Published online May 18, 2016. doi: 10.4254/wjh.v8.i14.616
Peer-review started: January 8, 2016
First decision: February 26, 2016
Revised: March 8, 2016
Accepted: April 20, 2016
Article in press: April 22, 2016
Published online: May 18, 2016
Processing time: 125 Days and 2 Hours
AIM: To investigate the combined diagnostic accuracy of acoustic radiation force impulse (ARFI), aspartate aminotransferase to platelet ratio index (APRI) and Forns index for a non-invasive assessment of liver fibrosis in patients with chronic hepatitis B (CHB).
METHODS: In this prospective study, 206 patients had CHB with liver fibrosis stages F0-F4 classified by METAVIR and 40 were healthy volunteers were measured by ARFI, APRI and Forns index separately or combined as indicated.
RESULTS: ARFI, APRI or Forns index demonstrated a significant correlation with the histological stage (all P < 0.001). According to the AUROC of ARFI and APRI for evaluating fibrotic stages more than F2, ARFI showed an enhanced diagnostic accuracy than APRI (P < 0.05). The combined measurement of ARFI and APRI exhibited better accuracy than ARFI alone when evaluating ≥ F2 fibrotic stage (Z = 2.77, P = 0.006). Combination of ARFI, APRI and Forns index did not obviously improve the diagnostic accuracy compared to the combination of ARFI and APRI (Z = 0.958, P = 0.338).
CONCLUSION: ARFI + APRI showed enhanced diagnostic accuracy than ARFI or APRI alone for significant liver fibrosis and ARFI + APRI + Forns index shows the same effect with ARFI + APRI.
Core tip: Chronic hepatitis B (CHB) is a major health problem in a lot of countries all over the world, particularly in China. An accurate staging of liver fibrosis is critical for prognosticating this disease. However, although it is still the golden standard, liver biopsy is hindered by its inherent drawbacks in clinical applications. In this study, we demonstrated that non-invasive methods, including acoustic radiation force impulse (ARFI), aspartate aminotransferase to platelet ratio index (APRI) and Forns index showed significant correlations with the histological staging results from liver biopsy. The combined measurement of ARFI and APRI had the best diagnostic accuracy, which provided an ideal and convenient non-invasive diagnostic method for the detection of hepatic fibrosis of CHB patients in clinical practice.
- Citation: Dong CF, Xiao J, Shan LB, Li HY, Xiong YJ, Yang GL, Liu J, Yao SM, Li SX, Le XH, Yuan J, Zhou BP, Tipoe GL, Liu YX. Combined acoustic radiation force impulse, aminotransferase to platelet ratio index and Forns index assessment for hepatic fibrosis grading in hepatitis B. World J Hepatol 2016; 8(14): 616-624
- URL: https://www.wjgnet.com/1948-5182/full/v8/i14/616.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i14.616
Chronic liver injury, such as chronic hepatitis B (CHB), may cause inflammation and necrosis of hepatocytes, leading to hepatic fibrosis. It is a long-term pathological change with certain possibility (about 20%) of progressing to liver cirrhosis[1]. Unlike cirrhosis, hepatic fibrosis is reversible at its early stage when proper clinical therapeutic interventions are applied[2]. Therefore, an accurate staging of liver fibrosis is critical for prognosticating this disease. To date, the gold standard for staging hepatic fibrosis is still the liver biopsy, which cannot be routinely performed because of its inherent limitations, such as pain, bleeding, inaccurate staging from sampling error, and variability of biopsy interpretation[3]. During the past decades, considerable efforts have been invested in developing non-invasive methods of assessments, which may provide accurate evaluation of liver fibrosis comparable to liver biopsy. Indeed, these non-invasive methods have several advantages such as high safety margin, simple, convenient, reproducible, and inexpensive.
Acoustic radiation force impulse (ARFI) is a new quantitative assessment method of estimating tissue stiffness through measurement of shear wave velocity (SWV, measured in m/s). Its quantitative representation is named as virtual touch tissue quantification, which gives an objective numerical evaluation of the tissue stiffness[4-6]. ARFI imaging offers a quantitative assessment of the hepatic parenchyma elasticity to non-invasively grade and stage hepatic fibrosis. It has been used to diagnose hepatic fibrosis of patients with CHB[7], hepatitis C[8], cirrhosis[9], and non-alcoholic fatty liver disease (NAFLD)[10]. In addition, ARFI is often performed with serum liver functions tests [e.g., alanine aminotransferase (ALT), aspartate aminotransferase (AST), total proteins, and albumin] to generate better prediction and evaluation of liver fibrosis[11]. Among these, AST platelet ratio (APRI) is a serum hepatic function test which has been proposed as a non-invasive tool for the assessment of liver fibrosis in CHB[12] or chronic hepatitis C[13]. Another important serum test is Forns index method, which uses simple obtained parameters including age, gamma-glutamyltransferase (GGT), cholesterol, and platelet count (PLT), but it requires a relatively complicated calculation[14]. One of the advantages of APRI and Forns index over the other non-invasive tests is that they are based on readily available blood tests and simple to use. Although these strategies have been widely applied in the past decade for hepatitis C evaluation[15,16], their accuracy for CHB grading are still not comparable with liver biopsy. Therefore, a combined use of these non-invasive methods may be another promising and practical diagnostic application in CHB patients. In the current study, we aimed to compare the accuracy among ARFI, APRI, Forns index and their combinations for non-invasive diagnosis grading and prognosis of human CHB-induced hepatic fibrosis.
This prospective study was approved by the ethical committee of Shenzhen Third People’s Hospital. All study procedures and methods were in accordance with the approved guidelines. All patients in this study were fully informed about the research protocol including the data handling and the privacy of personal data. After this procedure, patients signed the written consent. A total of 246 subjects were consecutively enrolled in this study, including 206 CHB subjects and 40 healthy subjects. These 206 CHB cases were selected from 245 CHB patients diagnosed by liver biopsy in Shenzhen Third People’s Hospital, from May 2011 to December 2014. Of the 206 CHB patients, there were 39 female cases (18.9%) and 167 male cases (81.1%). Inclusion criteria are: (1) patients must be 18-65 years old; (2) with hepatitis B surface antigen positive for more than 6 mo; (3) without receiving antiviral treatment before this study; (4) ALT and AST were < 2 × upper limit of normal (ULN) in the past 6 mo; (5) 18.5 < body mass index (BMI) < 31.0; (6) length of liver biopsy tissue ≥ 15 mm and contains at least 10 periportal areas; (7) hemoglobin > 90 g/L, prothrombin time 11-15.1 s; (8) activated partial thromboplastin time and thrombin time were at a normal range; and (9) cardiac and renal functions were normal. Negative for the following: Human immunodeficiency virus, hepatitis A virus, hepatitis C virus (HCV), hepatitis D virus, hepatitis E virus super-infection or co-infection, auto-immune liver diseases, alcoholic steatosis, NAFLD, hepatocellular carcinoma (HCC), pregnancy, ascites, as well as jaundice. Of the 245 eligible CHB patients, 39 were excluded because of the following: NAFLD (n = 10), received antiviral treatment before this study (n = 8), jaundice (n = 5), alcoholic steatosis (n = 6), HCV infection (n = 2), auto-immune liver disease (n = 1), with age < 18 (n = 4), with age > 65 (n = 1), and declined to participate (n = 2). Healthy group consisted of 40 volunteers, with 30 males and 10 females, aged range from 20-53 years old, with mean age of 39.8 ± 11.45 years and no hepatitis B virus (HBV) or HCV infection, no hypertension, diabetes, fatty liver and other apparent diseases. The BMI of healthy subjects were between 18.5 and 31.0. Other parameters were similar to the CHB patients. All CHB patients were examined by ARFI one day before or on the day of liver biopsy. All the subjects had blood or sera drawn for the detection of platelet and fibrotic serological markers.
Liver biopsy tissue specimens were collected by needle puncture (MN1613, Bard Biopsy Systems, Tempe, AZ) under the Color Doppler Ultrasound guidance in a separate clinic setting for diagnostic purposes. The liver specimen was 15-20 mm in length, including at least 10 portal vein areas. Then it was embedded by paraffin and stained by Sirius Red (Sigma-Aldrich, St. Louis, MO). Liver fibrosis stage was assessed by the METAVIR scoring system (F0 = no fibrosis; F1 = portal fibrosis without septa; F2 = portal fibrosis and a few septa; F3 = numerous fibrosis without cirrhosis; and F4 = cirrhosis)[17]. The METAVIR scoring system was previously used in other reports on CHB[18,19]. Two independent pathologists were responsible for the staging of all samples without additional information about the specimens they checked.
The detection of ARFI in the liver was performed under fasting conditions using Siemens Acuson S2000 with probe detector 4C1, frequency 2.0-4.0 MHz (Siemens Healthcare, Erlangen, Germany) according to routine instructions. ARFI was mainly conducted by a radiologist (Dong CF) with assistant from another physician and a nurse. Dong CF has 11-year experience in clinical radiology and 4-year experience in ARFI diagnosis. Form of the liver capsule and the echogenicity of hepatic parenchyma were recorded. Detection of SWV (m/s) of hepatic segments s5, s6, s7 and s8 was repeated for 3 times and the mean values were calculated. Thus, 12 measurements of hepatic segments s5, s6, s7, s8 were recorded. Our pilot study in healthy volunteers showed that when compared with conventional ARFI protocol (mean value from 10 measurements), the current protocol exhibited similar results with smaller standard deviation (1.08 ± 0.21 m/s vs 1.11 ± 0.12 m/s; t = 0.6794, P > 0.05). This is consistent with a report that showed the reproducibility of measurements in the right lobe was higher[20]. Images and data of ARFI were saved for analysis.
AST was determined in the same laboratory prior to the liver biopsy using Siemens ADVIA 2400 Chemistry system (Siemens Healthcare). Enzymatic activity was measured at 37 °C, according to International Federation of Clinical Chemistry standards. Platelet count was assessed by an automatic blood cell analyzer (XE-5000 Automated Hematology System, Sysmex, Lincolnshire, IL). The ULN range of AST was considered as 40 U/L.
APRI = AST(/ULN)/PLT(109/L) × 100.
Forns index = 7.811 - 3.131 × Ln(PLT) + 0.781 × Ln(GGT) + 3.467 × Ln(age) - 0.014 × (cholesterol)
A logistic regression analysis model for hepatic fibrosis ≥ F2 has been established by using the ENTER method.
Continuous normal distribution data were represented with means ± SD. Categorical normal distribution data were represented with median ± quartile (M ± Q). Kruskal-Wallis test was used to analyze the differences among these different groups. When there was a statistical significance (P < 0.05), a post-hoc Bonferroni test was applied to analyze data between two groups. P < 0.05 was considered to be statistically significant using a SPSS 13.0, IBM, Armonk, NY. The box plot was used to record the mean and degree of variation. MedCalc software (Ostend, Belgium) was used to draw receiver operating characteristic curve (ROC) and calculate cut-off value, sensitivity, specificity, positive predictive values, negative predictive values, AUROC of ARFI and APRI for every liver fibrotic stage. The ROC curve of two variables combination (ARFI + APRI and ARFI + Forns index) and three variables combination (ARFI + APRI + Forns index) for significant hepatic fibrosis (≥ F2) was also analyzed. When AUROC > 0.5, the closer of AUROC to 1, the better diagnostic outcome it provided. Comparison of AUROC among these parameters and their combination was analyzed by the Delong test[21].
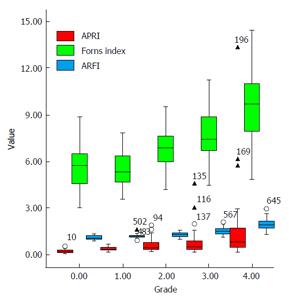
Basic information (e.g., age and gender) and assessment results of ARFI, APRI, and Forns index of all subjects were shown in Table 1. The average ages of subjects with significant or serious fibrosis (F2, F3 and F4) were significantly higher than subjects with mild fibrosis (F1) (P = 0.009 for F2 vs F3, P < 0.001 for F2 vs F4, and P < 0.001 for F3 vs F4). Also, male patients showed higher incidence of hepatic fibrosis (from F1 to F4) than female patients. The differences of ARFI results among F0, F1, F2, F3 and F4 groups were significant (P < 0.05). For Forns index, except for F0 and F1 group, the differences among other groups were significant (P < 0.05). Results of APRI indicated that only F4 showed significant change from other groups (F0, F1, F2 and F3) (all P < 0.001), while the F1, F2, and F3 groups showed significantly higher values than the F0 group (all P < 0.001) (Table 1).
| Group | Age (yr) | Gender (male/female) | BMI | ARFI | APRI | Forns index |
| F0 (n = 40) | 39.8 ± 11.45 | 30/10 | 22.91 ± 2.31 | 1.09 (1.01, 1.21) | 0.19 (0.14, 0.28) | 5.58 ± 1.33 |
| F1 (n = 41) | 33.07 ± 7.971 | 33/8 | 22.37 ± 2.24 | 1.19 (1.15, 1.28)1 | 0.34 (0.28, 0.44)1 | 5.60 ± 1.19 |
| F2 (n = 52) | 38.27 ± 7.662 | 43/9 | 22.26 ± 2.41 | 1.31 (1.21, 1.43)1,2 | 0.42 (0.32, 0.64)1 | 6.73 ± 1.091,2 |
| F3 (n = 59) | 39.83 ± 8.732 | 47/12 | 22.44 ± 2.57 | 1.52 (1.35, 1.64)1,2,3 | 0.45 (0.32, 0.86)1,2 | 7.58 ± 1.551,2,3 |
| F4 (n = 54) | 43.85 ± 10.811,2,3,4 | 44/10 | 22.35 ± 2.47 | 1.92 (1.74, 2.14)1,2,3,4 | 0.80 (0.51, 1.68)1,2,3,4 | 9.43 ± 2.301,2,3,4 |
| χ2/F | 7.907 | 0.947 | 0.477 | 176.043 | 107.992 | 49.501 |
| P value | < 0.001 | 0.918 | 0.753 | < 0.001 | < 0.001 | < 0.001 |
The median, quartile, minimum value, maximum value and outlier of ARFI, APRI and Forns index were shown in box type image (Figure 1). There was a high correlation between the staging of ARFI/APRI/Forns index and the hepatic histology, with correlation coefficient 0.845 (P < 0.001), 0.641 (P < 0.001) and 0.644 (P < 0.001), respectively (Table 2). In ENTER model, Y axis was the result from liver biopsy and the X axis was the results from ARFI + APRI or ARFI + Forns Index combined assessments. The equation for ARFI + APRI was y = -13.27 + 9.11 ARFI + 5.03 APRI, while the equation for ARFI + Forns index was y = -15.08 + 8.67 ARFI + 0.70 Forns index.
| Histological staging | Noninvasive test | Correlation (Spearman coefficient) | 95%CI | P value |
| METAVIR classification | ARFI | 0.845 | 0.805-0.877 | < 0.001 |
| APRI | 0.641 | 0.561-0.709 | < 0.001 | |
| Forns index | 0.644 | 0.564-0.711 | < 0.001 |
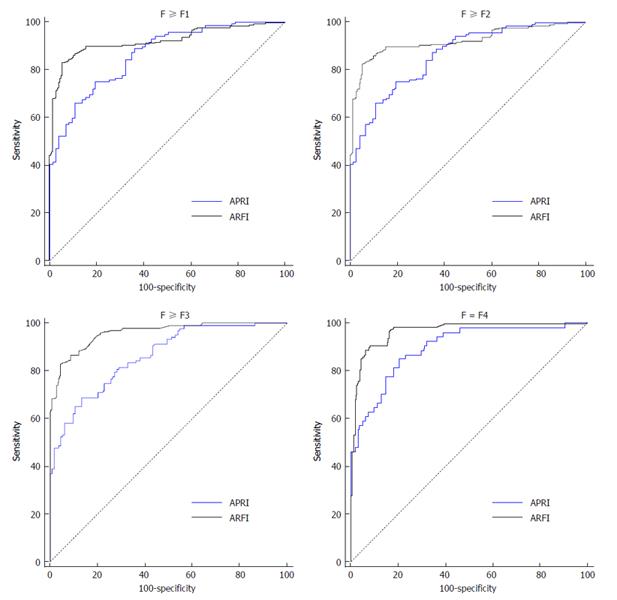
There were significantly different interval ranges between different liver fibrotic stages and the corresponding ARFI and APRI results. In order to determine the cut-off value of each fibrotic stage, we applied ROC to analyze the data from both ARFI and APRI (Figure 2). From the result, it showed that the diagnostic performance of ARFI for predicting stages more than F2, F3 and F4 was 91% (95%CI: AUROC = 0.87-0.95, P < 0.05), 94% (95%CI: AUROC = 0.90-0.96, P < 0.05), 96% (95%CI: AUROC = 0.93-0.98, P < 0.05), and the best cut-off value of F2, F3 and F4 was 1.29, 1.43 and 1.62 m/s. However, APRI measurement showed decreased accuracy of diagnosing significant fibrosis when compared with ARFI (Table 3).
| ≥ F1 | ≥ F2 | ≥ F3 | F4 | |
| ARFI | ||||
| Cut-off (m/s) | 1.26 | 1.29 | 1.43 | 1.62 |
| Sensitivity | 76.2% (69.80-81.90) | 83.6% (77.10-88.90) | 82.3% (74.00-88.80) | 90.7% (79.70-96.90) |
| Specificity | 95.0% (83.10-99.40) | 90.1% (89.50-97.60) | 89.5% (83.00-94.10) | 92.2% (87.40-95.60) |
| PPV | 99.1% (96.20-99.90) | 94.5% (91.90-99.10) | 86.9% (79.10-92.70) | 76.0% (64.40-86.30) |
| NPV | 35.9% (22.50-47.40) | 73.0% (63.10-81.40) | 85.6% (78.60-91.00) | 97.2% (93.70-99.10) |
| AUROC | 0.90 (0.86-0.94)a | 0.91 (0.87-0.95)a | 0.94 (0.90-0.96)a | 0.96 (0.93-0.98)a |
| APRI | ||||
| Cut-off (m/s) | 0.30 | 0.41 | 0.49 | 0.44 |
| Sensitivity | 84.0% (78.20-88.70) | 68.5% (60.80-75.50) | 63.7% (54.10-72.60) | 83.3% (70.70-92.10) |
| Specificity | 85.0% (70.20-94.30) | 82.7% (72.70-90.20) | 79.7% (71.90-86.20) | 67.2% (70.10-73.80) |
| PPV | 97.6% (94.20-99.30) | 89.0% (82.20-93.80) | 72.8% (62.90-81.20) | 41.7% (32.30-51.60) |
| NPV | 42.7% (30.00-56.10) | 56.3% (46.80-65.40) | 72.1% (64.00-79.20) | 93.5% (87.90-97.00) |
| AUROC | 0.92 (0.88-0.95)a | 0.84 (0.79-0.89)a | 0.79 (0.73-0.84)a | 0.82 (0.76-0.86)a |
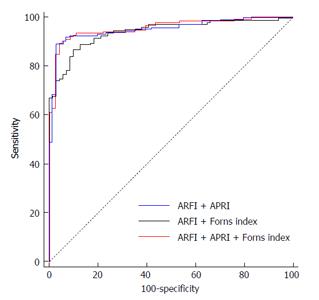
Firstly we established a logistic regression analysis model for hepatic fibrosis ≥ F2 in which the Y axis was the result from liver biopsy and the X axis was the results from combined ARFI + APRI/ARFI + Forns index assessment (Table 4). From the AUROC results of Table 5, when evaluating patients with hepatic fibrosis ≥ F2, there was a significant change between the AUROCs of ARFI + APRI and ARFI alone (0.940 and 0.913, respectively; Z = 2.77, P = 0.006), also between ARFI + Forns index and ARFI alone (0.933 and 0.913, respectively; Z = 2.091, P = 0.037), ARFI + APRI + Forns index and ARFI alone (0.944 and 0.913, respectively; Z = 2.893, P = 0.004), indicating an enhanced screening ability of the combined assessment than ARFI alone. However, the change between ARFI + APRI and ARFI + APRI + Forns index was not significant (0.940 and 0.944, respectively; Z = 0.958, P = 0.338), suggesting that Forns index cannot further improve the diagnostic accuracy for staging hepatic fibrosis ≥ F2 when using a combined method of ARFI + APRI (Figure 3).
| Combination | Variable | RC | SD of RC | Wald | P value | OR | 95%CI of OR |
| ARFI + APRI | ARFI | 9.11 | 1.48 | 37.68 | < 0.001 | 9085.54 | 494.92-166789.07 |
| APRI | 5.03 | 1.30 | 15.07 | < 0.001 | 153.01 | 12.07-1939.04 | |
| Constant | -13.27 | 1.95 | 46.09 | < 0.001 | - | - | |
| ARFI + Forns index | ARFI | 8.67 | 1.44 | 36.16 | < 0.001 | 5824.00 | 345.12-98280.97 |
| Forns index | 0.70 | 0.17 | 16.27 | < 0.001 | 2.01 | 1.43-2.82 | |
| Constant | -15.08 | 2.08 | 52.68 | < 0.001 | - | - |
| Comparison | AUROC | Difference | 95%CI | Z | P value | |
| Lower limit | Upper limit | |||||
| ARFI | 0.913 | 0.027 | 0.008 | 0.046 | 2.770 | 0.006 |
| ARFI + APRI | 0.940 | |||||
| ARFI | 0.913 | 0.020 | 0.001 | 0.040 | 2.091 | 0.037 |
| ARFI + Forns index | 0.933 | |||||
| ARFI | 0.913 | 0.031 | 0.010 | 0.053 | 2.893 | 0.004 |
| ARFI + APRI + Forns index | 0.944 | |||||
| ARFI + APRI | 0.940 | 0.007 | -0.011 | 0.025 | 0.728 | 0.466 |
| ARFI + Forns index | 0.933 | |||||
| ARFI + APRI | 0.940 | 0.005 | -0.005 | 0.014 | 0.958 | 0.338 |
| ARFI + APRI + Forns index | 0.944 | |||||
| ARFI + Forns index | 0.933 | 0.011 | -0.001 | 0.023 | 1.789 | 0.074 |
| ARFI + APRI + Forns index | 0.944 | |||||
To date, the gold standard for the diagnosis of liver fibrosis remains to be liver biopsy. In most circumstances, patients find it difficult to accept liver biopsy due to its complications. From 2009, with the introduction of ARFI, the clinical research on non-invasive assessment of fibrosis rapidly progressed. As an advanced imaging technology, ARFI is capable of providing biomechanical information on the tissue stiffness and elasticity using conventional ultrasound scanning of anatomical location and structure[22,23]. However, its utility, particularly in combination with other non-invasive methods in hepatitis B, has not been adequately evaluated.
In the current study, CHB patients with different stages of liver fibrosis were diagnosed by ARFI, APRI, Forns index and their combined assessments. Our results demonstrated that the mean SWV value from ARFI was highly correlated with the staging of liver fibrosis classified by liver biopsy (METAVIR classification). This result indicated that biomechanical properties (e.g., hepatic elasticity and stiffness) had progressed from liver fibrosis to cirrhosis during the development of CHB, which was consistent with the pathological progression of hepatocyte degeneration, necrosis, inflammation reaction, hepatocyte regeneration, formation of connective tissue fiber intervals, and liver lobule structural failure during the course of liver fibrosis of HBV infection[24].
With the progression of liver fibrosis from F2 to F4, the effectiveness of ARFI on the diagnosis of liver fibrosis also increased. That is, when the value of SWV was lower than 1.29 m/s (clinically F0 and F1 patients), hepatic fibrosis could be unlikely significant. SWV higher than 1.43 m/s could be likely considered as an indication for serious liver fibrosis (F3, sensitivity 82.3% and specificity 89.5%), and SWV > 1.62 m/s could be diagnosed as early cirrhosis (F4, sensitivity 90.7% and specificity 92.2%). In addition, when they were used independently, ARFI was the best way for the diagnosis of fibrosis ≥ F2; ARFI provides a dynamic technical support for non-invasive diagnosis of liver fibrosis. This result is in line with a report found that ARFI correlated well with liver biopsy and thus was a reliable ultrasound-based method for the assessment of advanced fibrosis induced by CHB[25].
Currently it is difficult for non-invasive diagnostic methods to differentiate F0 and F1 fibrotic stages. However, in this study, we found that there was a significant change of ARFI readings between the F0 and F1 groups (Table 1). It is known that stage F2 possesses significant diagnostic value in determining the progression of liver disease and anti-viral therapy choice. At this stage, patients have more risk in developing complications such as portal hypertension, cirrhosis, and HCC than patients without significant liver fibrosis[26]. If patients receive anti-viral therapy promptly during this period, it is possible to retard or even reverse the pathological progression of fibrosis[27]. Thus, early accurate diagnosis and appropriate therapy to patients at F2 fibrosis evidently decreases the morbidity and mortality of patients with CHB[28,29].
Similar to the FibroScan method which is partially affected by obesity[30], ARFI also has some disadvantages. For example, certain hepatic disorders (e.g., ascites and acute icteric hepatitis) may affect the ARFI results. However, in our study, all the enrolled subjects including obese patients with BMI of 30.81 successfully got SWV values. Thus, ARFI may have a wider application range than FibroScan. In general, ARFI overcome a spectrum of disadvantages of conventional ultrasound technologies, such as no manual operation of pressing, improved depth limitation (5 cm of the earlier machines and 8 cm of the newer machines) and location of imaging. Compared to other methods, ARFI has no pain, with good reproducibility of data and simple operation. Indeed, ARFI is potentially limited by patients with a BMI > 40 or after contrast-enhanced ultrasonography. Thus, its combination with other non-invasive methods is necessary to enhance the diagnostic accuracy[31].
Currently, serological diagnostic assays for non-invasive assessment of liver fibrosis are available including direct and indirect methods. The main purpose of these methods is to identify the existence of fibrosis but not the grading or staging. In this study, APRI and Forns index were also used to stage liver fibrotic stage. Although the sensitivity and specificity of these methods for the diagnosis of liver fibrosis was lower than ARFI, they partially reflected the pro-inflammatory response and hepatic compensation. The most important finding of this study was that combined measurement of ARFI and APRI exhibited better accuracy than ARFI or APRI alone when evaluating ≥ F2 fibrosis stage. Combination of ARFI, APRI and Forns index did not further improve the diagnostic effect than the combination of ARFI and APRI.
In conclusion, ARFI, APRI and Forns index correlated well with the histological liver fibrosis stages in CHB patients. ARFI showed better accuracy than APRI when evaluating F2, F3 and F4 stages. Combined check with ARFI and APRI showed a significant enhancement of diagnostic accuracy than ARFI or APRI alone. ARFI + APRI exhibited similar enhancement of diagnostic accuracy of hepatic fibrosis with ARFI + APRI + Forns index when evaluating fibrotic stages more than F2 in CHB patients. This study provides an ideal and convenient non-invasive diagnostic method for the detection of hepatic fibrosis of CHB patients in clinical practice.
Hepatitis B virus (HBV) infection-mediated chronic injury of hepatocytes induces fibrosis, which may progress to end-stage liver diseases like cirrhosis and hepatocellular carcinoma. Thus, accurate grading of hepatic fibrosis is important for the application of appropriate intervening strategy to retard the progression. To date, the “golden standard” of fibrotic grading is still liver biopsy, which wide clinical application is hindered by its inherent drawbacks. In recent years, biomechanical-based ultrasonic elastography received mass attention. However, several clinical studies found that the sole application of ultrasonic elastography may bring evident errors in diagnosing hepatic fibrosis. It is suggested that a combination of ultrasonic elastography and serum liver functions tests holds the potential to overcome those disadvantages.
There are an increasing number of hospitals using non-invasive ultrasonic elastography techniques, such as acoustic radiation force impulse (ARFI) and Fibroscan to grade hepatic fibrosis of chronic hepatitis B (CHB) patients in China and chronic hepatitis C patients in Western countries. Combination of different ultrasonic elastography techniques has been reported by a number of reports. However, few studies investigate the accuracy of the combination of ultrasonic elastography and serum liver functions tests.
This study evaluated the accuracy of one ultrasound elastography method (ARFI) and two serum biochemical tests [aspartate aminotransferase to platelet ratio index (APRI) and Forns index], as well as their combination in the assessment of liver fibrosis in CHB. The authors found that ARFI + APRI exhibited similar enhancement of diagnostic accuracy of hepatic fibrosis with ARFI + APRI + Forns index when evaluating fibrotic stages more than F2 in CHB patients.
The data in this study suggest that doctor can yield favorable outcomes through the accumulation of technical experience. Furthermore, this study also provides readers with important information regarding an ideal and convenient non-invasive diagnostic method for the grading of hepatic fibrosis of CHB patients.
ARFI imaging involves mechanically exciting a localized region of interest in the tissue with acoustic radiation force to induce a shear wave in the tissue. The displacement of the shear wave is tracked using a pulse-echo mode ultrasound at several lateral locations along the propagation path of the shear wave. By measuring the time to peak displacement at each location, the shear wave velocity was calculated, which is directly related to the elasticity of the tissue. APRI = AST(/ULN)/PLT(109/L) × 100. Forns index = 7.811 - 3.131 × Ln(PLT) + 0.781 × Ln(GGT) + 3.467 × Ln(age) - 0.014 × (cholesterol).
This is a good attempt by Dong et al to compare ARF1, APR1 and Forns to determine fibrosis stage in chronic HBV patients. As these are not new techniques for fibrosis evaluation and they wanted to establish that combination of ARF1/ APRI and ARF1/ Forns as better non-invasive technique.
P- Reviewer: Banerjee S, Malnick SDH, Pai CG S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Pinzani M, Vizzutti F. Fibrosis and cirrhosis reversibility: clinical features and implications. Clin Liver Dis. 2008;12:901-913, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Nguyen D, Talwalkar JA. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:2107-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Kaminuma C, Tsushima Y, Matsumoto N, Kurabayashi T, Taketomi-Takahashi A, Endo K. Reliable measurement procedure of virtual touch tissue quantification with acoustic radiation force impulse imaging. J Ultrasound Med. 2011;30:745-751. [PubMed] |
| 5. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010;9:289-293. [PubMed] |
| 9. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Demozzi E, Gallotti A, Pozzi Mucelli R. Acoustic radiation force impulse of the liver. World J Gastroenterol. 2013;19:4841-4849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3235] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 13. | Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, Kim DJ, Jun SY, Park CK. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 15. | Jeong JY, Kim TY, Sohn JH, Kim Y, Jeong WK, Oh YH, Yoo KS. Real time shear wave elastography in chronic liver diseases: accuracy for predicting liver fibrosis, in comparison with serum markers. World J Gastroenterol. 2014;20:13920-13929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 16. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 17. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 18. | Myers RP, Tainturier MH, Ratziu V, Piton A, Thibault V, Imbert-Bismut F, Messous D, Charlotte F, Di Martino V, Benhamou Y. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol. 2003;39:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 254] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Poynard T, Zoulim F, Ratziu V, Degos F, Imbert-Bismut F, Deny P, Landais P, El Hasnaoui A, Slama A, Blin P. Longitudinal assessment of histology surrogate markers (FibroTest-ActiTest) during lamivudine therapy in patients with chronic hepatitis B infection. Am J Gastroenterol. 2005;100:1970-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J, Bertrais S, Gallois Y, Calès P, Aubé C. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13220] [Cited by in RCA: 13268] [Article Influence: 358.6] [Reference Citation Analysis (0)] |
| 22. | Behler RH, Nichols TC, Zhu H, Merricks EP, Gallippi CM. ARFI imaging for noninvasive material characterization of atherosclerosis. Part II: toward in vivo characterization. Ultrasound Med Biol. 2009;35:278-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 632] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 25. | Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Poynard T, Halfon P, Castera L, Munteanu M, Imbert-Bismut F, Ratziu V, Benhamou Y, Bourlière M, de Ledinghen V. Standardization of ROC curve areas for diagnostic evaluation of liver fibrosis markers based on prevalences of fibrosis stages. Clin Chem. 2007;53:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 28. | Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Poynard T, Halfon P, Castera L, Charlotte F, Le Bail B, Munteanu M, Messous D, Ratziu V, Benhamou Y, Bourlière M. Variability of the area under the receiver operating characteristic curves in the diagnostic evaluation of liver fibrosis markers: impact of biopsy length and fragmentation. Aliment Pharmacol Ther. 2007;25:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |