in patients with SAS was significantly higher than in those without SAS (SAS-positive: 16.44 ± 7.99/h, SAS-negative: 9.29 ± 5.37/h, P = 0.017) (Table 2). Intraoperative AHI was significantly elevated by sedation in SAS-negative and mild SAS patients, but not in moderate and severe SAS patients. Thirty-one patients (88.6%) had intraoperative AHIs ≥ 5 according to the SAS. Among these, eleven patients (35.5%) had intraoperative AHIs ≥ 15 and < 30. Additionally, it was observed that AHIs exceeded 30 in 2 patients (6.5%; not shown in table). Based on these intraoperative AHI measurements, we attempted to determine the predictors of respiratory disturbances under sedation.
| n | % | |
| Gender | ||
| Male | 24 | 68.6 |
| Female | 11 | 31.4 |
| Age in yr | 73.2 ± 10.2 | |
| BMI in kg/m2 | 23.0 ± 3.7 | |
| Score of STOP questionnaire | ||
| 0 | 6 | 19.4 |
| 1 | 11 | 35.5 |
| 2 | 9 | 29 |
| 3 | 4 | 12.9 |
| 4 | 1 | 3.2 |
| Total dose of propofol in mg/h | 9.8 ± 3.8 | |
| Sedation period in min | 107.6 ± 44.0 |
| n (%) | Preoperative AHI | Intraoperative AHI | P value | |
| All patients | 35 | 9.25 ± 11.03 | 12.76 ± 7.59 | 0.049 |
| SAS | ||||
| Negative | 18 (51.4) | 2.55 ± 1.40 | 9.29 ± 5.37b | < 0.001 |
| Positive | 17 (48.6) | 16.34 ± 12.35 | 16.44 ± 7.99 | NS |
| Mild | 9 (52.9) | 8.41 ± 2.37 | 15.21 ± 8.08 | 0.042 |
| Moderate | 6 (35.3) | 18.55 ± 3.35 | 15.58 ± 7.60 | NS |
| Severe | 2 (11.8) | 45.40 ± 7.35 | 24.57 ± 7.67 | NS |
Single regression analysis was performed with intraoperative AHI as the objective variable and preoperative AHI, STOP questionnaire score, propofol dose/hour, age, sex, and BMI as the explanatory variables. A significant association was observed between the preoperative AHI (P = 0.0068), STOP questionnaire score (P = 0.0375), age (P = 0.0272), and BMI (P = 0.0299) (Table 3). In the multiple regression analysis, a significant difference was observed in preoperative AHI (P = 0.0296) when the STOP questionnaire score was removed from the above variables (Table 4). Age was included as an influential variable; however, no significant difference was found (P = 0.1240). Similarly, a significant difference was found between the STOP questionnaire score (P = 0.0069) and age (P = 0.0040) in the multiple regression analysis when preoperative AHI was excluded from the above variables (Table 5).
| P value | R2 | |
| Preoperative AHI | 0.0068 | 0.2016 |
| Age | 0.0272 | 0.1393 |
| BMI | 0.0299 | 0.1350 |
| STOP questionnaire | 0.0375 | 0.1408 |
| Dose of propofol | 0.0783 | 0.0910 |
| Gender | 0.2048 | 0.0483 |
| Partial regression coefficient | Standardized regression coefficient | |||||
| B | Standard error | β | t | P value | ||
| Intercept | 24.273 | 9.215 | 0 | 2.63 | 0.0129 | |
| Preoperative AHI | 0.252 | 0.111 | 0.366 | 2.28 | 0.0296 | |
| Age | -0.189 | 0.120 | -0.254 | -1.58 | 0.1240 | |
| Partial regression coefficient | Standardized regression coefficient | |||||
| B | Standard error | β | t | P value | ||
| Intercept | 34.319 | 8.401 | 0 | 4.09 | 0.0003 | |
| STOP questionnaire | 3.306 | 1.134 | 0.444 | 2.92 | 0.0069 | |
| Age | -0.364 | 0.116 | -0.477 | -3.13 | 0.0040 | |
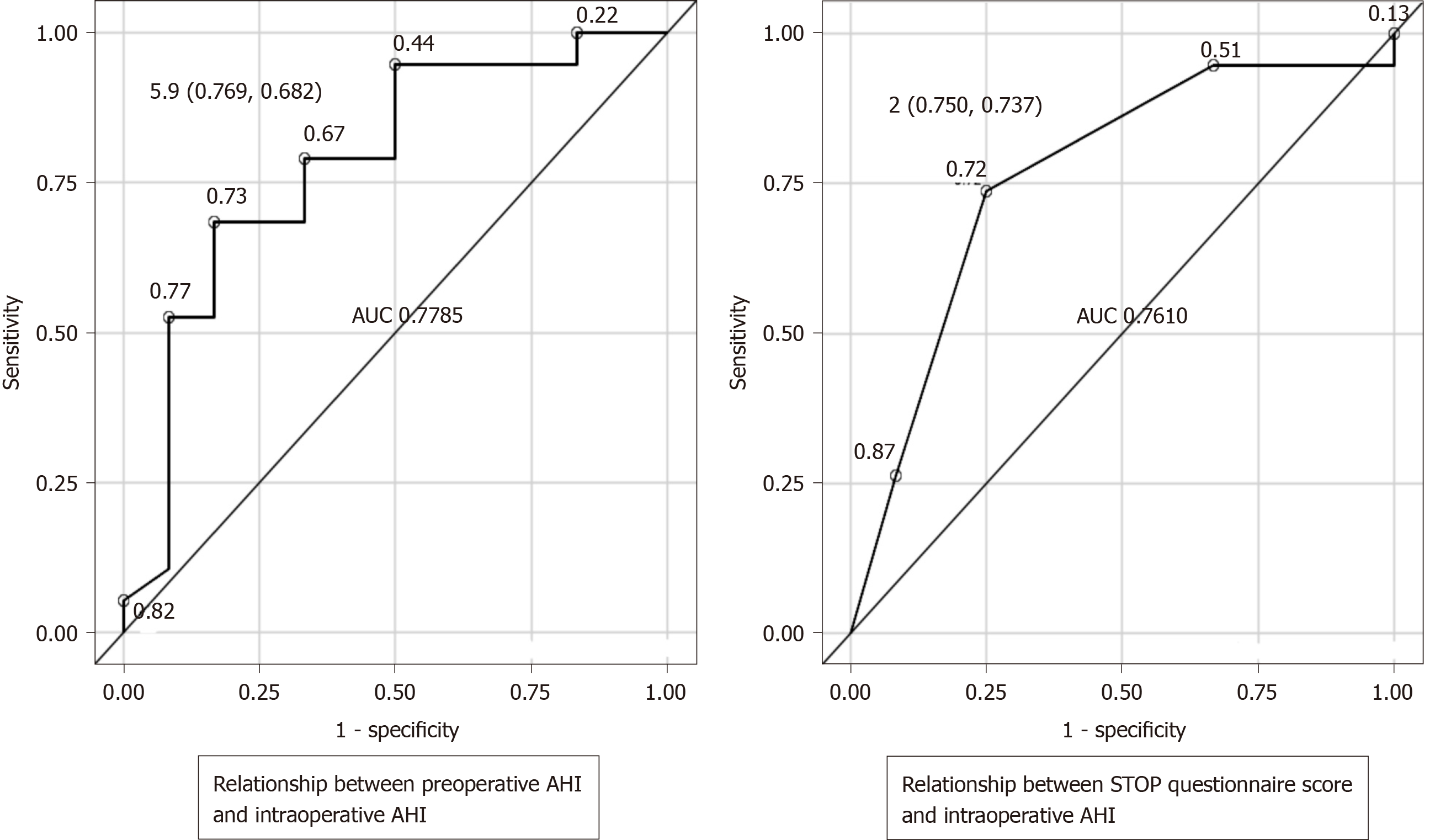
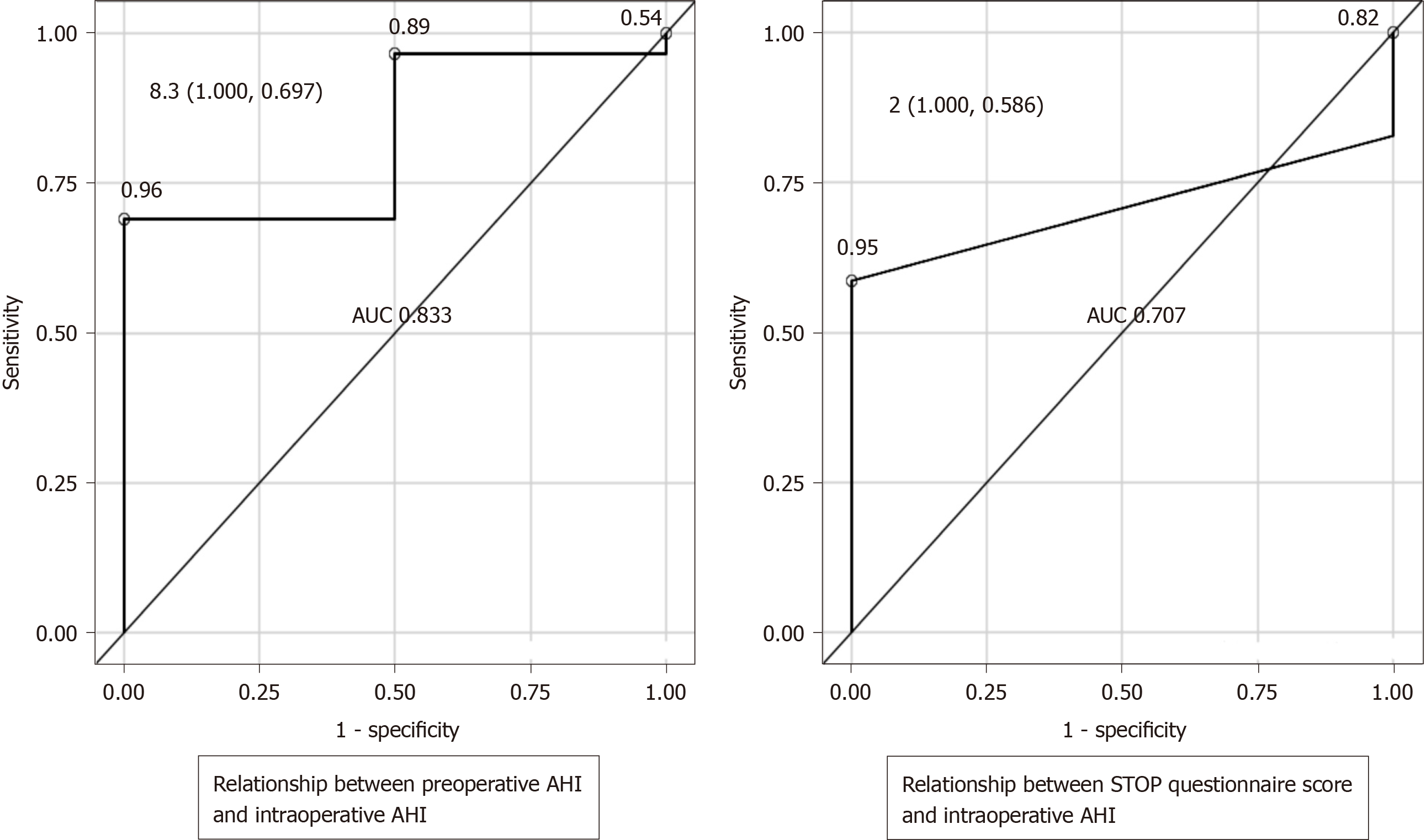
Receiver operating characteristic curves were created to evaluate the relationship between the preoperative screening tests and intraoperative AHI as the outcome. When the outcome was intraoperative AHI ≥ 15 and < 30 (SAS: Moderate criteria), if a preoperative AHI of 5.9 was considered as a cutoff, the sensitivity was 76.9%, the specificity was 68.2%, and the Youden's index was 0.451, which was then defined as the optimum cutoff. Similarly, if a STOP questionnaire score of 2 was taken as the cutoff value, the sensitivity was 75%, the specificity was 73.7%, and the Youden's index was 0.4868 (Figure 1). When the outcome was intraoperative AHI ≥ 30 (SAS: Severe criteria), if a preoperative AHI of 8.3 was taken as a cutoff value, the sensitivity was 100%, the specificity was 69.7%, and the Youden's index was 0.6970, which was then defined as the optimum cutoff value. Similarly, if a STOP questionnaire score of 2 was taken as the cutoff value, the sensitivity was 100%, the specificity was 58.6%, and the Youden's index was 0.5862 (Figure 2). Moreover, we compared the preoperative AHI and STOP questionnaire scores as potential preoperative screening tests to determine which would have a higher diagnostic ability. For intraoperative AHI ≥ 15 and < 30, the preoperative AHI showed an AUC of 0.778 and the STOP questionnaire score showed an AUC of 0.761, which were nearly equivalent; however, the difference was not statistically significant (P = 0.8921) (Figure 1). For intraoperative AHI ≥ 30, the preoperative AHI showed an AUC of 0.833, and the STOP questionnaire score showed an AUC of 0.707. Thus, the estimates of preoperative AHI were higher and the diagnostic ability was greater; however, these differences were not statistically significant (P = 0.4450) (Figure 2).
The establishment of predictive models for respiratory depression during sedation for ESD may increase the safety and comfort of the ESD procedure. In this prospective observational study, we found that (1) half of the patients undergoing ESD surgery experienced SAS preoperatively; (2) intraoperative AHI was significantly greater than the preoperative AHI, although these variables were significantly correlated with each other; and (3) both preoperative AHI and STOP questionnaire score were independent predictors of respiratory depression during sedation.
In the United States, among patients aged between 30 and 60 years, the prevalence of SAS is defined as AHI ≥ 5, and the clinical symptoms suggesting SAS are reported to be noted in 4% of men and 2% of women. Notably, when the asymptomatic patients are included, the incidence increases up to 24% in men and 9% in women[24]. This suggests that several patients undergoing ESD surgery remain undiagnosed. In fact, we found that about half of the patients were diagnosed with SAS based on preoperative PS. Obesity and aging are well-known risk factors for SAS and these were increasingly common in our ESD patients. Accordingly, preoperative SAS screening should be stressed more such that clinicians can predict and prepare for the risk of respiratory depression during ESD under sedation.
Preoperative SAS is an independent risk factor for postoperative respiratory complications[14-17]. However, whether preoperative SAS is a risk factor for hypoxemia during endoscopic procedures under sedation still remains controversial[20,21]. PSG is a standard diagnostic technique for evaluating the presence and severity of SAS. We previously demonstrated that PSG can detect respiratory disturbances under deep sedation more accurately and in more detail than pulse oximetry[13,22]. In this study, using the same PSG technique, we detected more episodes of respiratory depression during sedation than during natural sleep, particularly in patients without preoperative SAS and those with mild preoperative SAS. Notably, the severity of intraoperative SAS did not differ from the preoperative SAS severity in patients with preoperative moderate and severe SAS. Additional profound suppression of the upper airway muscle tone during deep sedation than that during natural sleep might account for the increased severity of SAS during sedation. The lateral decubitus position commonly used for gastric ESD procedure, which is known to improve the upper airway patency and AHI in SAS patients, might have prevented worsening of AHI in patients with moderate to severe SAS in this study[23]. In any case, it is advised to use adequate respiratory monitoring such as that involving capnogram, thermistor, and nasal pressure.
Our results indicate that both preoperative portable sleep monitor and STOP questionnaire are equally effective for predicting the occurrence of respiratory depression during sedation for gastric ESD. Although portable sleep monitoring is a simple clinical test that can be performed at the patient’s home, the device for the sleep study is costly and is not available at all medical facilities where endoscopic surgery is performed under sedation. In contrast, the STOP questionnaire consists of only four simple questions and can be used without special devices and laborious setting and analysis. In the original STOP questionnaire study[19], two patterns of the questionnaire (STOP with four questions and STOP-BANG with eight questions) were proposed and tested. Although STOP-BANG had better predictive performance, it requires additional measurements of BMI and neck circumference as well as information on age and sex. In an original study testing a general surgery population, the AUCs for predicting AHI ≥ 15 and AHI ≥ 30 during natural sleep were 0.722 and 0.769, respectively, when a STOP questionnaire score of 2 was used as the cutoff value. Using the same STOP questionnaire score cutoff value, we found that the AUCs for predicting intraoperative AHI ≥ 15 and AHI ≥ 30 were 0.761 and 0.707, respectively, in patients undergoing ESD surgery in agreement with the original STOP study. Accordingly, we consider the STOP questionnaire as a clinically relevant tool for predicting moderate to severe respiratory depression during sedation for ESD procedures in contrast to preoperative portable sleep monitoring. However, it should be noted that the performance of the STOP questionnaire is limited. A positive STOP questionnaire result may indicate that the patient may have respiratory depression during sedation, but it does not accurately predict the severity of respiratory depression. In contrast, a negative STOP questionnaire result may indicate that the patient would not develop severe respiratory depression; nevertheless, it does not guarantee stable respiration during sedation.
This study had several limitations. First, the total number of study patients was relatively small. Therefore, there is a possibility of bias in patient selection. In future studies, it will be necessary to expand the patient population and include more cases. Second, there were differences in the analysis method for AHI in PSG and PS. While the PSG was analyzed manually by a certified sleep technician, PS data were automatically analyzed by computer software. Thus, the AHI values might be different if the PS data are manually scored.
In conclusion, respiratory depression, characterized by obstructive apnea and hypopnea, commonly develops during ESD surgery under sedation. Additionally, the preoperative portable sleep monitor and STOP questionnaire scores accurately predict abnormal breathing. From the viewpoint of cost-effectiveness, the STOP questionnaire is a clinically useful tool for risk stratification of respiratory depression during ESD, leading to safe ESD procedures.
Recently, endoscopic treatments often take a long time under deep sedation. In these cases, there are many respiratory disturbances that cannot be detected.
In our previous study, polysomnography (PSG) could accurately identify the respiratory disturbances during endoscopic submucosal dissection (ESD) under deep sedation. We wanted to know the preoperative characteristics of patients who experienced intraoperative respiratory disturbances.
We established predictive models for respiratory depression during sedation for ESD.
Thirty-five adult patients undergoing sedation for gastric ESDs were studied. Preoperatively, a portable sleep monitor and STOP questionnaires were used. Respiration during sedation was assessed using a standard PSG. The apnea-hypopnea index (AHI) was obtained using a preoperative portable sleep monitor and PSG during ESD. A predictive model for the AHI during sedation was developed using either the preoperative AHI or STOP questionnaire score.
Half of the patients had a preoperative AHI greater than 5 /hour. The intraoperative AHI was significantly greater than the preoperative AHI (12.8 ± 7.6 events/h vs 9.4 ± 11.0 events/h, P = 0.049). Multiple linear regression analysis determined either STOP questionnaire score or preoperative AHI as an independent predictor for moderate to severe respiratory depression during sedation.
The cost-effective STOP questionnaire has performance for predicting abnormal breathing during sedation for ESD that is equivalent to that of preoperative portable sleep monitoring, and can be used as a routine screening tool prior to the ESD procedure.
The results of this study could increase the safety of ESD under sedation through the development of a clinically useful screening tool for predicting respiratory depression, which possibly leads to fatal outcomes during the procedure.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawara F S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LL
| 1. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Abraham NS, Fallone CA, Mayrand S, Huang J, Wieczorek P, Barkun AN. Sedation vs no sedation in the performance of diagnostic upper gastrointestinal endoscopy: a Canadian randomized controlled cost-outcome study. Am J Gastroenterol. 2004;99:1692-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Riphaus A, Wehrmann T, Weber B, Arnold J, Beilenhoff U, Bitter H, von Delius S, Domagk D, Ehlers AF, Faiss S, Hartmann D, Heinrichs W, Hermans ML, Hofmann C, In der Smitten S, Jung M, Kähler G, Kraus M, Martin J, Meining A, Radke J, Rösch T, Seifert H, Sieg A, Wigginghaus B, Kopp I; Sektion Enoskopie im Auftrag der Deutschen Gesellschaft für Verdauungs- und Stoffwechselerkrankungen e. V. (DGVS); Bundesverband Niedergelassener Gastroenterologen Deuschlands e. V. (Bng); Chirurgische Arbeitsgemeinschaft für Endoskopie und Sonographie der Deutschen Gesellschaft für Allgemein- und Viszeralchirurgie (DGAV); Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung e. V. (DCCV); Deutsche Gesellschaft für Endoskopie-Assistenzpersonal (DEGEA); Deutsche Gesellschaft für Anästhesie und Intensivmedizin (DGAI); Gesellschaft für Recht und Politik im Gesundheitswesen (GPRG). [S3-guidelines--sedation in gastrointestinal endoscopy]. Z Gastroenterol. 2008;46:1298-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, Aisenberg J. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 7. | American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1313] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 8. | Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5:527-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 9. | Sharma VK, Nguyen CC, Crowell MD, Lieberman DA, de Garmo P, Fleischer DE. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | ASGE Ensuring Safety in the Gastrointestinal Endoscopy Unit Task Force. Calderwood AH, Chapman FJ, Cohen J, Cohen LB, Collins J, Day LW, Early DS. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014;79:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Fu ES, Downs JB, Schweiger JW, Miguel RV, Smith RA. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 2004;126:1552-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Lynn LA, Curry JP. Patterns of unexpected in-hospital deaths: a root cause analysis. Patient Saf Surg. 2011;5:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Urahama R, Uesato M, Aikawa M, Yamaguchi Y, Hayano K, Matsumura T, Arai M, Kunii R, Isono S, Matsubara H. Polysomnographic assessment of respiratory disturbance during deep propofol sedation for endoscopic submucosal dissection of gastric tumors. World J Gastrointest Endosc. 2018;10:340-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Meoli AL, Rosen CL, Kristo D, Kohrman M, Gooneratne N, Aguillard RN, Fayle R, Troell R, Kramer R, Casey KR, Coleman J; Clinical Practice Review Committee; American Academy of Sleep Medicine. Upper airway management of the adult patient with obstructive sleep apnea in the perioperative period--avoiding complications. Sleep. 2003;26:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Mickelson SA. Anesthetic and postoperative management of the obstructive sleep apnea patient. Oral Maxillofac Surg Clin North Am. 2009;21:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Hwang D, Shakir N, Limann B, Sison C, Kalra S, Shulman L, Souza Ade C, Greenberg H. Association of sleep-disordered breathing with postoperative complications. Chest. 2008;133:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 18. | Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1845] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 19. | Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1252] [Cited by in RCA: 1436] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 20. | Andrade CM, Patel B, Vellanki M, Kumar A, Vidyarthi G. Safety of gastrointestinal endoscopy with conscious sedation in obstructive sleep apnea. World J Gastrointest Endosc. 2017;9:552-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Liou SC, Hsu CM, Chen C, Su MY, Chiu CT. Assessment of the Berlin Questionnaire for evaluation of hypoxemia risk in subjects undergoing deep sedation for screening gastrointestinal endoscopy. Ther Clin Risk Manag. 2018;14:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Urahama R, Uesato M, Aikawa M, Kunii R, Isono S, Matsubara H. Occurrence of Cortical Arousal at Recovery from Respiratory Disturbances during Deep Propofol Sedation. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002;97:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2765] [Cited by in RCA: 2763] [Article Influence: 120.1] [Reference Citation Analysis (0)] |