Published online Mar 18, 2017. doi: 10.4254/wjh.v9.i8.427
Peer-review started: August 8, 2016
First decision: September 28, 2016
Revised: December 2, 2016
Accepted: December 13, 2016
Article in press: December 14, 2016
Published online: March 18, 2017
Processing time: 223 Days and 0.1 Hours
To identify predictive factors associated with long-term patient and graft survival (> 15 years) in liver transplant recipients.
Medical charts of all de novo adult liver transplant recipients (n = 140) who were transplanted in Hamburg between 1997 and 1999 were retrospectively reviewed. In total, 155 transplantations were identified in this time period (15 re-transplantations). Twenty-six orthotopic liver transplant (OLT) recipients were early lost to follow-up due to moving to other places within 1 year after transplantation. All remaining 114 patients were included in the analysis. The following recipient factors were analysed: Age, sex, underlying liver disease, pre-OLT body mass index (BMI), and levels of alanine aminotransferase (ALT), bilirubin, creatinine and gamma-glutamyltransferase (gamma-GT), as well as warm and cold ischemia times. Furthermore, the following donor factors were assessed: Age, BMI, cold ischemia time and warm ischemia time. All surviving patients were followed until December 2014. We divided patients into groups according to their underlying diagnosis: (1) hepatocellular carcinoma (n = 5, 4%); (2) alcohol toxic liver disease (n = 25, 22.0%); (3) primary sclerosing cholangitis (n = 6, 5%); (4) autoimmune liver diseases (n = 7, 6%); (5) hepatitis C virus cirrhosis (n = 15, 13%); (6) hepatitis B virus cirrhosis (n = 21, 19%); and (7) other (n = 35, 31%). The group “other” included rare diagnoses, such as acute liver failure, unknown liver failure, stenosis and thrombosis of the arteria hepatica, polycystic liver disease, Morbus Osler and Caroli disease.
The majority of patients were male (n = 70, 61%). Age and BMI at the time point of transplantation ranged from 16 years to 69 years (median: 53 years) and from 15 kg/m2 to 33 kg/m2 (median: 24), respectively. Sixty-six OLT recipients (58%) experienced a follow-up of 15 years after transplantation. Recipient’s age (P = 0.009) and BMI (P = 0.029) were identified as risk factors for death by χ2-test. Kaplan-Meier analysis confirmed BMI or age above the median as predictors of decreased long-term survival (P = 0.008 and P = 0.020). Hepatitis B as underlying disease showed a trend for improved long-term survival (P = 0.049, χ2-test, P = 0.055; Kaplan-Meier analysis, Log rank). Pre-transplant bilirubin, creatinine, ALT and gamma-GT levels were not associated with survival in these patients of the pre-era of the model of end stage liver disease.
The recipients’ age and BMI were predictors of long-term survival after OLT, as well as hepatitis B as underlying disease. In contrast, donors’ age and BMI were not associated with decreased survival. These findings indicate that recipient factors especially have a high impact on long-term outcome after liver transplantation.
Core tip: Due to organ shortage and epidemiological developments, the number of older potential orthotopic liver transplant (OLT) recipients increased greatly over the last decades. In order to identify predictors for long-term survival after liver transplantation, we analysed all adult, first OLTs performed at the University Medical Center Hamburg-Eppendorf between 1997 and 1999 and compared these findings with the Eurotransplant database. Our study shows that recipient’s age and body mass index as well as hepatitis B as underlying disease are predictors of long-term survival after OLT.
- Citation: Pischke S, Lege MC, von Wulffen M, Galante A, Otto B, Wehmeyer MH, Herden U, Fischer L, Nashan B, Lohse AW, Sterneck M. Factors associated with long-term survival after liver transplantation: A retrospective cohort study. World J Hepatol 2017; 9(8): 427-435
- URL: https://www.wjgnet.com/1948-5182/full/v9/i8/427.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i8.427
Survival after liver transplantation has strongly improved in the last decades, but factors associated with long-term survival have not been well defined yet. Research on the factors associated with best long-term outcome is, therefore, essential for an optimal use of the donated organs. This is even more relevant since age of donors and recipients is increasing. This development is mostly due to the organ shortage as well as epidemiological developments.
The majority of deaths after older potential orthotopic liver transplant (OLT) occur within the first months after transplantation. This is predominantly caused by pulmonary infections, sepsis or multiple organ failure[1]. An analysis of a large cohort from the Eurotransplant database included more than 90000 patients that were liver transplanted between 1968 and 2009[1]. Within this cohort the early mortality was 6%, 9% and 12% for 1-, 3- and 6-mo mortality in patients who were liver transplanted after the year 2000[1].
Although several transplant centres worldwide now have more than 20 years of clinical experience in the field of liver transplantation, only few studies have analysed the long-term outcomes in OLT recipients[2,3].
Several donor and recipient factors, including age and body mass index (BMI), are well-known to influence short-term survival[4]. Their relevance for long-term outcome has not been studied in detail yet. However, the negative influence of obesity on survival in non-transplant recipients is a well-known fact, since the Framingham study of the 1990s[5]. The World Health Organization has defined obesity as a condition of excessive accumulation of body fat, causing severe damage to health (http://www.who.org). In fact, the prevalence of obesity is increasing worldwide and is a major threat to liver transplant recipients as well as the health of the general population. Common co-morbidities associated with obesity are hypertension, coronary heart disease, heart failure, stroke, hyperuricemia, dyslipidemia, insulin resistance and glucose intolerance. In addition, within the Framingham study, it was shown that fluctuations in body weight in non-transplant patients were associated with an increased mortality, independent of obesity and the trend of body weight over time[5].
In contrast to the general population, the role of bodyweight in liver transplant recipients is less clear. Werneck et al[6] demonstrated in a study including 136 liver transplant recipients that there was no significant difference between obese and normal weight patients regarding length of stay in the Intensive Care Unit or in 2-year survival. On the other hand, Sawyer et al[4] demonstrated a decreased short-term survival in obese patients in comparison to normal weight liver transplant recipients.
In addition to BMI, ages of donor and recipient have been discussed controversially within the last years[7]. Recipients’ age is also known to have an influence on the outcome of liver transplantation. Schoening et al[2] studied the 20-year survival rate of 313 liver transplant recipients. Those authors divided their cohort into three sub-groups: Patients below the age of 30, between 30 years and 55 years, and patients above 55 years. Patients below the age of 30 lived significantly longer after transplantation, as compared to the other two groups. However, no analysis was performed in which the patients were divided according to the median age in that study. Furthermore, the long-time survival of transplant recipients was compared with a “virtual control group”, based on the life expectancy in the general population. While patients younger than 55 years showed a decreased survival, as compared to the general population, there was no difference in life expectancy between patients older than 55 years and the general population.
The aim of the present study was to identify factors associated with long-term patient and graft survival (> 15 years) in liver transplant recipients and compare these to the Eurotransplant database. This study focused specifically on recipient’s age and BMI, as the influence of these factors is still not well defined.
This study was performed at the University Medical Center Hamburg-Eppendorf, a tertiary centre in North Germany. Since the first liver transplantation in Hamburg was performed in 1984, more than 2000 liver transplantations have been performed at this centre.
Medical charts of all de novo adult liver transplant recipients (n = 140), who were transplanted in Hamburg between 1997 and 1999, were retrospectively reviewed (Figure 1). In total, 155 transplantations were identified in this time period (15 re-transplantations). Twenty-six OLT recipients were early lost to follow-up due to moving to other places within 1 year after transplantation (Figure 1). All remaining 114 patients were included in the analysis. The following recipient factors were analysed: Age, sex, underlying liver disease, pre-OLT BMI, and levels of alanine aminotransferase (ALT), bilirubin, creatinine and gamma-glutamyltransferase (gamma-GT), as well as warm and cold ischemia times. Furthermore, the following donor factors were assessed: Age, BMI, cold ischemia time and warm ischemia time. All surviving patients were followed-up until December 2014. We divided patients into groups according to their underlying condition (Table 1): (1) hepatocellular carcinoma (HCC) (n = 5, 4%); (2) alcohol toxic liver disease (n = 25, 22.0%); (3) primary sclerosing cholangitis (n = 6, 5%); (4) autoimmune liver diseases (n = 7, 6%); (5) hepatitis C virus (HCV) cirrhosis (n = 15, 13%); (6) hepatitis B virus (HBV) cirrhosis (n = 21, 19%); and (7) other (n = 35, 31%). The group “other” included rare diagnoses, such as acute liver failure, unknown liver failure, stenosis and thrombosis of the arteria hepatica, polycystic liver disease, Morbus Osler and Caroli disease.
| Patients who survived (n = 68) | Patients who died (n = 46) | P-value (χ2 test) | |
| Male | 39 (57%) | 31 (67%) | NS |
| Age, yr (median, SD) | 16-65 (50.5, 13) | 17-69 (56.0, 12) | 0.009 |
| BMI, range kg/m2 (median, SD) | 18-33 (23.1, 3) | 15-29 (25.9, 4) | 0.029 |
| Pre-LTx creatinine, mg/dL (median, SD) | 0.4-3.5 (1.0, 0.5) | 0.3-2.9 (1.1, 0.6) | NS |
| GFR, mL/min (median, SD) | 15.3-230.2 (73.3, 38.1) | 22.6- 240.4 (62.5, 48.0) | NS |
| ALT, U/L (median, SD) | 4-2610 (35.5, 449.5) | 6-1566 (19.5, 339.0) | NS |
| Gamma-GT, U/L (median, SD) | 7-374 (47.0, 8) | 13-184 (43.0, 45) | NS |
| Bilirubin, mg/dL (median, SD) | 0.4-28.1 (2.4, 5.7) | 0.4-28.3 (2.4, 5.8) | NS |
| Warm ischemia time, min (median, SD) | 25-100 (50.0, 18) | 22-75 (54.0, 15) | NS |
| Cold ischemia time, min (median, SD) | 242-940 (542.5, 157) | 174-825 (521.0, 146) | NS |
| Donor age, yr (median, SD) | 12-70 (36.5, 16) | 13-75 (41.0, 1) | NS |
| Donor BMI, kg/m2 (median, SD) | 17-30 (23.5, 3) | 18-31 (24.2, 2) | NS |
| Underlying diagnosis n (%) | |||
| HCC | 2 (3) | 3 (6) | NS |
| Alcohol toxic liver cirrhosis | 12 (18) | 13 (27) | NS |
| PSC | 4 (6) | 2 (4) | NS |
| Autoimmune | 5 (8) | 2 (4) | NS |
| HCV cirrhosis | 9 (14) | 6 (13) | NS |
| HBV infection | 16 (24) | 5 (10) | 0.049 |
| Other | 18 (27) | 17 (35) | NS |
In addition to patient survival, the graft survival was also analysed. By definition, graft loss resulted in re-transplantation or death. The factors that were significantly associated with graft survival in our cohort were then compared with a large cohort of 2971 patients from Eurotransplant, which had been transplanted within the same period (1997-1999).
Categorical variables were compared using χ2 test. Metric data were compared using the non-parametric Mann-Whitney test. Survival analysis was performed utilizing Kaplan-Meier analysis. All investigated factors were tested utilizing univariate and multivariate models.
As metric values did not fulfil the criteria for a normal distribution (Kolmogorov Smirnov test P < 0.01), median values instead of mean values were depicted. All statistical analyses were performed utilizing SPSS (version 13.0) and P-values < 0.05 were considered to be statistically significant.
For this retrospective, observational study neither informed consent nor approval of the ethics committee was needed according to the Professional Code of the German Medical Association (article B.III. § 15.1) and to the recommendations of our local ethical committee (Ethikkommission der Ärztekammer Hamburg).
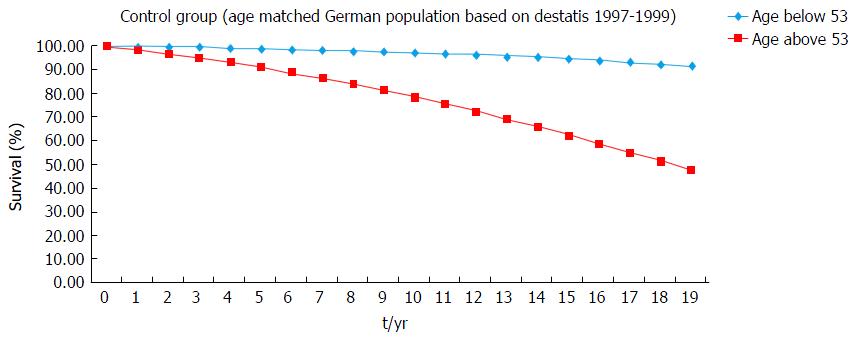
To discuss the survival of transplant patients with an age below and above the median of age (53 years) we constructed an imaginary control cohort. Therefore, we analysed the survival of historical data (https://www.destatis.de) of an age-matched cohort of the healthy German population.
In addition, to improve reliability of data, we compared our results with data from a cross-sectional Eurotransplant cohort including 2971 patients who underwent liver transplantation between 1997 and 1999. Eurotransplant kindly supported us with de-personalized data that were already arranged and categorized according to our median values of age and BMI and to status of HBV positivity. To compare this cohort with our own cohort, survival of these patients was analysed up to the same time point (until December 2014).
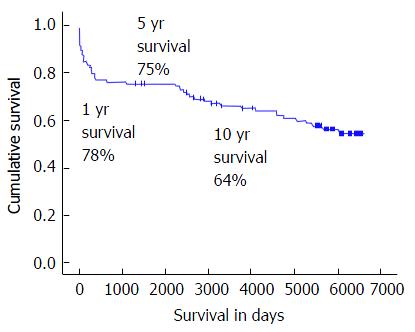
Overall, 114 OLT recipients were included in the study (Table 1). The majority of the patients were male (n = 70, 61%). The age and BMI at the time of transplantation ranged from 16 years to 69 years (median: 53 years) and from 15.1 kg/m2 to 33.3 kg/m2 (median: 24 kg/m2), respectively. See Table 1 for an overview of the overall investigated factors. The median follow-up was 5139 d. Sixty-six (58%) OLT recipients experienced a follow-up of 15 years after OLT (Figure 1). The 1-, 5- and 10-year patient survival rates were 78%, 74% and 64% (Figure 1).
Graft survival 15 years post-OLT was 53%. Fifty-three patients experienced a graft loss either by death (34%) or re-transplantation (13%). Characteristics of patients with graft survival and those with graft loss are depicted in Table 1.
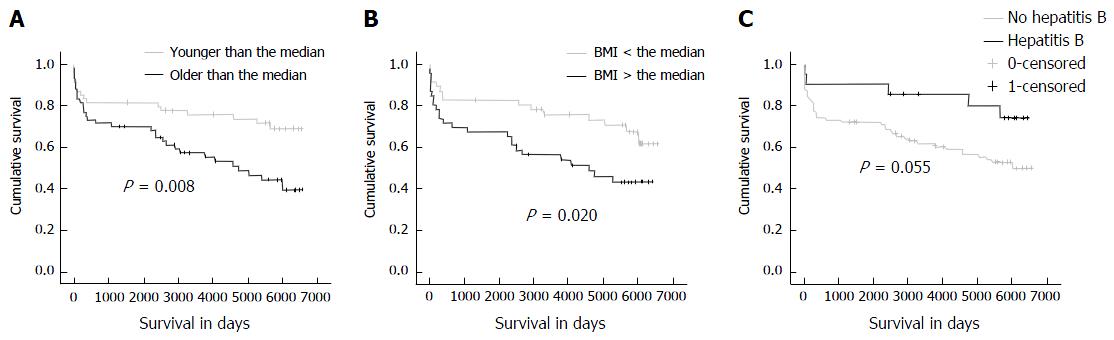
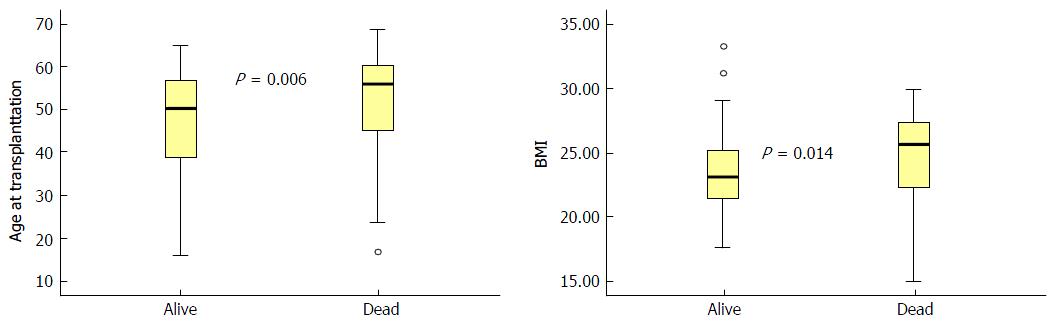
During the observational period, the mortality rate was significantly higher in patients with an age above the median (53 years) at transplantation as compared to patients younger than the median (P = 0.009). The Kaplan-Meier analysis confirmed that older patients had a decreased patient survival rate (P = 0.008; Figure 2). Furthermore, the median age at the time of transplantation was higher in patients who deceased within 15 years of follow-up in comparison with patients who were still alive at the end of the study period (P = 0.006, Mann-Whitney test; Figure 3). These findings were confirmed in the cross-sectional Eurotransplant cohort (n = 2973) transplanted in the same period, with a follow-up of 15-17 years. In this cohort, 625/1145 (55%) patients with an age above 53 years died within the 15-year to 17-year follow-up period, while only 653/1809 (36%) patients with an age below 53 years died (P < 0.001; Table 2).
| Patients who survived n (%) | Patients who died n (%) | P-value (χ2 test) | |
| Age below 53 yr (n = 1809) | 1156 (64) | 653 (36) | |
| Age above 53 yr (n = 1145) | 520 (45) | 625 (55) | < 0.001 |
| BMI below 24 kg/m2 (n = 1454) | 880 (61) | 574 (39) | |
| BMI above 24 kg/m2 (n = 1493) | 796 (53) | 697 (47) | < 0.001 |
| Hepatitis B as underlying disease (n = 255) | 170 (67) | 85 (33) | |
| Non-hepatitis B patients (n = 1705) | 946 (55) | 759 (45) | < 0.001 |
In a sub-analysis, we defined age above 60 years as “old” and analysed the groups of transplant younger (n = 89) and older (n = 25) than this threshold, separately. In patients older than 60 years, the patient survival rate was significantly lower as compared to younger patients (χ2 test P = 0.007, Kaplan-Meier analysis P = 0.002). Donor age (12-75 years, median: 40) was not significantly correlated with patient survival. A multivariate analysis confirmed age as an independent factor associated with graft survival (P < 0.01).
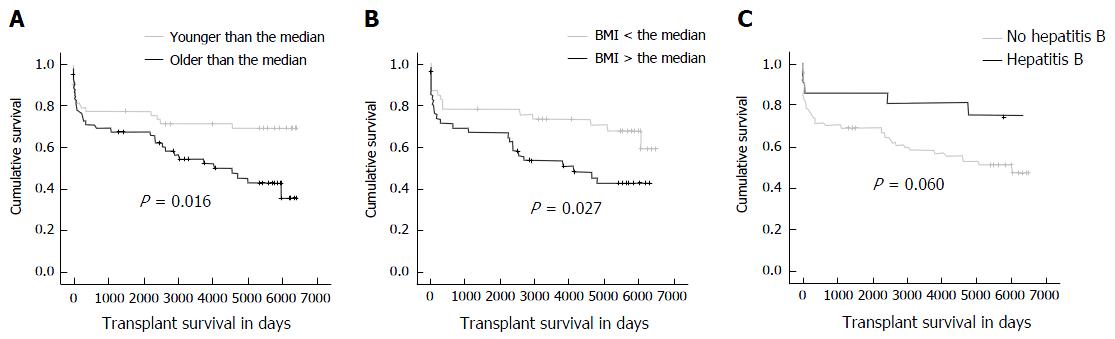
Patients with an older age at the time of transplantation had a significantly worse graft survival, compared to patients younger than the median (Figure 4). This was confirmed by χ2 test (P = 0.017) and Mann-Whitney test (P = 0.017). Looking at the subgroup of patients older than 60 years, there was a significantly lower graft survival according to Kaplan-Meier survival analysis (P = 0.05) but not according to the χ2-test. Donor’s age was not related to graft survival in this study (P = ns). There was no significant association between patients who survived more than 1 year and had age above the median (χ2 test P = 0.498).
Patients with a BMI above the median (24 kg/m2) displayed a higher mortality than patients with a BMI below the median (P = 0.029). This reduced survival rate was confirmed by the Kaplan-Meier analysis (P = 0.020; Figure 2). Additionally, BMI at the time of transplantation was higher in patients who died within 15 years of follow-up in comparison to patients who survived (P = 0.014; Figure 3). This was confirmed in the Eurotransplant control cohort (n = 2971). Patients with a BMI below 24 kg/m2 showed an improved survival rate in comparison with patients with a BMI above this threshold (P≤ 0.001). In detail, 61% with a BMI below 24 kg/m2 survived, while 53% with a BMI above 24 kg/m2 survived (Table 2).
A sub-analysis of patients with severe obesity and a BMI above 30 kg/m2 was not possible as only two patients fulfilled this criterion.
There was no significant association between patients who survived more than 1 year and had BMI above the median (χ2 test P = 0.449). Notably, there was no significant association between age and BMI of the recipient (R = 0.114, P = 0.278), so that BMI seemed to be independent of age. Unfortunately, a multivariate analysis did not confirm BMI as an independent factor associated with decreased survival; perhaps, significance was missing due to the limited number of factors.
In contrast, the BMI of the donor was not associated with survival of the recipient (P = ns).
Patients having a BMI above the median (24 kg/m2) had a significantly worse graft survival, compared to patients with a BMI lower than the median (χ2 test: 0.009, Mann-Whitney test: 0.047). On the other hand, in this study, donor’s BMI did not have an influence on graft survival.
The only underlying aetiology of cirrhosis which was statistically significantly associated with outcome was hepatitis B. Patients with hepatitis B as an underlying disease tended to have an improved patient survival in comparison to patients with other underlying diseases (P = 0.049 in the categorical analysis and P = 0.055 in the Kaplan-Meier analysis; Figure 2C). Three out of 21 liver transplant recipients with hepatitis B suffered from acute, fulminant hepatitis B, leading to acute liver failure and transplantation, while the majority (n = 18) had been transplanted due to chronic hepatitis B with cirrhosis. Regarding the BMI, there was no difference between HBV-positive and HBV-negative patients (t-test, 2-sided, unequal variance, P = 0.38), so that other reasons must be responsible for the survival benefit.
All HBV-positive liver transplant recipients received intravenous immunoglobulins, hepatitis B immune globulin (HBIG), to avoid reinfection of the graft.
In addition to patient survival, graft survival of patients with hepatitis B as underlying disease was also improved, compared to patients with other diagnoses (χ2 test: 0.018, Mann-Whitney test: 0.018). The Eurotransplant control cohort confirmed that patients with hepatitis B had an improved survival in comparison to the remaining patients (Table 2).
Neither recipient’s laboratory parameters prior to transplantation (ALT, gamma-GT, bilirubin, creatinine) nor warm ischemia time or cold ischemia time influenced patient survival significantly.
In the current situation of tremendous organ shortage, it is important to identify patients who benefit most from a liver transplantation and also to detect risk factors associated with poor outcome. The main findings of this study were that recipients’ age and BMI are relevant for prediction of long-term patient survival as well as graft survival. Interestingly, neither other recipient factors such as bilirubin, creatinine, ALT nor donor factors, such as age and BMI, were associated with decreased survival. Another interesting finding was that OLT recipients with hepatitis B as underlying disease had improved survival rates.
The association of recipients’ age and BMI and patient and graft survival was proven by univariate analysis for both factors. However, in multivariate analysis only age remained a significant predictor. On the other hand, it was unexpected that there was no significant association between survival of recipient’s and donor’s age and BMI. This finding is in contrast to numerous previous studies which demonstrated a significantly decreased survival in recipients of older donations within a large ET-DRI study[8]. Recently, a large analysis of more than 41000 liver transplant recipients receiving a donation after circulatory death showed that recipients of livers from donors with an age below 50 years had a higher survival rate, compared to recipients of livers from donors with an age above 60. However, several studies indicated that older grafts can be used safely with a careful selection of patient and donor in the majority of cases[9-13]. Based on the published literature, strict recommendations for the acceptance or refusal of potential liver donors cannot be made. The authors concluded that careful donor organ and recipient selection can lead to excellent results[14].
In contrast to donor’s age, our study highlighted the value of recipient’s age as a predictor of survival. We identified a threshold of 53 years for recipient’s age and a BMI of 24 kg/m2 as relevant risk factors. These findings were confirmed in the analysis of the Eurotransplant cohort of 2971 patients. Perhaps a larger cohort might also confirm a relevant aspect of donor age on survival. However, our study did not find such an association.
Within a previous German study with a follow-up period of 20 years and 313 liver transplant recipients, the survival of elderly transplant recipients (> 55 years) was reduced within the first year after transplantation, but long-term survival was similar to the general population[2]. Our observation that there is a relevant difference regarding survival between OLT recipients above and below the median age of 53 years (Figure 2A) is well in line with this study. However, we could not find a significant association between 1-year patient survival and age or BMI above the median. Therefore, these factors might be associated with long-term but not with short-term survival. Further studies are needed to elucidate this aspect.
Earlier studies showed inconsistent results concerning BMI and survival. A study by Fujikawa et al[15] investigated the impact of obesity on clinical and financial outcome after liver transplantation and showed no influence on either patient survival or hospital costs. Also, it is conceivable that obese recipients were selected more carefully with respect to other risk factors. In contrast, the study by Rustgi et al[16] observed a worse survival rate in patients having a BMI > 35. Our study confirms the finding that a higher BMI of the recipient is associated with a decreased survival. Only three of the patients in our study displayed malnutrition with a BMI < 18; thus, no interpretation of a possible effect of malnutrition and survival was possible for our cohort.
In order to strengthen our data, we compared the survival rate of our patients (younger or older than the median of 53 years) with two control groups (as described in the methods). There were no significant differences between all three groups (Figure 5 and Table 2). However, these are hypothetical control cohorts and more detailed statistical analyses were not possible.
Three independent statistical tests (Kaplan-Meier survival analysis/Log rank, χ2 test, Mann-Whitney test) confirmed the association between recipient’s age or BMI and decreased patient and graft survival rates. However, there was no correlation between age and BMI indicating that these factors are independently associated with lower survival. Unfortunately, a multivariate analysis makes no sense due to the low number of significant factors in the univariate analysis. It is not surprising that older or overweight patients depict a shorter survival. This has been a well-known fact for many years.
Interestingly, hepatitis B was associated with an improved long-term patient survival in our cohort. This should be interpreted carefully as there are only 21 HBV patients in our study population. However, this observation might be due to the regularly applied immunoglobulin preparations, HBIG, that these patients still get at our institution[17-19]. However, currently this is only one hypothetical explanation of the observed survival benefit of hepatitis B patients.
In addition, our study cohort analysis of the Eurotransplant control cohort also shows an increased survival for transplant recipients with underlying hepatitis B in comparison to the remaining patients (P < 0.001). This observation is in line with an analysis of the survival of liver transplant recipients with hepatitis B, based on the European liver transplant registry[20]. Within this study investigating the outcome of liver transplant recipients with hepatitis B as underlying disease within a period of approximately 20 years (1988-2010), it could be shown that the survival of HBV-positive transplant recipients strongly improved within these 2 decades[20]. This has been assumed to be caused by the prevention of hepatitis B re-infection by immunoglobulins[20]. However, this hypothesis still needs to be confirmed by further studies.
The results of this study might be helpful to identify patients with better chances of long-term survival. Our overall 15-year patient survival rate (Figure 1) of 58% is well in line with previous reports depicting a 20-year survival rate of approximately 50% after liver transplantation[2,3]. However, in the current era of model of end-stage liver disease (MELD)-allocation, which favours the sickest patients, such survival rates might not be met in future studies. Upcoming studies are needed to investigate not only short but also long-term survival of patients who received a liver transplantation in the MELD-era. Perhaps the MELD score is a valuable tool for identifying the sickest patients, but it might not be the best predictor of long-term outcome. Furthermore, according to previous studies, it has been shown that prognosis of the patient is far more related to clinical parameters than laboratory data[17]. The study of Aloia et al[18] also showed a decreased value of the MELD score in contrast to parameters such as ventilator status, diabetes mellitus, HCV, creatinine levels and recipient’s and donor’s age.
Our study has some limitations. It is based on patients who underwent liver transplantation in the pre-MELD era and at a time when less patients received organs with extended donor criteria. Furthermore, the number of patients with HCC was only 4% (5/114, 4%). In our study, at present these numbers are much higher.
Unfortunately, multivariate analysis of our data was prone to errors due to the small number of patients in comparison to the multiple variables. Thus, it can be said that the analysed cohort was too small for the investigation of the variables. This was a retrospective analysis and, therefore, there is some lack of information considering the long time period of observation (15-17 years). However, there are not many studies dealing with such long-term data as presented in this collective. In the future, more research, especially on the potential influence of immunoglobulins on the HBV patient’s outcome, is necessary.
In conclusion, age and BMI of OLT recipients were predictors of long-term survival, while pre-transplant bilirubin, creatinine, ALT and gamma-GT were not associated with patient survival or graft survival (pre-MELD era). Age and BMI of the donor had no relevant influence on patient or graft survival in this cohort. OLT recipients with hepatitis B as underlying disease displayed an improved survival. The relevance of this observation still needs to be determined.
We thank Eurotransplant for providing data for the control cohort of 2971 patients.
Predictive factors associated with long-term patient and graft survival (> 15 years) in liver transplant recipients are not well defined. This study evaluates the possible association between various factors and survival.
The role of age and body mass index (BMI) for the outcome of liver transplant recipients still needed to be shown.
This is the first study demonstrating a relevant association between age above 53 years or a BMI above 24 kg/m2 with decreased graft survival. These thresholds were confirmed in an independent large Eurotransplant cohort to be associated with decreased graft survival. Furthermore, there was a weaker association between underlying hepatitis B and improved graft survival. The pathological mechanism and relevance of this finding still needs to be shown.
Future studies will focus in detail on patients with an age above 53 years or a BMI above 24 kg/m2 to verify the authors’ findings. If their data can be confirmed, this will help transplant physicians worldwide to predict the risk of liver transplant recipients.
Liver transplant recipients and their survival as well as graft survival, defined as period until death or re-transplantation were studied.
Pischlke et al analyzed the clinical data of the patients who underwent liver transplantation during 1997 to 1999. This article is interesting.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chuang WL, Komatsu H, Sugawara Y, Yin DP S- Editor: Qiu S L- Editor: Filipodia E- Editor: Li D
| 1. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 639] [Article Influence: 49.2] [Reference Citation Analysis (2)] |
| 2. | Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, Guckelberger O, Puhl G, Seehofer D, Neuhaus P. Twenty-year longitudinal follow-up after orthotopic liver transplantation: a single-center experience of 313 consecutive cases. Am J Transplant. 2013;13:2384-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Dopazo C, Bilbao I, Castells LL, Sapisochin G, Moreiras C, Campos-Varela I, Echeverri J, Caralt M, Lázaro JL, Charco R. Analysis of adult 20-year survivors after liver transplantation. Hepatol Int. 2015;9:461-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable long-term function in severely obese patients undergoing liver transplantation. Clin Transplant. 1999;13:126-130. [PubMed] |
| 5. | Lissner L, Odell PM, D’Agostino RB, Stokes J, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 414] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Werneck M, Afonso RC, Coelho GR, Sboarini C, Coelho MP, Thomé T, Lisboa LF, Ferraz Neto BH. Obese and nonobese recipients had similar need for ventilatory support after liver transplantation. Transplant Proc. 2011;43:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Anderson CD, Vachharajani N, Doyle M, Lowell JA, Wellen JR, Shenoy S, Lisker-Melman M, Korenblat K, Crippin J, Chapman WC. Advanced donor age alone does not affect patient or graft survival after liver transplantation. J Am Coll Surg. 2008;207:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, Porte RJ, Rogiers X, Ringers J. The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Borchert D, Glanemann M, Mogl M, Langrehr JM, Neuhaus P. Older liver graft transplantation, cholestasis and synthetic graft function. Transpl Int. 2005;18:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Borchert DH, Glanemann M, Mogl M, Langrehr J, Neuhaus P. Adult liver transplantation using liver grafts from donors over 70 years of age. Transplant Proc. 2005;37:1186-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Cescon M, Grazi GL, Cucchetti A, Ravaioli M, Ercolani G, Vivarelli M, D’Errico A, Del Gaudio M, Pinna AD. Improving the outcome of liver transplantation with very old donors with updated selection and management criteria. Liver Transpl. 2008;14:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Serrano MT, Garcia-Gil A, Arenas J, Ber Y, Cortes L, Valiente C, Araiz JJ. Outcome of liver transplantation using donors older than 60 years of age. Clin Transplant. 2010;24:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Dudek K, Kornasiewicz O, Remiszewski P, Zieniewicz K, Wróblewski T, Krawczyk M. Results of liver transplantation from old donors. Transplant Proc. 2014;46:2762-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Scalea JR, Redfield RR, Foley DP. Liver transplant outcomes using ideal donation after circulatory death livers are superior to using older donation after brain death donor livers. Liver Transpl. 2016;22:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Fujikawa T, Fujita S, Mizuno S, Shenkman E, Vogel B, Lipori P, Hemming AW, Nelson D, Reed AI. Clinical and financial impact of obesity on the outcome of liver transplantation. Transplant Proc. 2006;38:3612-3614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Rustgi VK, Marino G, Rustgi S, Halpern MT, Johnson LB, Tolleris C, Taddei TH. Impact of body mass index on graft failure and overall survival following liver transplant. Clin Transplant. 2004;18:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Patkowski W, Zieniewicz K, Skalski M, Krawczyk M. Correlation between selected prognostic factors and postoperative course in liver transplant recipients. Transplant Proc. 2009;41:3091-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Aloia TA, Knight R, Gaber AO, Ghobrial RM, Goss JA. Analysis of liver transplant outcomes for United Network for Organ Sharing recipients 60 years old or older identifies multiple model for end-stage liver disease-independent prognostic factors. Liver Transpl. 2010;16:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Lin CC, Yong CC, Chen CL. Active vaccination to prevent de novo hepatitis B virus infection in liver transplantation. World J Gastroenterol. 2015;21:11112-11117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Burra P, Germani G, Adam R, Karam V, Marzano A, Lampertico P, Salizzoni M, Filipponi F, Klempnauer JL, Castaing D. Liver transplantation for HBV-related cirrhosis in Europe: an ELTR study on evolution and outcomes. J Hepatol. 2013;58:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |