Published online Nov 16, 2018. doi: 10.4253/wjge.v10.i11.348
Peer-review started: May 12, 2018
First decision: July 9, 2018
Revised: August 8, 2018
Accepted: October 8, 2018
Article in press: October 10, 2018
Published online: November 16, 2018
Processing time: 188 Days and 16.1 Hours
To prospectively evaluate the efficacy of submucosal injection of platelet-rich plasma (PRP) on endoscopic resection of large sessile lesions.
Eleven patients were submitted to endoscopic mucosal resection (EMR) with prior injection of PRP, obtained at the time of endoscopy. Patients were followed during 1 mo. The incidence of adverse events (delayed bleeding or perforation) and the percentage of mucosal healing (MHR) after 4 wk were registered.
EMR was performed in 11 lesions (46.4 mm ± 4 mm, range 40-70 mm). Delayed bleeding or perforation was not observed in any patient. Mean ulcerated area at baseline was 22.7 cm2 ± 11.7 cm2 whereas at week 4 were 2.9 cm2 ± 1.5 cm2. Patients treated with PRP showed a very high MHR after 4 wk (87.5%).
PRP is an easy-to-obtain solution with proven and favourable biological activities that could be used in advanced endoscopic resection.
Core tip: This was a prospective single-center study to evaluate the efficacy of submucosal injection of platelet-rich plasma (PRP) on 11 patients submitted to endoscopic resection of large lesions. PRP as lifting solution proved absence of delayed bleeding or perforation and strong healing activity.
- Citation: Lorenzo-Zúñiga V, de Vega VM, Bartolí R, Marín I, Caballero N, Bon I, Boix J. Submucosal injection of platelet-rich plasma in endoscopic resection of large sessile lesions. World J Gastrointest Endosc 2018; 10(11): 348-353
- URL: https://www.wjgnet.com/1948-5190/full/v10/i11/348.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i11.348
Submucosal injection of fluid solutions is crucial to prevent of delayed perforation (DP) in advanced resection techniques, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), by avoiding deep thermal injury. The perforation rate is traditionally considered as a quality of standard practice. It has a rate of 0.03%-0.8% during diagnostic procedures and 0.15%-3% during therapeutic procedures[1]. Otherwise, delayed bleeding (DB) is a well-known and the most frequent adverse event after these resections, with an incidence of 2.6%–9.7%, not prevented by adding adrenaline to the submucosal fluid cushion or applying argon plasma coagulation, because these methods only decrease the incidence of early bleeding[2-4]. There is no scientific evidence to recommend the systematic closure of the eschars with hemostatic clips to prevent DB because they are ineffective in large mucosal defects and increase procedure costs[5].
The ideal submucosal solution should provide a sustained lift, facilitate en-bloc or oligopiecemeal resection, be inexpensive, widely available and have few adverse effects[1]. The optimal fluid to lift the lesion is still a matter of debate. Platelet-rich plasma (PRP), as autologous concentrated in plasma, has demonstrated strong healing properties as a shield over the eschars after EMR in preclinical models[6,7]. PRP solution has showed the best electrical and rheological properties to perform safety endoscopic resections[8].
Therefore, we aim to evaluate the efficacy of submucosal injection of PRP on EMR of large sessile lesions.
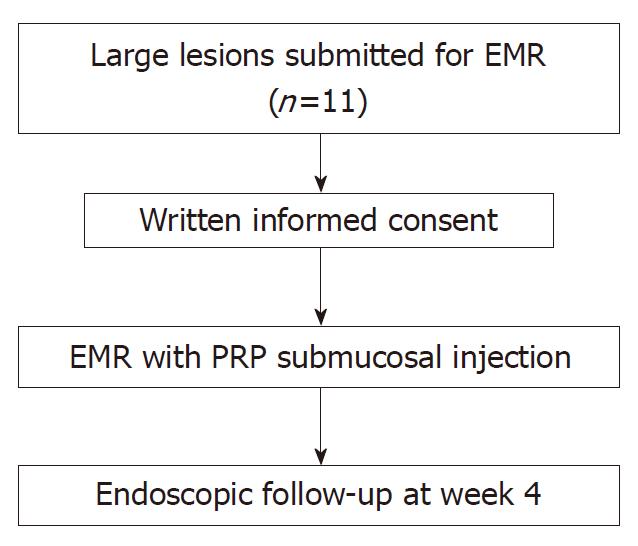
This study was registered at ClinicalTrials.gov under the identifier NCT02931149 (EndoPRP study), was conducted from August 2016 to March 2017. Subjects eligible for the study were men and women aged 18 and older who were submitted for EMR of sessile lesions larger than 35 mm. We obtained a written informed consent in all participants. The Healthcare Ethics Committee of our institution (University Hospital Germans Trias i Pujol) approved the study protocol (IRB approval PT-16-002 on July 8, 2016), and was performed in accordance with the Declaration of Helsinki.
This was a non-randomized prospective single-center study. We performed an expanded access study (compassionate use) of PRP outside of a clinical trial because we wanted to generate information with a small number of individual patients. Patients were allocated to receive PRP as submucosal injection of PRP prior to EMR (Figure 1). After the procedure, all patients were followed during 4 wk. EMR was performed with blended current controlled by a microprocessor (ME 402 maxium KLS martin, Tuttlingen. Germany). The device used in all patients was a circular polyfilament snare 25 mm in diameter (SnareMaster, Olympus. Tokyo). After the procedure coagulation of the base with APC was performed in all cases.
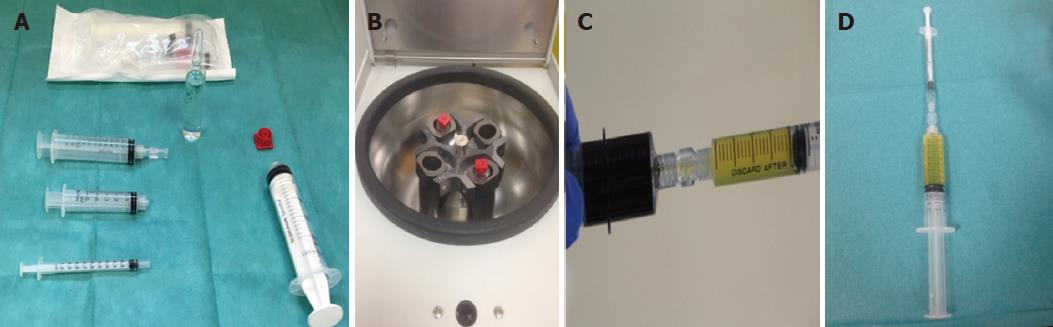
We obtained PRP with OLIN-1 kit (a single-use sterile product), that comes in both a 20 mL and 40 mL format, from a sample of patient’s blood (18-36 mL) drawn at our Endoscopy Unit prior to perform the EMR (Figure 2). Peripheral blood was centrifuged (2500 rpm/8 min at room temperature). Depending on the size of the lesions, smaller or larger than 40 mm, we used 18 or 36 mL of blood (1 or 2 kits). A 20-mL syringe prefilled with 2 mL acid citrate dextrose (15% vol./vol.) was used for the standardized blood draw. Syringes were centrifuged obtaining two different layers; erythrocytes (± 45% of volume) placed at the bottom, and PRP (55% vol., around 8 mL) on the top. PRP was activated with the addition of 20 mmol/L CaCl2 just before the administration. A sample of 10 μL of blood and plasma were used to take a measurement of baseline blood platelet count and platelet count in PRP.
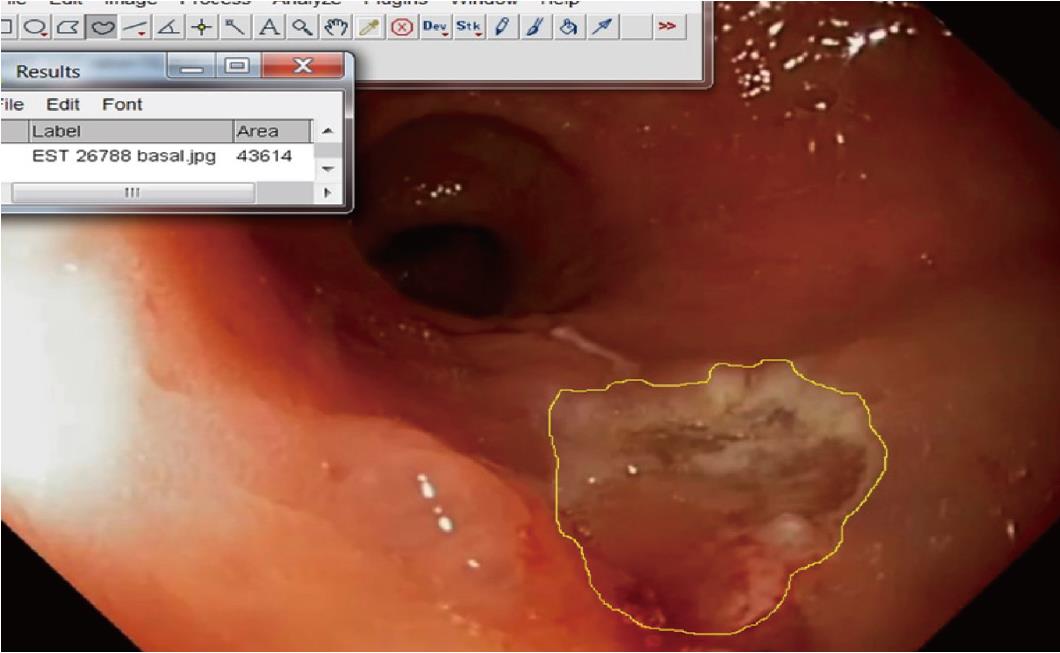
The primary outcome was the assessment of the incidence of adverse events (DB or DP). Secondary objective was the evaluation of mucosal healing rate (MHR), calculated as a percentage of mucosal restoration after 1 mo. Measurement of mucosal lesion and mucosal defect was carried out as comparison with opened forceps (7 mm) or by direct measurement with the specimen before pinning the specimen. We calculated the mean ulcerated and mucosal healing rate by the use of ImageJ public software (Image Processing and Analysis in Java; https://imagej.nih.gov/ij/)(Figure 3).
Unless otherwise indicated, results are expressed as mean ± SE or proportions as required. Statistical analyses were carried out with SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL, United States).
A total of 11 EMRs large colorectal or gastric lesions were performed in 11 patients (Table 1). There were 6 (54.5%) females and their mean age was 68.3 years (range 53 to 84 years). More than half were located in rectum or in left colon, mean basal platelet count was of 175 × 109/L, whereas obtained PRP was 2 times the basal value. The mean lesion size was 46.4 mm (SD, 11.4 mm; range 40-70 mm). Oligopiecemeal technique with complete resection was reached in all cases. Histology showed absence of deep submucosal involvement in all patients.
| EMR with PRP | |
| No. of patients | 11 |
| Mean age (yr) | 68.3 ± 9.48 |
| Men/women | 5/6 |
| Mean size of lesions (mm) | 46.4 ± 11.4 |
| Basal platelet count (109/L) | 175.4 ± 47.2 |
| PRP count (109/L) | 362.8 ± 98.7 |
| Site, n (%) | |
| Antrum | 2 (18.2) |
| Rectum | 4 (36.4) |
| Left colon | 3 (27.2) |
| Right colon/cecum | 2 (18.2) |
| Histology, n (%) | |
| Tubular adenoma | 3 (27.3) |
| Tubulovillous adenoma | 1 (9.1) |
| Villous adenoma | 0 (0) |
| Serrated adenoma | 4 (36.3) |
| Intramucosal adenocarcinoma | 3 (27.3) |
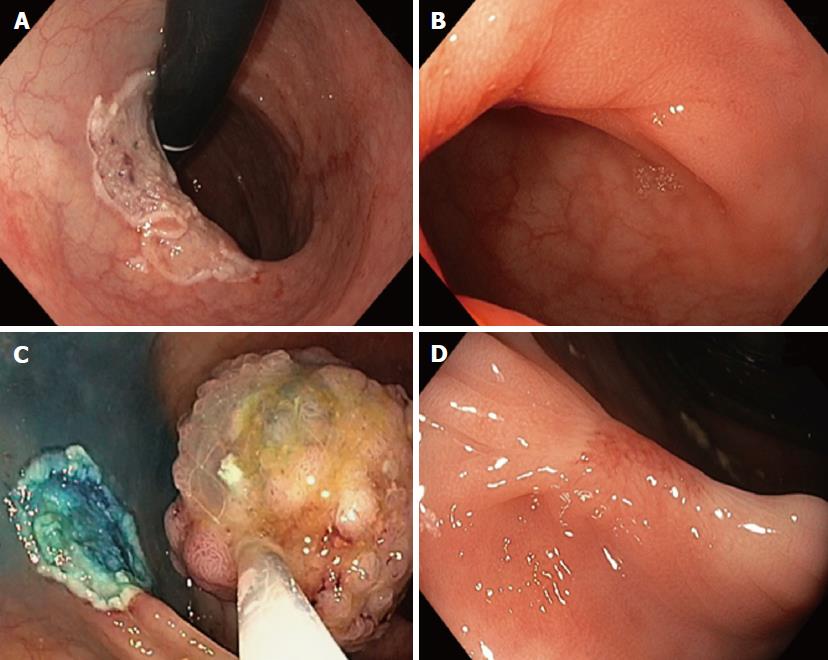
Patient outcomes are summarized in Table 2. DP or DB was not observed in any case. PRP does not prolong EMR time. No evidence of stricture was found during the follow-up. Mean ulcerated area at baseline was 22.7 cm2 ± 11.7 cm2 whereas after 4 wk was 2.9 cm2 ± 1.5 cm2. The percentage of mucosal healing at week 4 was of 87.5% (Figure 4).
| EMR with PRP | |
| Delayed perforation, n (%) | 0 (0) |
| Delayed bleeding, n (%) | 0 (0) |
| Mean ulcerated area at baseline (cm2) | 22.7 ± 11.7 |
| Mean ulcerated area at 4 wk (cm2) | 2.9 ± 1.5 |
| Mucosal healing rate (%) | 87.5 |
In this prospective study, we found that submucosal injection of PRP has proven efficacy in EMR of lesions larger than 35 mm, showing strong healing activity. Otherwise, the use of a submucosal fluid cushion rich in platelets prevents the incidence of DB or DP.
EMR and ESD as resection techniques can produce adverse events, such as perforation or bleeding. Post-EMR bleeding occurs in 5%-7% lesions ≥ 20 mm, whereas perforation is an uncommon event with an incident of 1.4%-1.5%[1]. Mucosal elevation through the injection of a solution into the submucosal space can reduce the incidence of these events and improve the technical feasibility of the procedure[9]. Normal saline is the most widely used solution but is not the most convenient for large lesions due to the maintenance of the fluid cushion. According to this, we should use other biocompatible lifting solutions easy to prepare and to administrate.
Use of PRP involves taking a sample of a patient’s blood prior to the endoscopic procedure and concentrating autologous platelets by centrifugation. PRP fluid contains at least 2 times peripheral blood platelets value and high levels of growth factors essential for mucosal healing, which are released from the alpha granules of activated platelets[10,11]. The rationale for use PRP as solution to perform submucosal injection in endoscopic resection techniques lies in the exponential release of multiple bioactive factors, and subsequently, enhances the natural healing process, as well as in its haemostatic properties, with very low risk of fibrotic healing or strictures. In gastrointestinal disorders PRP has demonstrated efficacy in the prevention of DP[7] and wound healing in primary colonic anastomosis[12]. Previous reports have confirmed that surgical sites enhanced with PRP heal at rates two to three times those of untreated surgical sites and anabolic effects are directly correlated to platelet number[13].
Our study has tested the efficacy to use PRP in EMR of lesions large lesions, obtained through an inexpensive kit, showing strong anabolic effects. Otherwise, PRP has favourable biological and rheological properties as compared with other solutions as hyaluronic acid[14]. This faster and stronger healing activity acts as mechanical defense that prevents the appearance of delayed adverse events. Regarding DB, PRP by mimicking the last step of the coagulation cascade, the formation of a fibrin clot, submucosal injection develops a more stable shield than prevent this complication.
Our study has some limitations because with this small number of patients we need larger studies to validate these findings and to perform a comparison study with other lifting solutions. PRP is an easy-to-obtain solution with proven favourable biological activities that could be applied as submucosal injection prior to endoscopic resection of large lesions. These data emphasize the need for continuing research in this topic.
Submucosal injection of fluid solutions is crucial to prevent of adverse events in endoscopic resections. Platelet-rich plasma (PRP) has demonstrated strong healing properties in preclinical models.
PRP solution proved excellent electrical and rheological properties to perform safety endoscopic resections. PRP could be an ideal lifting solution in therapeutic endoscopy.
The primary outcome was the assessment of the incidence of adverse events (delayed bleeding or delayed perforation). Secondary objective was the evaluation of mucosal healing rate (MHR), calculated as a percentage of mucosal restoration after 1 mo.
This was a non-randomized prospective single-center study (ClinicalTrials.gov NCT02931149). Subjects eligible for the study were men and women aged 18 and older who were submitted for endoscopic resection (EMR) of sessile lesions larger than 35 mm. Patients were allocated to receive PRP as submucosal injection of PRP prior to EMR.
EMR was performed in 11 lesions (46.4 mm± 4 mm, range 40-70 mm). Delayed bleeding or perforation was not observed in any patient. Mean ulcerated area at baseline was 22.7 cm2 ± 11.7 cm2 whereas at week 4 were 2.9 cm2 ± 1.5 cm2. Patients treated with PRP showed a very high MHR after 4 wk (87.5%).
The new finding of this study is that PRP is lifting solution with proven and favourable biological activities that could be used in advanced endoscopic resection.
We need larger studies to validate these findings and to perform a comparison study with other lifting solutions.
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chow WK, Gkekas I, Roy PK, Zhang QS S- Editor: Ma YJ L- Editor: A E- Editor: Song H
| 1. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 766] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 2. | Burgess NG, Metz AJ, Williams SJ, Singh R, Tam W, Hourigan LF, Zanati SA, Brown GJ, Sonson R, Bourke MJ. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12:651-61.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 4. | Albéniz E, Fraile M, Ibáñez B, Alonso-Aguirre P, Martínez-Ares D, Soto S, Gargallo CJ, Ramos Zabala F, Álvarez MA, Rodríguez-Sánchez J. A Scoring System to Determine Risk of Delayed Bleeding After Endoscopic Mucosal Resection of Large Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013;77:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Bon I, Bartolí R, Lorenzo-Zúñiga V. Endoscopic shielding technique, a new method in therapeutic endoscopy. World J Gastroenterol. 2017;23:3761-3764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lorenzo-Zúñiga V, Boix J, Moreno de Vega V, Bon I, Marín I, Bartolí R. Efficacy of platelet-rich plasma as a shielding technique after endoscopic mucosal resection in rat and porcine models. Endosc Int Open. 2016;4:E859-E864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bon I, Lorenzo-Zúñiga V, Moreno de Vega V, Rodríguez A, De la Ossa N. D., Marín I, Boix J, Bartolí R. Comparative study of electrical and rheological properties of different solution to perform submucosal injection. UEG J. 2017;5:A454. |
| 9. | Ferreira AO, Moleiro J, Torres J, Dinis-Ribeiro M. Solutions for submucosal injection in endoscopic resection: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E1-E16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009;40:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Zhou B, Ren J, Ding C, Wu Y, Chen J, Wang G, Gu G, Li J. Protection of colonic anastomosis with platelet-rich plasma gel in the open abdomen. Injury. 2014;45:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kumar KA, Rao JB, Pavan Kumar B, Mohan AP, Patil K, Parimala K. A prospective study involving the use of platelet rich plasma in enhancing the uptake of bone grafts in the oral and maxillofacial region. J Maxillofac Oral Surg. 2013;12:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Russo F, D’Este M, Vadalà G, Cattani C, Papalia R, Alini M, Denaro V. Platelet Rich Plasma and Hyaluronic Acid Blend for the Treatment of Osteoarthritis: Rheological and Biological Evaluation. PLoS One. 2016;11:e0157048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |