Copyright
©The Author(s) 2015.
World J Gastrointest Endosc. Mar 16, 2015; 7(3): 192-205
Published online Mar 16, 2015. doi: 10.4253/wjge.v7.i3.192
Published online Mar 16, 2015. doi: 10.4253/wjge.v7.i3.192
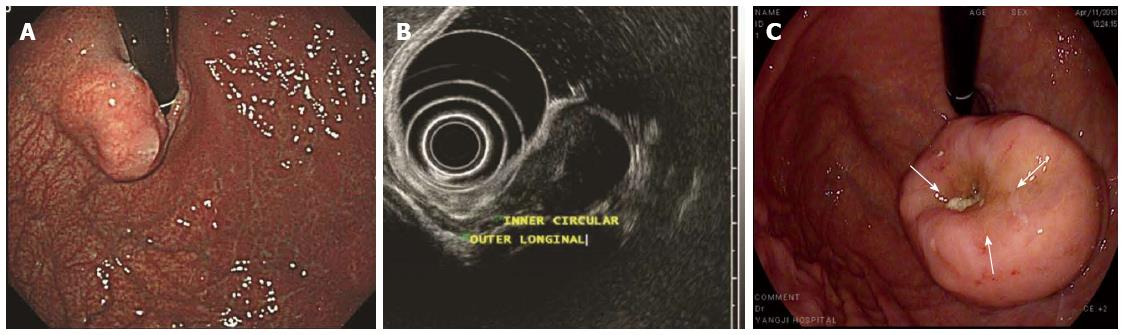
Figure 1 Features of gastrointestinal stromal tumors.
A: An approximately 2-cm elevated lesion covered with nearly intact mucosa was observed at the cardia; B: EUS demonstrated a 21-mm, generally homogenous hypoechoic, well circumscribed pear-shaped lesion originating from the inner circular layer of the proper muscle layer. Inside the lesion, a hyperechoic septum-like structure was noticed; C: There was a small deep focal ulceration at the center of the gastrointestinal stromal tumor (GIST) (white arrows).
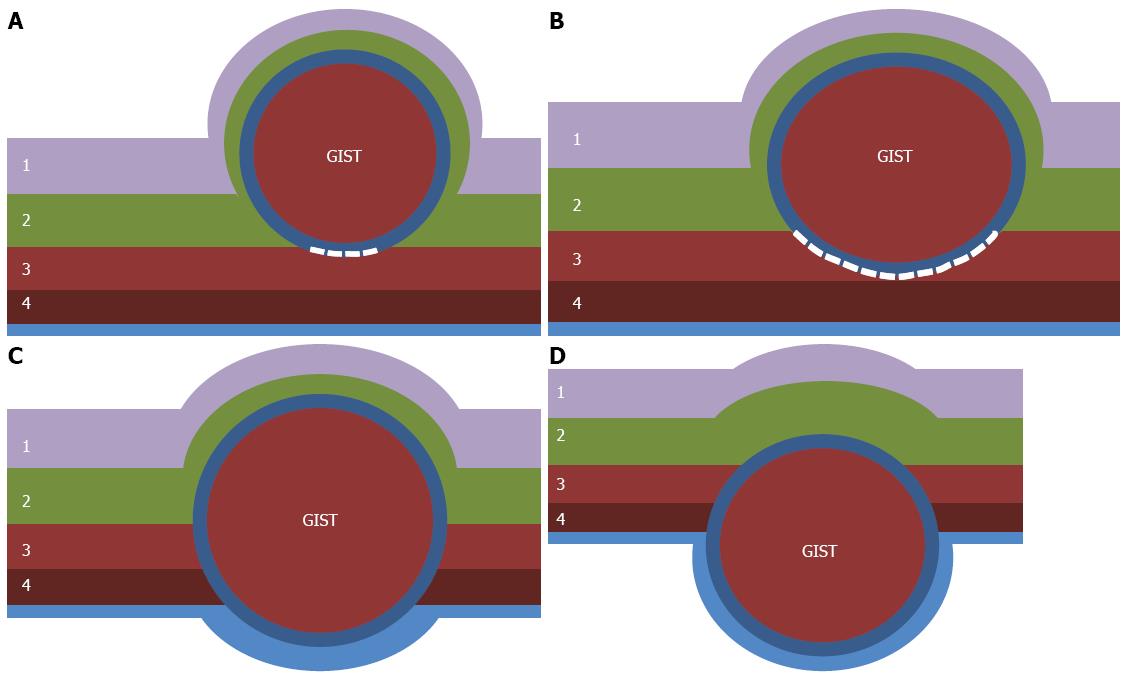
Figure 2 Classification of gastrointestinal stromal tumors according to the location in the gastric wall.
A: Type I is a gastrointestinal stromal tumor (GIST) that has a very narrow connection with the proper muscle layer and protrudes into the luminal side like a polyp; B: Type II has a wider connection with the proper muscle layer and protrudes into the luminal side at an obtuse angle; C: Type III is located in the middle of the gastric wall; D: Type IV protrudes mainly into the serosal side of the gastric wall. White dotted lines indicate the area dissected from the proper muscle layer. 1: Mucosa; 2: Submucosa; 3: Circular layer of proper muscle; 4: Longitudinal layer of proper muscle.
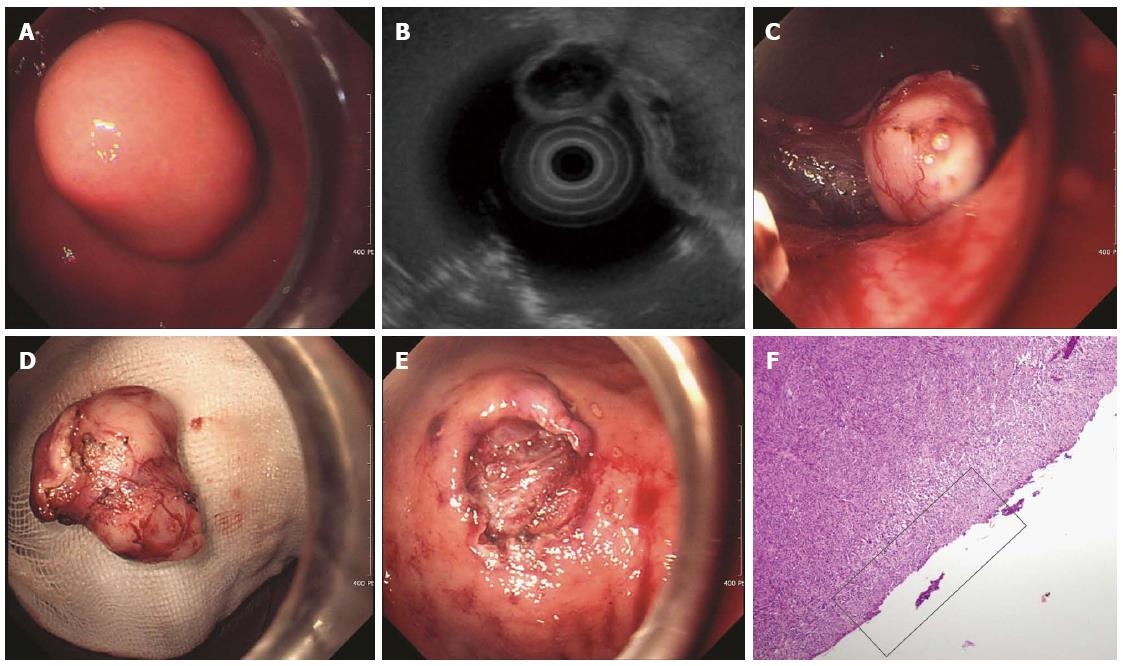
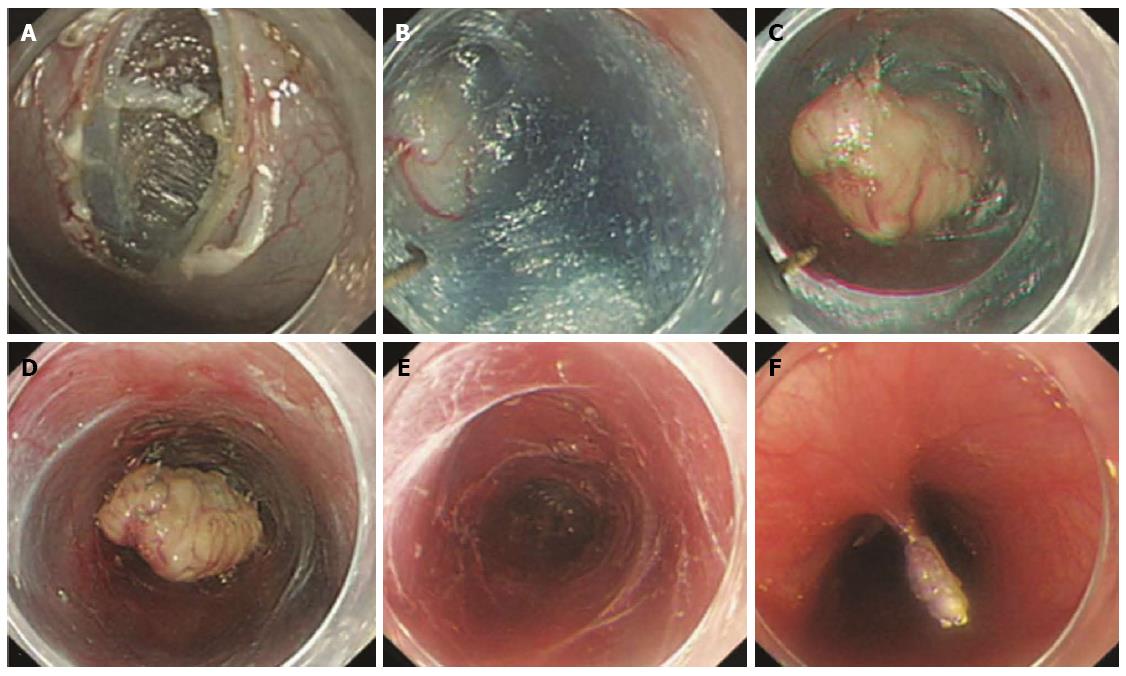
Figure 3 Endoscopic enucleation using the standard endoscopic submucosal dissection technique.
A: An approximately 2.5-cm subepithelial tumor was identified at the greater curvature side of the upper body of the stomach; B: A 2.6-cm mixed echogenic tumor with a slightly irregular border arising from the proper muscle layer was noticed; C: Endoscopic enucleation using the endoscopic submucosal dissection technique was performed; D: En bloc resection was achieved; E: There was no perforation at the operation site; F: On pathologic examination, a vertical resection margin was apparently involved with tumor cells (red boxed area); R1 resection was confirmed. (Courtesy of Kyung Oh Kim, Gil hospital, Incheon, South Korea).
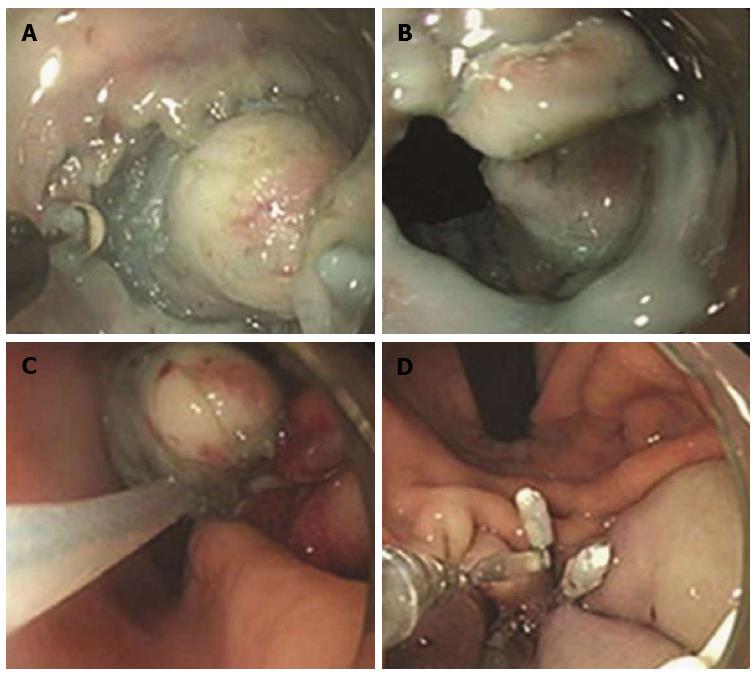
Figure 4 Endoscopic muscularis dissection of an esophageal subepithelial tumor originating from the proper muscle layers.
A: Endoscopic view of the esophageal submucosal tumor; B: Exposure of the tumor using a longitudinal incision; C: Blunt dissection of the tumor as deep as the proper muscle layer with a transparent hood; D: Stopping bleeding after blunt dissection; E: The whole tumor was removed F: Linear clipping was performed to close the submucosal entry site (adopted from Liu et al[44]).
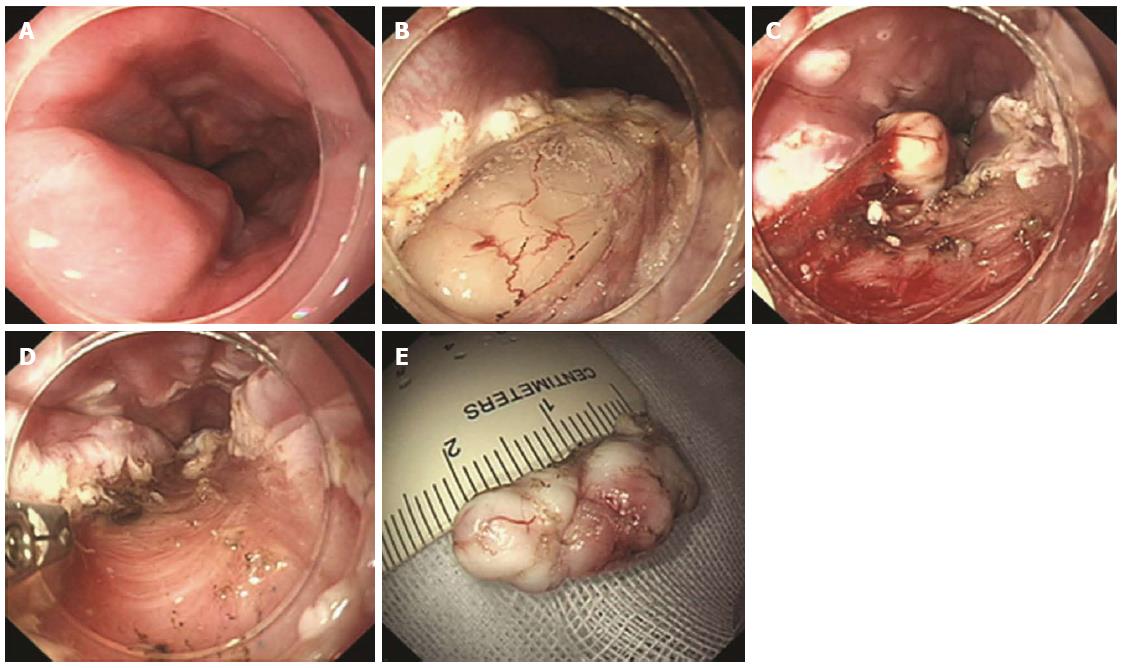
Figure 5 Endoscopic submucosal tunnel dissection procedure with longitudinal access.
A: A 2-cm longitudinal mucosal incision was created, approximately 5 cm over the submucosal tumor; B: Submucosal dissection was performed, making a submucosal tunnel until the tumor was visible; C: Dissection was performed along the margin of the tumor; D: Dissection was completed; E: After removing the tumor, potential bleeding foci were coagulated; F: Closing the entry with clips (adopted from Gong et al[46]).
Figure 6 Procedure for endoscopic full-thickness resection of gastric subepithelial tumors originating from the proper muscle layer.
A: A circumferential incision was made as deep as the proper muscle layer around the lesion with an IT knife; B: The tumor protruded into the peritoneal cavity after active perforation due to the incision into the serosal layer around the lesion; C: The tumor and surrounding tissue were pulled into the gastric cavity; D: The gastric wound was successfully closed with several metallic clips (adopted from Zhou et al[52]).
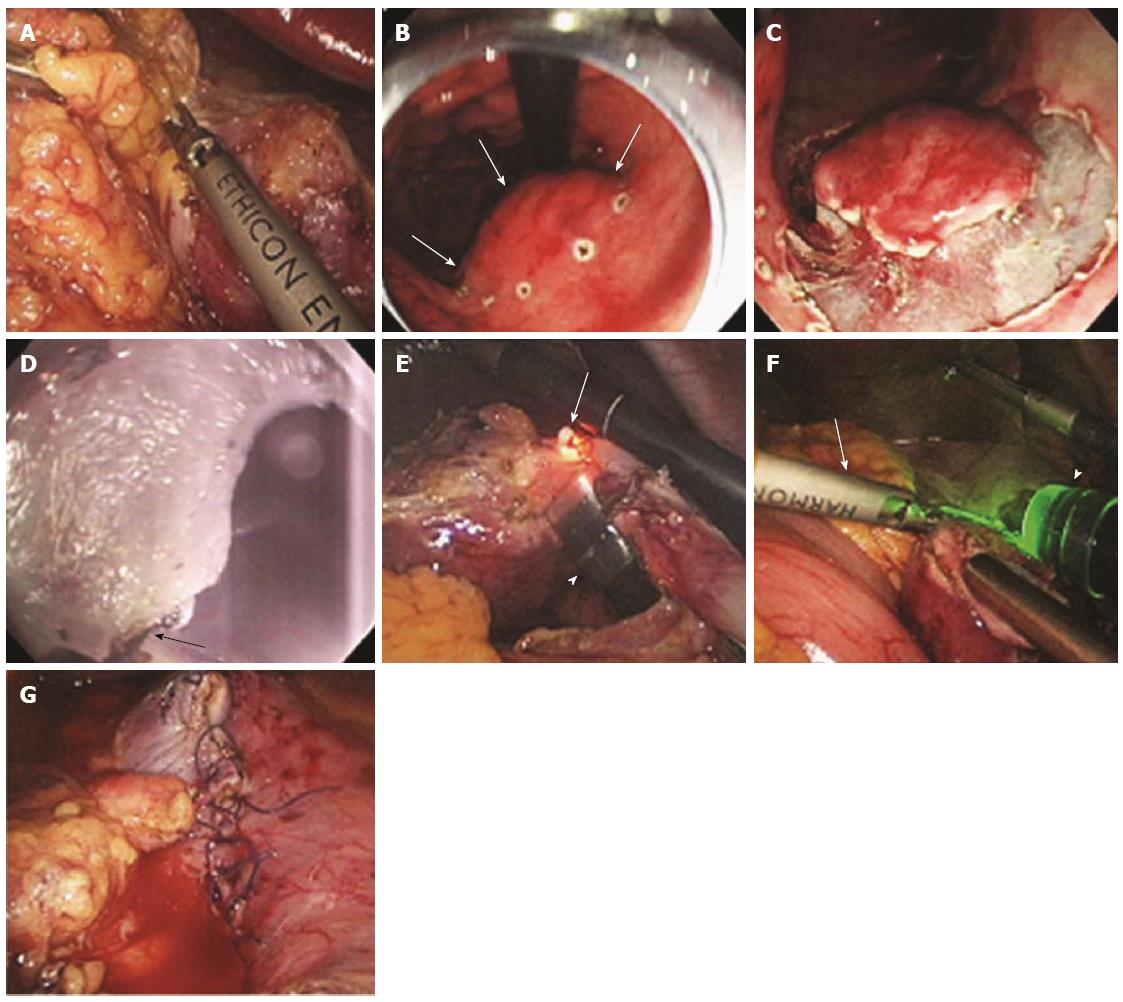
Figure 7 Procedure for laparoscopy-assisted endoscopic full-thickness resection.
A: Laparoscopic view while the lesser omentum attached around the tumor site was dissected; B: Endoscopic view after marking around the gastric subepithelial tumor (white arrows) located on the lesser curvature side of the gastric body; C: Gastroscopic view after incision as deep as the submucosal layer around the lesion; D: Gastroscopic view of the full-thickness incision from inside the stomach using the IT knife (white arrow); E: Laparoscopic view of the full-thickness incision from inside the stomach using the IT knife (arrow, the tip of the IT knife; arrowhead, the gastroscope); F: Laparoscopic view of the remaining full-thickness incision from outside the stomach using a Harmonic ACE (arrow); G: Laparoscopic view after laparoscopic hand-sewn closure of the gastric-wall defect (adopted from Abe et al[58]).
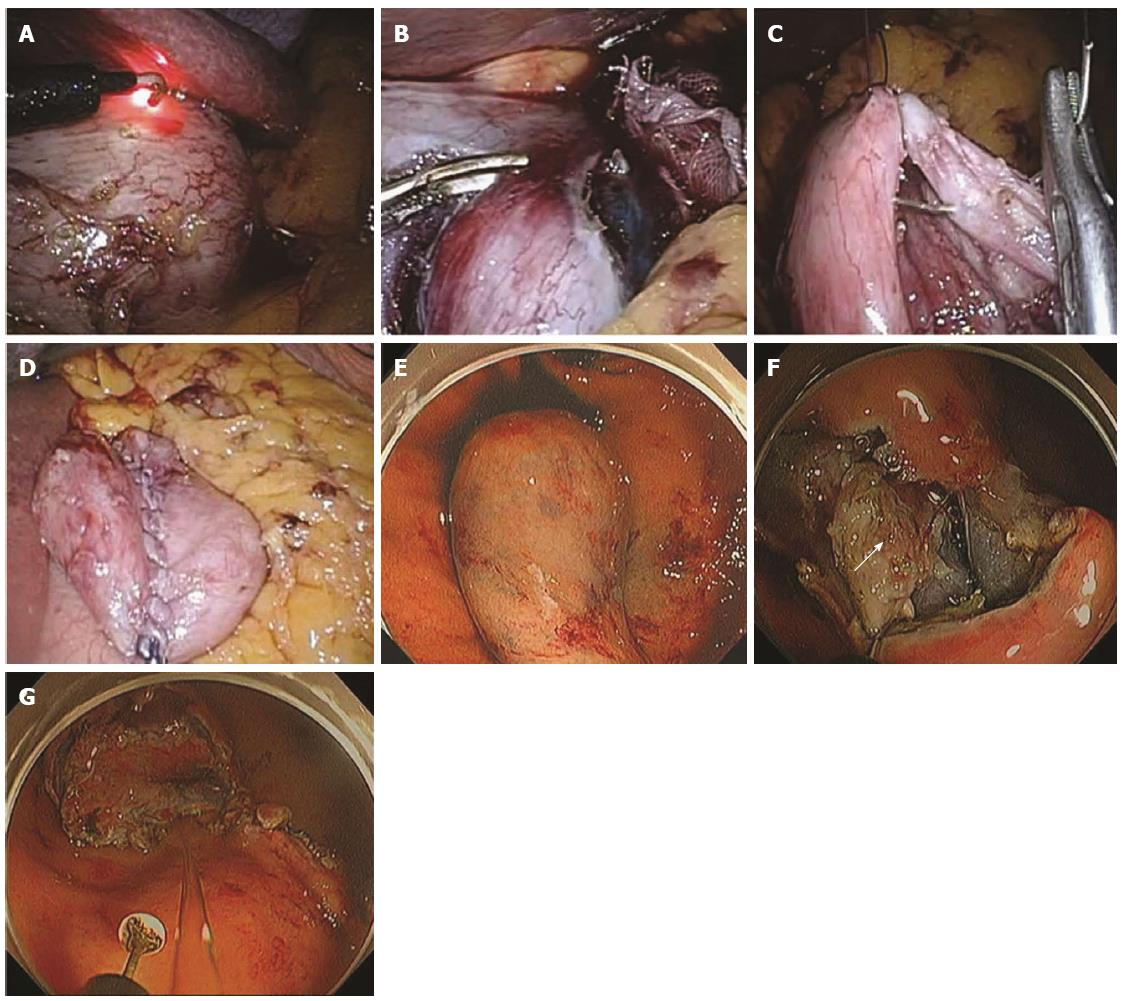
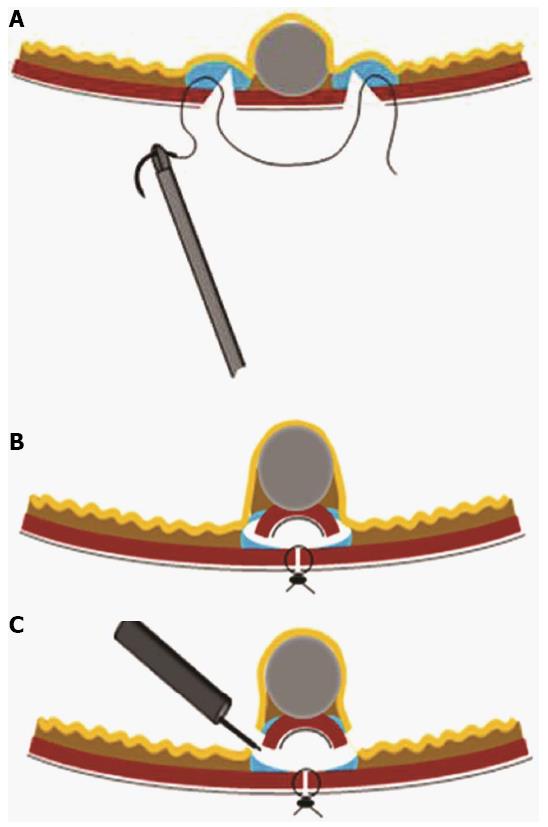
Figure 8 Procedure for non-exposed wall-inversion surgery.
A: Laparoscopic markings on the serosal surface guided by light from the fiber-optic probe shining through the gastric endoscope; B: Circumferential seromuscular layer dissection outside the serosal markings; C: Seromuscular layer suture closure; D: Laparoscopic view of inversion of the dissected area; E: Endoscopic view of massive protrusion of the inverted tissue; F: Serosal surface (arrow) identified during mucosubmucosal layer dissection; G: Flipped tissue to be resected (adopted from Mitsui et al[61]).
Figure 9 Scheme of the procedure for non-exposed wall-inversion surgery.
A: Seromuscular layer suture after submucosal injection and seromuscular cutting; B: Divided seromuscular layer inversion after laparoscopic seromuscular closure; C: Mucosubmucosal layer dissection (adopted from Mitsui et al[61]).
- Citation: Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc 2015; 7(3): 192-205
- URL: https://www.wjgnet.com/1948-5190/full/v7/i3/192.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i3.192