Published online Aug 8, 2017. doi: 10.4254/wjh.v9.i22.967
Peer-review started: January 20, 2017
First decision: March 13, 2017
Revised: March 19, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: August 8, 2017
Processing time: 200 Days and 15.2 Hours
To determine the predictive performance of cholinesterase compared to existing prognostic models in evaluating liver function in patients with chronic hepatitis D.
In an observational, cross-sectional and retrospective study, consecutive patients with hepatitis D cirrhosis were evaluated. Demographic, clinical and laboratory parameters were recorded. Serum cholinesterase levels were correlated with existing scoring models for chronic liver disease and Liver function tests. Receiver operating characteristic (ROC) curves were constructed to find an optimal cholinesterase level predicting ascites, Child Turcotte Pugh (CTP) score ≥ 10, model for end stage liver disease (MELD) score ≥ 15, baseline-event-anticipation (BEA) score for hepatitis D ≥ 5 and the aspartate transaminase to Platelet Ratio Index (APRI) ≥ 1.5.
This study investigated 233 patients with chronic liver disease due to hepatitis D; 192 were male, median age 42 (16-69 years). Fifty patients had ascites and 15 had encephalopathy. One hundred and sixty-seven (71.7%) were in Child class A, 52 (22.3%) in Child class B and 14 (5.0%) in class C. A MELD score of 15 or more was seen in 24 patients. Cholinesterase levels correlated well with the INR, albumin, CTP score, MELD, MELD sodium, BEA and APRI scores (P < 0.001 each). Area under the ROC curve for ascites, CTP ≥ 10, MELD ≥ 15, BEA ≥ 5, APRI ≥ 1.5 was 0.836, 0.966, 0.913, 0.871 and 0.825 respectively (P < 0.001 each). Cut off values of cholinesterase (IU/L) for predicting ascites, CTP ≥ 10, MELD ≥ 15, BEA ≥ 5 and APRI ≥ 1.5 were < 3812, < 2853, < 2829, < 4719 and < 3954 with a sensitivity of 80%, 100%, 91.67%, 82.50%, 58.0% and specificity of 81.97%, 84.79%, 87.56%, 77.06% and 55.64% respectively.
Serum cholinesterase demonstrates promising correlations with serum albumin, INR and CTP, MELD, BEA and APRI scores and is predictive of liver reserves in hepatitis D cirrhosis.
Core tip: Prognostic models to assess liver function in patients with chronic liver disease are used extensively in clinical settings. These systems employ multiple clinical and laboratory parameters to evaluate liver reserves and predict outcome. In our study we assessed cholinesterase as an independent predictor of hepatic reserves. We found that its values correlated strongly with Liver function tests and with the existing scoring models. Thereafter, we defined optimal cholinesterase levels corresponding to the different stages and classes of the scoring systems and hence the severity of chronic liver disease. The study’s subjects were patients suffering from cirrhosis due to hepatitis D.
- Citation: Abbas M, Abbas Z. Serum cholinesterase: A predictive biomarker of hepatic reserves in chronic hepatitis D. World J Hepatol 2017; 9(22): 967-972
- URL: https://www.wjgnet.com/1948-5182/full/v9/i22/967.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i22.967
Prognostic models to evaluate liver function include the Child Turcotte Pugh (CTP) score, the Model for Endstage Liver Disease (MELD) score and the aspartate transaminase to Platelet Ratio Index (APRI). The CTP score is often used to assess the risk of surgery in patients with cirrhosis and it correlates with survival[1]. The MELD score is used by the United Network of Organ Sharing (UNOS) to prioritize patients awaiting cadaveric liver transplant[2]. An increase in the MELD score is associated with an increasing severity of hepatic dysfunction and an increased three-month mortality risk. The APRI is considered as an alternative to liver biopsy for predicting liver fibrosis[3]. However, its role in some etiologies is controversial. Increasing levels > 1.5 may show decreasing hepatocyte mass and increasing fibrosis. Recently, a baseline-event-anticipation (BEA) score has been developed for hepatitis D to define clinical parameters associated with worse outcomes[4].
Commonly used liver synthetic function tests include serum albumin and prothrombin time and international normalized ratio. Serum butyrylcholinesterase, commonly known as serum cholinesterase, is an enzyme synthesized by hepatocytes and has the half-life of eleven days[5]. Its serum level is decreased in chronic liver damage, infections, and malnutrition[6].
Chronic hepatitis D is a severe disease with rapid progression of fibrosis leading to cirrhosis, decompensation and hepatocellular carcinoma[7]. The role of cholinesterase to assess the liver reserves in hepatitis D patients has not been well defined. The objective of this study is to determine the performance of cholinesterase in predicting liver function compared to existing synthetic liver function tests and scoring models in patients with hepatitis D and cirrhosis.
This observational, cross-sectional study examined the efficacy of cholinesterase as a liver function test to assess the synthetic reserve in a retrospective fashion. Two hundred and thirty-three consecutive patients presenting to the liver clinic with cirrhosis due to chronic hepatitis D were evaluated. Available baseline demographic and clinical parameters were recorded. Serum cholinesterase levels were checked as a routine test to evaluate liver function.
Data were expressed as the number of subjects with percentages for nominal variables. These variables were compared by χ2 or Fisher exact test. Continuous variables were presented as means with standard deviation, and compared using Student t test, Mann-Whitney U test and ANOVA. Correlations were tested using tests Pearson’s correlation test. Receiver operating characteristic (ROC) curves were constructed to determine optimal cholinesterase levels predicting multiple state variables such as MELD score ≥ 15. Areas under receiver operating characteristic curves, sensitivity and specificity were used to examine the accuracy of the cholinesterase for various predictions. The state variables examined included Ascites, MELD score ≥ 15, MELD score > 10, APRI ≥ 1.5, BEA ≥ 5, CTP ≥ Class B and CTP ≥ Class C. Cutoff cause were determined by Youden’s J statistic (verified by a unit weighted ROC cutoff based on minimizing the distance from the point representing perfect classification to the ROC curve). Statistical analyses were performed using SPSS 23.0 software (IBM SPSS Statistics, New York, NY, United States). All tests were 2-tailed and a P value < 0.05 was required for statistical significance.
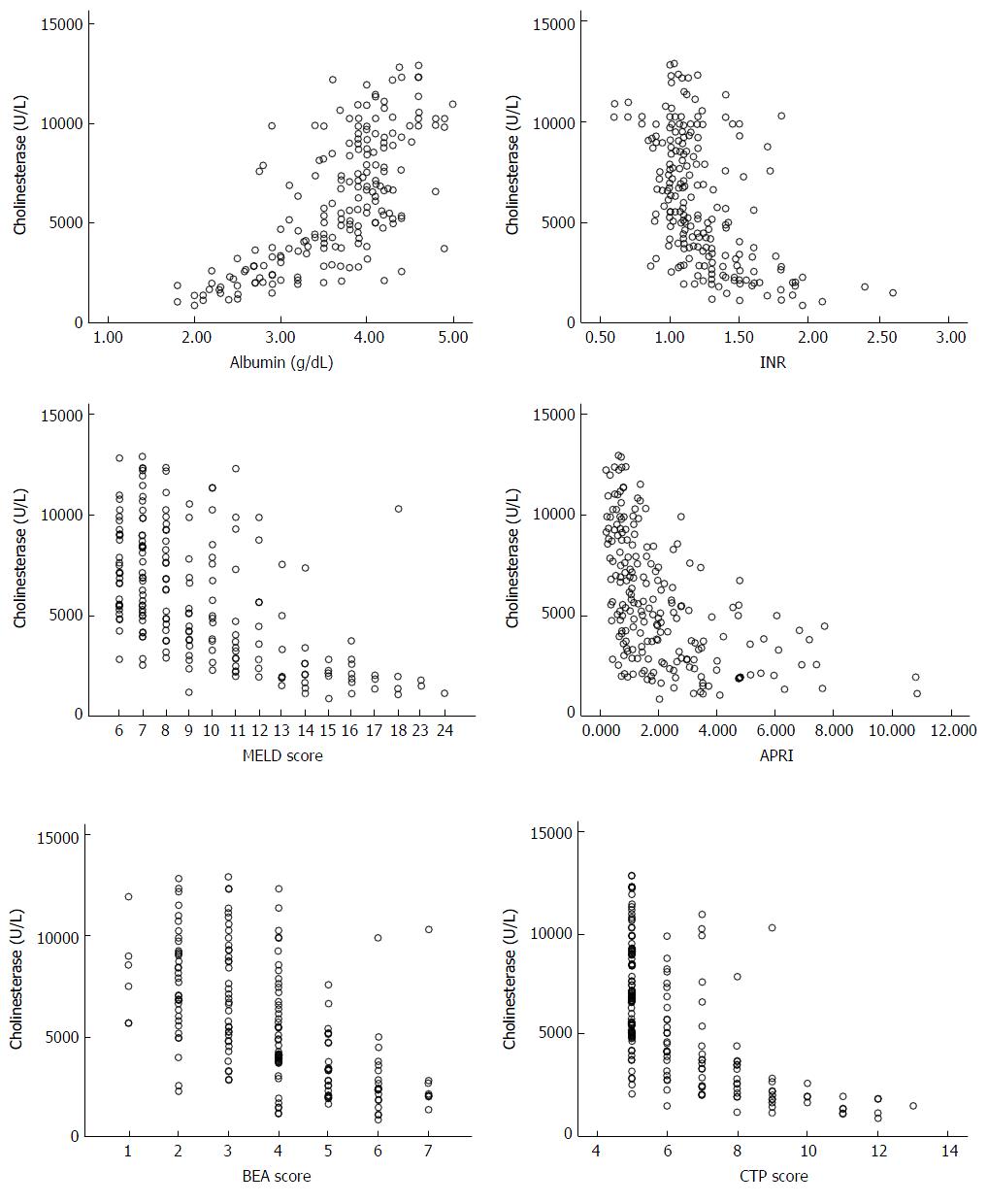
Out of 233 patients with chronic liver disease due to hepatitis D, 192 (82.4%) were male, the median age was 42 (range 16-69 years). Fifty (21.5%) patients had ascites and 15 (6.4%) encephalopathy. One hundred and sixty-seven (71.7%) were classified into Child class A, 52 (22.3%) into Child class B and 14 (5.0%) into class C. A MELD score of 15 or more was seen in 24 (10.3%). Cholinesterase levels (mean ± SEM) in males were 6177 ± 228 and in females were 5151 ± 452 (P = 0.06). A statistically significant difference was not found between gender and BMI at any stage of the disease. The baseline characteristics of the study patients are recorded in Table 1. Cholinesterase levels correlated with the albumin, INR, CTP score, MELD, MELD sodium, APRI and BEA scores with the Pearson correlation coefficient, r values of 0.724, -0.520, -0.624, -0.561, -0.533, -0.531, and -0.591 respectively (P < 0.001 each) (Figure 1). The mean cholinesterase levels for each class are shown in Table 2.
| No. of patients | 233 |
| Male:female | 192:41 |
| Age (yr) | 42 (16-69) |
| BMI (kg/m2) | 23.4 (14.3-40) |
| Ascites | 50 (21.5) |
| Encephalopathy | 15 (6.4) |
| Bilirubin (mg/dL) | 0.90 (0.2-6.9) |
| Albumin (g/dL) | 3.8 (1.8-5.0) |
| INR | 1.13 (0.6-2.6) |
| Creatinine (mg/dL) | 0.8 (0.4-1.96) |
| Sodium (mmol/L) | 139 (120-150) |
| AST (IU/L) | 53 (10-638) |
| Platelets (× 109/L) | 120 (22-388) |
| Cholinesterase (IU/L) | 5508 (861-12891) |
| Child class | |
| A | 167 (71.7) |
| B | 52 (22.3) |
| C | 14 (5.0) |
| CTP score | 5 (5-13) |
| MELD score | 8 (6-24) |
| MELD sodium | 9 (6-26) |
| MELD score 15 or more | 24 (10.3) |
| APRI | 1.26 (0.19-10.8) |
| APRI 1.5 or more | 100 (42.9) |
| BEA class | |
| A | 6 (2.6) |
| B | 164 (70.4) |
| C | 63 (27) |
| BEA score | 4 (1-7) |
| Parameter | Values (U/L) | P value |
| Child class | ||
| A (CTP up to 6) | 7058 ± 208 | |
| B (CTP 7-9) | 3773 ± 372 | < 0.001 (A vs B) |
| C (CTP ≥ 10) | 1605 ± 129 | < 0.001 (B vs C) |
| MELD score | ||
| ≥ 15 | 2285 ± 373 | |
| < 15 | 6423 ± 206 | < 0.001 |
| APRI | ||
| ≥ 1.5 | 4002 ± 219 | |
| < 1.5 | 7498 ± 251 | < 0.001 |
| BEA score | ||
| A | 8036 ± 967 | |
| B | 6993 ± 222 | 0.337 (A vs B) |
| C | 3211 ± 258 | < 0.001 (B vs C) |
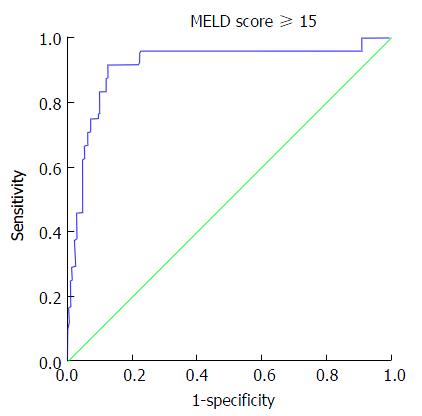
Area under the ROC curve for ascites, CTP ≥ 10, MELD ≥ 15, BEA ≥ 5, APRI ≥ 1.5 was 0.836, 0.966, 0.913, 0.871 and 0.825 respectively (P < 0.001 each) (Table 3 and Figure 2). Cut off values of cholinesterase (IU/L) for predicting ascites, CTP ≥ 10, MELD ≥ 15, BEA ≥ 5 and APRI ≥ 1.5 were < 3812, < 2853, < 2829, < 4719 and < 3954 with a sensitivity of 80%, 100%, 91.67%, 82.50%, 58.0% and specificity of 81.97%, 84.79%, 87.56%, 77.06 and 55.64% respectively (Table 4). Mean serum cholinesterase decreased with reduced hepatic reserves. For example patients in Child Class A had a mean cholinesterase of 7058 compared to those in Class B with 3773 and Class C with 1605. This is also reflected in the optimal cutoff values of < 3812 at Child Class B (CTP score 7-9) and < 2853 at Child Class C (CTP ≥ 10) and similarly a value of < 4719 at MELD ≥ 10 and < 2829 at MELD ≥ 15.
| State variable | AUC | Std. error | Asymptotic sig. (P value) | 95%CI |
| Ascites | 0.836 | 0.038 | < 0.001 | 0.762-0.910 |
| CTP ≥ 7 | 0.889 | 0.029 | < 0.001 | 0.832-0.946 |
| CTP ≥ 10 | 0.966 | 0.013 | < 0.001 | 0.940-0.992 |
| MELD ≥ 10 | 0.798 | 0.034 | < 0.001 | 0.731-0.864 |
| MELD ≥ 15 | 0.913 | 0.038 | < 0.001 | 0.838-0.987 |
| APRI ≥ 1.5 | 0.825 | 0.026 | < 0.001 | 0.773-0.877 |
| BEA ≥ 5 | 0.871 | 0.028 | < 0.001 | 0.816-0.926 |
| State variable | Cholinesterase cut off value (IU/L) | Sensitivity, % (95%CI) | Specificity, % (95%CI) | Likelihood ratio |
| Ascites | < 3812 | 80.00 (66.28- 89.97) | 81.97 (75.62-87.25) | 4.436 |
| CTP ≥ 7 | < 3812 | 79.10 (67.43-88.08) | 67.47 (59.78-74.53) | 2.432 |
| CTP ≥ 10 | < 2853 | 100.00 (79.41-100.00) | 84.79 (79.31-89.29) | 6.576 |
| MELD ≥ 10 | < 4719 | 72.09 (61.38-81.23) | 80.27 (72.91-86.37) | 3.654 |
| MELD ≥ 15 | < 2829 | 91.67 (73.00-98.97) | 87.56 (82.31-91.71) | 7.369 |
| BEA ≥ 5 | < 4719 | 82.54 (70.90-90.95) | 77.06 (70.00-83.15) | 3.598 |
| APRI ≥ 1.5 | < 3954 | 58.00 (47.71-67.80) | 55.64 (46.78-64.25) | 1.307 |
Traditional liver function tests and scoring systems used to stage severity of the liver disease face several inherent limitations. For example, the LFTs investigated in this study maybe abnormal in illnesses not associated with liver dysfunction. Aminotransferase levels may increase in non-hepatic disease such as myocardial infarction[8] while bilirubin maybe altered by hemolysis. Moreover, the CTP score includes subjective parameters such as the degree of ascites and encephalopathy[9] and these findings may be altered substantially by medical interventions. Furthermore, its role is limited due to a ceiling and floor effect: An inability to discriminate values for bilirubin > 3.0 mg /dL, INR greater > 2.3 and albumin less < 2.8 g/dL. Finally, the CTP score does not include creatinine for the assessment of renal function, another major marker of the severity of the disease.
The MELD score has been criticized for several different reasons[10-13]. It is vulnerable to variations in laboratory measurements and does not include portal hypertensive complications (e.g., ascites, encephalopathy, variceal bleeding, and spontaneous bacterial peritonitis). Again, it suffers from a floor and ceiling effect: Patients with the combination of an INR of ≤ 1, creatinine ≤ 1 mg/dL, and bilirubin ≤ 1 mg/dL receive the minimum score of 6 MELD points, while UNOS set an upper limit for the MELD score at 40 points. Modifications of the MELD scoring system have been implemented by introducing the MELD sodium, by reweighting MELD components (lower weights ascribed to serum creatinine and international normalized ratio (INR) and a higher weight to serum bilirubin), by refitting MELD [by implementing new upper and lower bounds for creatinine (0.8 and 3.0 mg/dL, respectively) and for INR (1.0 and 3.0, respectively)], and by dynamic changes in MELD scoring (Delta MELD).
The scoring systems use multiple clinical and laboratory parameters to evaluate liver reserves and predict outcomes. In our study we assessed cholinesterase as an independent test for liver function and hepatic reserves.
Cholinesterase levels have been assessed to predict survival in patients with Parenchymal cirrhosis[14], predict outcome in graft-vs-host disease[15], distinguish between liver disease and non-liver disease aberration in liver function tests[16] and differentiate cirrhosis from non-cirrhosis[17]. Serum cholinesterase levels have also been found to correlate with CTP Class[18,19]. In addition, cholinesterase levels have been shown to recover with improvements in hepatic function[20] at a rate exceeding recovery from organophosphate poisoning.
Our study showed that cholinesterase levels could be used in conjunction with existing scoring systems as a prognostic marker of hepatic reserves. However, serum cholinesterase levels may be affected by gender, nutritional status and carcinomas[6]. We did not find any differences related to gender and body mass index. None of our patients had malignancy while all of the patients included in this study were suffering from cirrhosis related to hepatitis D. So differences in the etiology of the liver disease could not affect the results of this study. The prevalence of inherited atypical cholinesterase has been reported to be low in multiple studies[21]. So any genetic variations are less likely to influence the results of this study.
In conclusion, serum cholinesterase is an excellent biomarker of the synthetic function of liver in CLD with hepatitis D. Cholinesterase levels should be routinely checked to assess liver function and may be incorporated in MELD scoring. It can be effectively used to follow the staging of liver disease in hepatitis D. Our results should be validated in other cohorts and etiologies of CLD.
Chronic hepatitis D is a severe disease with rapid progression of fibrosis leading to cirrhosis, decompensation and hepatocellular carcinoma. Commonly used liver synthetic function tests include serum albumin and prothrombin time and international normalized ratio. The objective of this study was to determine the performance of cholinesterase levels in predicting liver function compared to the existing scoring models in patients with hepatitis D and cirrhosis.
The authors defined optimal cholinesterase levels corresponding to the different stages and classes of the scoring systems assessing the severity of chronic liver disease.
Serum cholinesterase demonstrated promising correlations with serum albumin, international normalized ratio and Child Turcotte Pugh, Model for Endstage Liver Disease, baseline-event-anticipation and aspartate transaminase to Platelet Ratio Index scores.
Serum cholinesterase levels can be effectively used to monitor the staging of liver disease in hepatitis D. These results may be validated in other cohorts and etiologies of chronic liver disease to predict the liver reserves.
Serum butyrylcholinesterase, commonly known as serum cholinesterase, is an enzyme synthesized by hepatocytes and has the half-life of eleven days
The authors investigated the role of cholinesterase levels as predictor of hepatic reserves in chronic hepatitis D patients. This paper is generally well conducted and straightforward. The authors concluded that cholinesterase levels can be considered a biomarker of liver function in these patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cunha C S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Franzetta M, Raimondo D, Giammanco M, Di Trapani B, Passariello P, Sammartano A, Di Gesù G. Prognostic factors of cirrhotic patients in extra-hepatic surgery. Minerva Chir. 2003;58:541-544. [PubMed] |
| 2. | Freeman RB, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L; UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851-858. [PubMed] [DOI] [Full Text] |
| 3. | Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. [PubMed] [DOI] [Full Text] |
| 4. | Calle Serrano B, Großhennig A, Homs M, Heidrich B, Erhardt A, Deterding K, Jaroszewicz J, Bremer B, Koch A, Cornberg M. Development and evaluation of a baseline-event-anticipation score for hepatitis delta. J Viral Hepat. 2014;21:e154-e163. [PubMed] [DOI] [Full Text] |
| 5. | Ostergaard D, Viby-Mogensen J, Hanel HK, Skovgaard LT. Half-life of plasma cholinesterase. Acta Anaesthesiol Scand. 1988;32:266-269. [PubMed] |
| 6. | Santarpia L, Grandone I, Contaldo F, Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4:31-39. [PubMed] [DOI] [Full Text] |
| 7. | Abbas Z, Afzal R. Life cycle and pathogenesis of hepatitis D virus: A review. World J Hepatol. 2013;5:666-675. [PubMed] [DOI] [Full Text] |
| 8. | Rej R. Aminotransferases in disease. Clin Lab Med. 1989;9:667-687. [PubMed] |
| 9. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [PubMed] [DOI] [Full Text] |
| 10. | Trotter JF, Brimhall B, Arjal R, Phillips C. Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transpl. 2004;10:995-1000. [PubMed] [DOI] [Full Text] |
| 11. | Gotthardt D, Weiss KH, Baumgärtner M, Zahn A, Stremmel W, Schmidt J, Bruckner T, Sauer P. Limitations of the MELD score in predicting mortality or need for removal from waiting list in patients awaiting liver transplantation. BMC Gastroenterol. 2009;9:72. [PubMed] [DOI] [Full Text] |
| 12. | Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3:50-60. [PubMed] [DOI] [Full Text] |
| 13. | Lau T, Ahmad J. Clinical applications of the Model for End-Stage Liver Disease (MELD) in hepatic medicine. Hepat Med. 2013;5:1-10. [PubMed] [DOI] [Full Text] |
| 14. | Garello E, Battista S, Bar F, Niro GA, Cappello N, Rizzetto M, Molino G. Evaluation of hepatic function in liver cirrhosis: clinical utility of galactose elimination capacity, hepatic clearance of D-sorbitol, and laboratory investigations. Dig Dis Sci. 1999;44:782-788. [PubMed] |
| 15. | Bacigalupo A, Oneto R, Bruno B, Lamparelli T, Gualandi F, Bregante S, Raiola AM, Di Grazia C, Dominietto A, Lombardi A. Serum cholinesterase is an early and sensitive marker of graft-versus host-disease (GVHD) and transplant-related mortality (TRM). Bone Marrow Transplant. 2001;28:1041-1045. [PubMed] [DOI] [Full Text] |
| 16. | Ogunkeye OO, Roluga AI. Serum cholinesterase activity helps to distinguish between liver disease and non-liver disease aberration in liver function tests. Pathophysiology. 2006;13:91-93. [PubMed] [DOI] [Full Text] |
| 17. | Ramachandran J, Sajith KG, Priya S, Dutta AK, Balasubramanian KA. Serum cholinesterase is an excellent biomarker of liver cirrhosis. Trop Gastroenterol. 2014;35:15-20. [PubMed] |
| 18. | Meng F, Yin X, Ma X, Guo XD, Jin B, Li H. Assessment of the value of serum cholinesterase as a liver function test for cirrhotic patients. Biomed Rep. 2013;1:265-268. [PubMed] [DOI] [Full Text] |
| 19. | Temel HE, Temel T, Cansu DU, Ozakyol A. Butrylcholinesterase activity in chronic liver disease patients and correlation with Child-Pugh classification and MELD scoring system. Clin Lab. 2015;61:421-426. [PubMed] |
| 20. | Brown SS, Kalow W, Pilz W, Whittaker M, Woronick CL. The plasma cholinesteerases: a new perspective. Adv Clin Chem. 1981;22:1-123. [PubMed] |
| 21. | Steegmüller H. On the geographical distribution of pseudocholinesterase variants. Humangenetik. 1975;26:167-185. [PubMed] |