Published online May 18, 2016. doi: 10.4254/wjh.v8.i14.625
Peer-review started: January 27, 2016
First decision: March 9, 2016
Revised: March 30, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: May 18, 2016
Processing time: 107 Days and 18.5 Hours
AIM: To investigate the efficacy of pegylated interferon alfa (PEG-IFNα) therapy with and without entecavir in patients with chronic hepatitis D.
METHODS: Forty hepatitis D virus (HDV) RNA positive patients were randomized to receive either PEG-IFNα-2a 180 μg weekly in combination with entecavir 0.5 mg daily (n = 21) or PEG-IFNα alone (n =19). Patients who failed to show 2 log reduction in HDV RNA level at 24 wk of treatment, or had detectable HDV RNA at 48 wk of therapy were considered as treatment failure. Treatment was continued for 72 wk in the rest of the patients. All the patients were followed for 24 wk post treatment. Intention to treat analysis was performed.
RESULTS: The mean age of the patients was 26.7 ± 6.8 years, 31 were male. Two log reduction in HDV RNA levels at 24 wk of therapy was achieved in 9 (43%) patients receiving combination therapy and 12 (63%) patients receiving PEG-IFNα alone (P = 0.199). Decline in hepatitis B surface antigen (HBsAg) levels was insignificant. At the end of treatment, HDV RNA was negative in 8 patients (38%) receiving combination therapy and 10 patients (53%) receiving PEG-IFNα-2a alone. Virological response persisted in 7 (33%) and 8 (42%) patients, respectively at the end of the 24 wk follow-up period. One responder patient in the combination arm lost HBsAg and became hepatitis B surface antibody positive. Six out of 14 baseline hepatitis B e antigen reactive patients seroconverted and four of these seroconverted patients had persistent HDV RNA clearance.
CONCLUSION: Administration of PEG-IFNα-2a with or without entecavir, resulted in persistent HDV RNA clearance in 37% of patients. The addition of entecavir did not improve the overall response.
Core tip: Chronic hepatitis D is a difficult to treat infection. Six months post treatment response is seen only in one quarter of the patients treated with pegylated interferon alfa (PEG-IFNα). In an attempt to improve the response of PEG-IFN, we combined entecavir. This is the first study to evaluate the efficacy of PEG-IFN with entecavir compared to PEG-IFN alone for the treatment of hepatitis D infection. Our study showed that the combination treatment did not have any additional benefit in terms of hepatitis D virus RNA suppression and hepatitis B surface antigen reduction as compared to PEG-IFN alone.
- Citation: Abbas Z, Memon MS, Umer MA, Abbas M, Shazi L. Co-treatment with pegylated interferon alfa-2a and entecavir for hepatitis D: A randomized trial. World J Hepatol 2016; 8(14): 625-631
- URL: https://www.wjgnet.com/1948-5182/full/v8/i14/625.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i14.625
The prevalence of hepatitis B surface antigen (HBsAg) positive individuals in Pakistan is 2.5%[1] and it is estimated that 5 million persons are HBsAg positive. In a large series, hepatitis D virus (HDV) antibodies in HBsAg positive individuals were found to be present in 16.6% cases[2]. So there is a large pool of patients exposed to hepatitis D in this country.
Chronic hepatitis D is a difficult to treat infection. Standard interferon-alfa is not an ideal treatment[3]. Recent few trials with pegylated interferon alfa (PEG-IFNα) have shown a better response of 25%-30% six months post treatment[4,5]. In an attempt to improve the response of PEG-IFNα, the International Hepatitis Delta Network evaluated adefovir and later tenofovir in combination with PEG-IFNα in HIDIT-1 and HIDIT-2 studies[6,7]. It was expected that HIDIT-2 will yield better results due to use of potent nucleotide analogue in combination with PEG-IFNα for a longer duration of therapy.
Recently presented results of HITID-2 trial[7] showed that 96 wk of PEG-IFNα-2a and tenofovir therapy was associated with a high frequency of serious adverse events. Combination treatment had similar effects on HBsAg reduction as compared to PEG-IFNα alone. More than one third of the on-treatment responders experienced a posttreatment HDV RNA relapse despite prolonged therapy. The results of the long term post treatment follow-up are awaited. Combination therapy with tenofovir did not provide obvious benefits in hepatitis D patients with low baseline hepatitis B virus (HBV)-DNA levels and prolongation of treatment to 96 wk did not provide higher off-treatment HDV RNA responses (compared to 48 wk in the HIDIT-1 study).
The aim of this study is to evaluate the efficacy of PEG-IFNα-2a with entecavir for the treatment of chronic hepatitis D. The reason for choosing entecavir was that HIDIT-2 study using tenofovir was in progress with high hopes and no data was available for entecavir in combination with PEG-IFNα-2a in the chronic hepatitis D setting. This drug, particularly in combination with PEG-IFNα-2a, have shown good results in HBsAg decline[8].
This randomized study compared the efficacy of PEG-IFNα-2a plus entecavir vs PEG-IFNα-2a alone for the treatment of chronic hepatitis D. Patients were randomized 1:1 into two groups. Duration of treatment was 72 wk with a post-treatment follow-up of 24 wk.
Inclusion criteria were age 15-60 years, anti-HDV anti body positive and detectable serum HDV RNA at enrolment by real time polymerase chain reaction (PCR), elevated alanine aminotransferase (ALT) on two occasions in last 3 mo during screening phase, patients with compensated liver disease, i.e., Child Pugh class A, hemoglobin > 12.0 g/dL for males and > 11.0 g/dL for females at screening, total leucocyte count > 3.000/mm3, and neutrophils > 1500/mm3, platelets > 80.000/mm3, serum creatinine level < 1.5 mg/dL and liver biopsy within 6 mo prior to randomization.
Exclusion criteria were patients who had received therapy for chronic hepatitis D, co-infection with hepatitis C or human immunodeficiency virus, serum total bilirubin greater than twice the upper limit of normal at screening, evidence of decompensated liver disease (Childs B-C), history or other evidence of a medical condition associated with chronic liver disease (e.g., Wilson’s disease, hemochromatosis, autoimmune hepatitis, alcoholic liver disease, alpha1 anti-trypsin deficiency, toxin exposures, thalassemia), women with ongoing pregnancy or who are breast feeding, evidence of drug and/or alcohol abuse, history of severe cardiac or pulmonary disease, inability or unwillingness to provide informed consent or abide by the requirements of the study.
The study was conducted at the Orthopaedic and Medical Institute, Karachi, Liver Stomach Clinic, Karachi and Asian Institute of Medical Sciences, Hyderabad, Pakistan. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and principles of Good Clinical Practice. Patients gave the informed consent and the ethics committee approved the study.
The dose of PEG-IFNα-2a in each arm was 180 μg weekly (Pegasys®, F. Hoffmann-La Roche Ltd, Basel). Entecavir was given in a dose of 0.5 mg per oral daily in the combination arm.
Virological response was defined as HDV RNA clearance at the end of treatment and at follow-up six months post treatment. Biochemical response was normalization of ALT at the end of treatment and at follow-up.
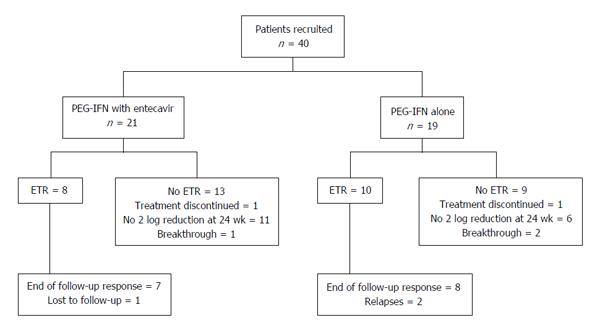
Treatment failure was defined as failure to show 2 log reduction in HDV RNA level at 24 wk of treatment, or presence of detectable HDV RNA at 48 wk of therapy or relapse at 24 wk post treatment follow-up. Development of decompensation (ascites or hepatic encephalopathy) during the treatment, drop outs, and lost to follow-up were also considered as treatment failure in an intention to treat analysis (Figure 1).
Randomization (1:1 allocation) was computer-generated. The investigators were not involved in sequence generation or allocation concealment steps and were provided with sealed envelopes containing the treatment code to administer, in increasing numbers, according to chronological inclusion in the study.
Viral nucleic acids were isolated from patients’ serum samples by High Pure Viral Nucleic Acid Kit, according to the manufacturer’s instructions (Roche Diagnostics, United States). Serum HBV DNA levels were measured using the Cobas TaqMan (Roche Diagnostics Systems, Basel, Switzerland) with a lower limit of quantification 20 IU/mL. For HDV RNA, qualitative test was based on the reverse transcription PCR of the target gene. Quantification of HDV RNA was done by real time PCR having a lower limit of detection of 500 IU/mL. Hepatitis B e antigen (HBeAg) and antibody against HBeAg (anti-HBe) status was determined using enzyme immunoassays. Serum HBsAg was quantified using the Architect HBsAg assay (Abbott Laboratories, Abbott Park, IL, United States). Grading of inflammation and the staging of fibrosis was performed according to the Batt’s and Ludwig’s classification[9].
The Patients were educated regarding administration of subcutaneous pegylated interferon and oral entecavir, expected adverse events, schedule for laboratory monitoring, and clinic appointment. Patients were evaluated as outpatients for safety, tolerance and efficacy every 4 wk during treatment until week 72 and then at 92 wk, i.e., 24 wk post treatment during the follow-up period. At each visit complete blood count and biochemistry was assessed. HDV RNA levels were measured at baseline, 24, 48, 72 and 96 wk. HBsAg levels were measured at baseline and 24 wk. HBeAg and anti-HBe antibodies were checked at baseline. In case of HBeAg reactive, tests for HBeAg and anti-HBeAg done were checked at 24, 48 and 72 wk to document seroconversion.
Adverse events and clinical laboratory parameters were recorded. Serious adverse events were defined as those that were fatal, life-threatening, required inpatient hospitalization or discontinuation of treatment. These included decompensation of liver disease and mortality. Non-serious adverse events and laboratory abnormalities leading to dose modifications and premature withdrawal from therapy were noted.
Data were expressed as the number of subjects with percentages for nominal variables. These variables were compared by χ2 or Fisher exact test. Continuous variables were presented as mean (standard deviation), and compared using Mann-Whitney U test. The degree of the relationship between linear related variables was measured by the Pearson r correlation test. Statistical analyses were performed using SPSS 20.0 software (IBM SPSS Statistics, New York, NY, United States). All tests were 2-tailed and a two-tailed P value < 0.05 was required for statistical significance. Intention-to-treat analysis was done to include all randomized patients.
A total of 40 patients with chronic hepatitis D was included during the study period of 2012-2014. Twenty-one patients were treated with PEG-IFNα plus entecavir (combination arm) and 19 with PEG-IFNα alone (monotherapy arm). The mean age of the patients was 26.7 ± 6.8 years, 31 were male and 14 were HBeAg reactive; 7 in each arm.
Demographic and baseline clinical characteristics of the patients are shown in Table 1. Age, gender, body mass index, degree of fibrosis and inflammation on liver biopsy, ALT, HBeAg status, and HDV RNA levels were comparable to the combination and monotherapy arm patients. However, platelet count in monotherapy arm was lower than in the combination arm. Baseline HBsAg and HBV DNA levels were correlated (Pearson correlation = 0.625, P < 0.001). Liver biopsy was available in all cases as one of the inclusion criteria. Mild inflammation was seen in 6 and moderate to severe in 34 patients. Stage of the disease was 0-2 in 18 and 3-4 in 22 patients. Cirrhosis of the liver as evident from histology, ultrasound or clinical examination was present in 8 (20%) patients.
| PEG-IFNα with entecavir (n = 21) | PEG-IFNα alone (n = 19) | P value | |
| Age (mean, yr) | 26.4 ± 6.4 | 27 ± 7.4 | 0.946 |
| Gender (male:female) | 16:5 | 15:4 | 1.00 |
| Body mass index (kg/m2) | 21.8 ± 3.6 | 23.6 ± 4.3 | 0.151 |
| Hemoglobin (g/dL) | 13.7 ± 1.59 | 13.9 ± 1.3 | 0.473 |
| Total leucocyte count (× 106/L) | 7.1 ± 2.0 | 6.7 ± 1.9 | 0.626 |
| Platelets count (× 109/L) | 237 ± 83 | 185 ± 59 | 0.0231 |
| Total bilirubin (mg/dL) | 0.67 ± 0.34 | 0.70 ± 0.38 | 0.828 |
| ALT (IU/L) | 87 ± 55 | 89 ± 41 | 0.379 |
| GGT (IU/L) | 49 ± 41 | 69 ± 72 | 0.255 |
| Inflammatory grade on biopsy | 0.186 | ||
| 0-1 | 5 (31) | 1 (5) | |
| 2-3 | 16 (69) | 18 (95) | |
| Fibrosis stage on biopsy | 0.105 | ||
| 0-2 | 12 (57) | 6 (32) | |
| 3-4 | 9 (43) | 13 (68) | |
| Cirrhosis | 2 (10) | 6 (32) | 0.120 |
| HBeAg reactive | 7 (33) | 7 (37) | 0.816 |
| HDV RNA (mean log10 IU/mL) | 7.5 ± 1.1 | 6.9 ± 1.2 | 0.119 |
| HBV DNA detected | 5 (24) | 5 (26) | 1.00 |
| HBV DNA (mean log10 IU/mL) | 1.08 ± 2.10 | 1.48 ± 2.70 | 0.656 |
| HBsAg (mean log10 IU/mL) | 4.50 ± 0.42 | 4.20 ± 0.64 | 0.068 |
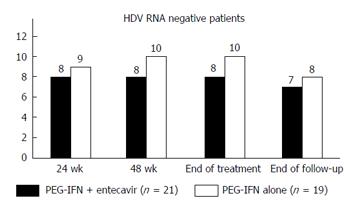
Two log reduction in HDV RNA levels at 24 wk of therapy was achieved in 9 (43%) patients receiving combination therapy and in 12 (63%) patients receiving PEG-IFNα alone (P = 0.199) (Figure 1). There was no significant difference in the HBsAg log10 levels after six months of therapy; 4.13 ± 0.91 in the combination arm vs 4.01 ± 0.51 in the monotherapy arm (P = 0.608), and a mean decline in HBsAg levels (P = 0.579). At the end of treatment, HDV RNA was negative in 8 patients (38%) receiving combination therapy and in 10 patients (53%) receiving PEG-IFNα-2a alone by intention to treat analysis (P = 0.356). ALT normalization was seen in 4 (19%) patients of the combination arm and 7 (37%) patients of the monotherapy arm (P = 0.293). One 29-year-old male patient in the combination arm lost HBsAg and became anti-HBs antibody positive. His baseline parameters were HBsAg 2903 IU/mL (log10 = 3.46), HBeAg negative with undetectable HBV DNA, and HDV RNA 158000 IU/ml (log10 = 5.20). At 24 wk of treatment, his HBsAg level was 11.6 IU/mL, and HDV RNA negative. HBsAg became undetectable at 48 wk and he developed anti-HBs antibodies.
Two patients in the monotherapy arm relapsed during the 24 wk post-treatment period and one patient in the combination arm was lost to follow-up, decreasing the persistent virological clearance 24 wk post treatment to 7 (33%) in the combination arm and 8 (42%) in the monotherapy arm (P = 0.567) (Table 2 and Figure 2) in an intention to treat analysis. End of follow-up biochemical response was seen in only patients with the virologic response; 5/21 (24%) and 7/19 (37%) patients of combination arm and monotherapy arm respectively (P = 0.369).
| Variable | Responders (n = 15) | Non-responders (n = 25) | P value |
| Age (mean, yr) | 27.9 ± 8.4 | 25.9 ± 5.7 | 0.654 |
| Gender (male:female) | 12:3 | 19:6 | 1.00 |
| Body mass index (kg/m2) | 23.8 ± 4.2 | 21.9 ± 3.8 | 0.158 |
| ALT (mean IU/L) | 103 ± 44 | 80 ± 50 | 0.0331 |
| GGT (mean IU/L) | 45 ± 21 | 66 ± 72 | 0.700 |
| Inflammatory activity on biopsy | 0.493 | ||
| 0-1 | 3 (20) | 3 (12) | |
| 2-3 | 12 (80) | 22 (88) | |
| Fibrosis on biopsy | 0.412 | ||
| 0-2 | 8 (53) | 10 (40) | |
| 3-4 | 7 (47) | 15 (60) | |
| Cirrhosis | 2 (13) | 6 (24) | 0.686 |
| HBeAg reactive | 4 (27) | 10 (40) | 0.502 |
| Baseline HDV RNA | 7.01 ± 1.25 | 4.61 ± 1.91 | 0.072 |
| Baseline HBsAg | 4.38 ± 0.63 | 4.32 ± 0.53 | 0.727 |
| Treatment arm | 0.567 | ||
| PEG-IFNα + entecavir (n = 21) | 7 (33) | 14 (67) | |
| PEG-IFNα alone (n = 19) | 8 (42) | 11 (58) | |
| Two log of HDV RNA reduction at week 24 | 15 (100) | 6 (24) | < 0.0011 |
| Baseline HBeAg reactive | 4 (27) | 10 (40) | 0.502 |
| 24 wk HBsAg level | 3.97 ± 1.01 | 4.31 ± 0.45 | 0.476 |
| One log reduction of HBsAg level at 24 wk | 5 (33) | 4 (16) | 0.255 |
| Patients with decrease in HBsAg level at 24 wk from baseline | 12 (80) | 12 (48) | 0.056 |
| HBeAg reactive patients (n = 14) who seroconverted | 4/4 (100) | 2/10 (20) | 0.0151 |
Though there were no statistical differences in log reduction of HBsAg levels at 24 wk of treatment between responders and non responders, there was a trend of decrease in HBsAg levels (P = 0.056). Out of 14 HBeAg reactive patients, HBeAg seroconversion was seen in 2 patients on combination arm and 4 patients of monotherapy arm. Both patients of the combination arm and 2 patients of the monotherapy arm achieved persistence HDV RNA clearance during the follow-up period while one patient relapsed and another had virological breakthrough during treatment. In contrast, 7 out of 8 patients, who did not seroconvert, were null responders and one patient could not complete treatment due to complications of treatment (combination arm).
One patient from the combination arm could achieve only one log reduction in the HDV RNA levels at 24 wk of treatment, and was considered as a non-responder according to the protocol. He was taken off the study and was considered a treatment failure in an intention to treat analysis. However, he continued to receive PEG-IFNα monotherapy for another 24 wk and became HDV RNA negative at the end of treatment and the response persisted 24 wk post treatment.
The side effects reported by these patients were usually of PEG-IFN and included nausea, weakness, fever, decreased appetite, bloating, body aches, headaches, weight loss. These side effects did not require a dose reduction. One patient developed transient neutropenia and responded to subcutaneous filgrastim. Two patients discontinued treatment; one in each arm during the treatment before 24 wk of therapy. One patient in the monotherapy arm developed ascites while treatment was stopped in another patient in the combination arm due to severe depression.
We used PEG-IFNα in combination with entecavir to treat HDV patients for the first time. The results of our study are in congruence with the previous studies that the combination treatment of nucleos(t)ides with PEG-IFNα does not have any edge over PEG-IFN monotherapy in terms of sustained clearance of HDV RNA[10].
Some of the previous studies, including one of ours, have shown that response to the treatment can be predicted by the HDV RNA assessment at six months, and may give a clue whether to stop treatment[11,12]. Patients with negative HDV RNA at six months are more likely to have sustained virologic response[5] while non-responders could be identified by a less than 3 log decrease of HDV RNA at 6 mo of treatment[11]. We used two log reduction at 24 wk as a criterion to continue treatment in our protocol. One of our patients from the combination arm had one log reduction at 24 wk, was taken off the study as non-responder but he continued treatment and showed persistent virological clearance. As there are not many treatment options for chronic hepatitis D, we may suggest that the patients, even with one log reduction in the viral load at 24 wk of therapy, who continue to show steady decline in HDV RNA levels may remain on treatment.
We had a low relapse rate in this study compared to our previous experience[5] as according to the protocol only better responders continued treatment, i.e., patients who had a 2 log reduction in HDV RNA levels at 24 wk of treatment. Moreover, treatment was extended to 72 wk instead of stopping at 48 wk. It may be beneficial to extend treatment duration beyond 48 wk in good responders to decrease the relapse rate, i.e., patients who show a reduction in HDV RNA and HBsAg levels, and HBeAg reactive patients who seroconvert during the treatment. However, proper way to judge this dictum could be a randomized trial comparing 48 wk vs 72-96 wk of therapy. Extending duration of therapy may also useful in patients with slow but steady decline of HDV RNA and HBsAg levels.
Heller et al[13] studied prolonging therapy of chronic hepatitis D with PEG-IFNα for up to 5 years. Only three of 12 patients treated achieved a complete virologic response, endpoint defined as the combination of undetectable HDV RNA with loss of HBsAg and anti- HBsAg seroconversion in serum. Thus, given the poor response rates, and long-term risks of interferon-based therapies, we have to be selective in choosing our patients for a prolonged therapy. The long term results of HIDIT-1 study[6] where patients were treated for 48 wk vs HIDIT-2 study[7] when the treatment was extended for 92 wk were not much different. Thus, given the poor response rates, and long-term risks of interferon-based therapies, we have to be selective in choosing our patients for a prolonged therapy and we cannot make it a rule. Optimized HBsAg titer monitoring and checking HDV RNA levels may improve the outcome[14,15].
In our study, HBeAg reactive patients who seroconverted during the treatment had a less chance of relapse. One of our responder patients in the combination arm, who had a lower HBsAg level as compared to the average of the cohort, lost HBsAg during the treatment. Interferon based therapy is known to induce HBsAg seroconversion and it is usually associated with low pretreatment HBsAg levels[16].
We did not check for genotypes of HBV and HDV for this study as it is already known that the genotype of hepatitis D is 1[17] and of hepatitis B is D in our region[18]. We followed our patients for six months post treatment. Late HDV RNA relapses may occur after PEG-IFNα therapy of hepatitis delta and thus the term “sustained virological response” should be used with caution in HDV infection[19]. There was a possibility of a higher relapse rate in our patients if we had followed up our patients for a longer period.
In conclusion, combination treatment did not show any additional benefit in terms of HDV RNA suppression and HBsAg reduction as compared to PEG-IFNα alone. Liver fibrosis and HBsAg levels did not predict HDV RNA response. HDV RNA response at 24 wk, HBeAg seroconversion and any reduction in HBsAg levels during treatment may predict the patients who are going to have a better outcome.
We are thankful to Dr. Syed Salman Ali and Dr. Muhammad Mustafa for their help as coordinator in the initial phases of study.
Chronic hepatitis D is a difficult to treat infection. Six months post treatment response is seen only in one quarter of the patients treated with pegylated interferon alfa (PEG-IFNα).
In an attempt to improve the response of PEG-IFN, the authors combined entecavir which is a reverse transcriptase inhibitor.
This is the first study to evaluate the efficacy of subcutaneous PEG-IFN with oral entecavir compared to PEG-IFN alone for the treatment of hepatitis D infection.
This study showed that the combination treatment did not have any additional benefit in terms of hepatitis D virus (HDV) RNA suppression and hepatitis B surface antigen reduction as compared to PEG-IFN alone.
HDV is a small spherical enveloped RNA virus. It is considered to be a subviral satellite because it can propagate only in the presence of the hepatitis B virus. There is no satisfactory treatment available to treat this infection. However, PEG-IFN is often used.
The paper indicates in a randomized trial that the addition of entecavir to PEG-IFN does not increase efficacy vs PEG-IFN monotherapy in the treatment of chronic hepatitis D. The data are valuable as they extend and confirm previous anecdotal reports.
P- Reviewer: Cunha C, Gatselis NK, Rizzetto M S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
| 1. | Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HU. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr Health J. 2010;16 Suppl:S15-S23. [PubMed] |
| 2. | Mumtaz K, Hamid SS, Adil S, Afaq A, Islam M, Abid S, Shah HA, Jafri W. Epidemiology and clinical pattern of hepatitis delta virus infection in Pakistan. J Gastroenterol Hepatol. 2005;20:1503-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Abbas Z, Khan MA, Salih M, Jafri W. Interferon alpha for chronic hepatitis D. Cochrane Database Syst Rev. 2011;CD006002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Wedemeyer H. Hepatitis D revival. Liver Int. 2011;31 Suppl 1:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther. 2014;19:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 359] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 7. | Wedemeyer H, Yurdaydin C, Ernst S, Caruntu FA, Curescu MG, Yalcin K, Akarca US, Gurel SG, Zeuzem S, Erhardt A, Luth S, Papatheodoridis GV, Keskin O, Port K, Radu M, Celen MK, Ildeman R, Stift J, Heidrich B, Mederacke I, Hardtke S, Koch A, H. P. Dienes HP, Manns MP, HIDIT-2 Study Group.. Prolonged therapy of hepatitis delta for 96 weeks with pegylated-interferon-a-2a plus tenofovir or Placebo does not prevent HDV RNA relapse after Treatment: the HIDIT-2 study. J Hepatolol. 2014;60:S2-S3. |
| 8. | Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, Streinu-Cercel A, Wang JY, Idilman R, Reesink HW. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study). Hepatology. 2015;61:1512-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [PubMed] |
| 10. | Yurdaydin C. Treatment of chronic delta hepatitis. Semin Liver Dis. 2012;32:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, Heintges T, Häussinger D. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int. 2006;26:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, Pham BN, Maylin S, Bedossa P, Dény P. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology. 2006;44:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R, McBurney R, Schmid P, Albrecht J, Kleiner DE. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014;40:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Manesis EK, Schina M, Le Gal F, Agelopoulou O, Papaioannou C, Kalligeros C, Arseniou V, Manolakopoulos S, Hadziyannis ES, Gault E. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir Ther. 2007;12:381-388. [PubMed] |
| 15. | Ouzan D, Pénaranda G, Joly H, Halfon P. Optimized HBsAg titer monitoring improves interferon therapy in patients with chronic hepatitis delta. J Hepatol. 2013;58:1258-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Manesis EK, Hadziyannis ES, Angelopoulou OP, Hadziyannis SJ. Prediction of treatment-related HBsAg loss in HBeAG-negative chronic hepatitis B: a clue from serum HBsAg levels. Antivir Ther. 2007;12:73-82. [PubMed] |
| 17. | Moatter T, Abbas Z, Shabir S, Jafri W. Clinical presentation and genotype of hepatitis delta in Karachi. World J Gastroenterol. 2007;13:2604-2607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Abbas Z, Muzaffar R, Siddiqui A, Naqvi SA, Rizvi SA. Genetic variability in the precore and core promoter regions of hepatitis B virus strains in Karachi. BMC Gastroenterol. 2006;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Heidrich B, Yurdaydın C, Kabaçam G, Ratsch BA, Zachou K, Bremer B, Dalekos GN, Erhardt A, Tabak F, Yalcin K. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014;60:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |