Published online Apr 28, 2015. doi: 10.4254/wjh.v7.i6.910
Peer-review started: September 2, 2014
First decision: November 27, 2014
Revised: January 10, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: April 28, 2015
Processing time: 241 Days and 12.8 Hours
Advanced cholangiocarcinoma is associated with poor prognostic survival and has limited therapeutic options available at present. The importance of angiogenesis and expression of pro-angiogenic factors in intrahepatic forms of cholangiocarcinoma suggest that therapies targeting angiogenesis might be useful for the treatment of this disease. Here we report three cases of patients with advanced intrahepatic cholangiocarcinoma progressive after standard chemotherapy and treated with sunitinib 50 mg/d in 6-wk cycles of 4 wk on treatment followed by 2 wk off treatment (Schedule 4/2). In all three patients, sunitinib treatment was associated with a sustained disease control superior to 4 mo, patients achieving either a partial response or stable disease. A reduction in tumor size and density was observed in all cases, suggesting tumor necrosis as a result of sunitinib treatment in these patients. In addition, sunitinib was generally well tolerated and the occurrence of side effects was managed with standard medical interventions, as required. Our results suggest that sunitinib therapy may be associated with favorable outcomes and tolerability in patients with advanced cholangiocarcinoma. Those observations contributed to launch a prospective phase II multicenter trial investigating sunitinib in advanced intrahepatic cholangiocarcinoma (SUN-CK study; NCT01718327).
Core tip: No systemic therapy after progression on platinum-based chemotherapy is currently approved. Based on imaging hypervascular pattern and molecular expression of vascular endothelial growth factor, we evaluated sunitinib, a multikinase inhibitor as second line treatment in patients with advanced intrahepatic cholangiocarcinoma. We report 3 cases of disease control lasting 4-16 mo that provide the rational for developing prospective clinical trials with sunitinib in second line for advanced intrahepatic cholangiocarcinoma.
- Citation: Dreyer C, Sablin MP, Bouattour M, Neuzillet C, Ronot M, Dokmak S, Belghiti J, Guedj N, Paradis V, Raymond E, Faivre S. Disease control with sunitinib in advanced intrahepatic cholangiocarcinoma resistant to gemcitabine-oxaliplatin chemotherapy. World J Hepatol 2015; 7(6): 910-915
- URL: https://www.wjgnet.com/1948-5182/full/v7/i6/910.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i6.910
While cholangiocarcinoma is the second-most common primary hepatic tumor after hepatocellular carcinoma (HCC), it is a rare disease for which there are few therapeutic options[1]. The only curative treatment is surgical resection; however, this is only viable for localized disease. Resectable cholangiocarcinoma is associated with frequent recurrences and a five-year survival rate of 20%-40% following surgery[1]. Treatment with cisplatin plus gemcitabine is associated with moderate efficacy when disease recurs[2]; however, for patients presenting with disease progression following first-line therapy, there is currently no consensus on the best treatment option.
Angiogenesis and the expression of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), play an important role in the pathogenesis of biliary tract cancers, including cholangiocarcinoma[3-5]. In addition, an association between microvessel density and inferior curative resection rate and local recurrence has also been observed[6]. In addition, our pathology team and other have showed that intrahepatic forms of cholangiocarcinoma are associated with high VEGF expression in comparison to hilar cholangiocarcinoma and that VEGF expression level correlates with poor prognosis[7,8]. Anti-angiogenic agents, such as the humanized anti-VEGF receptor (VEGFR) monoclonal antibody, bevacizumab, in combination with the receptor tyrosine kinase inhibitor erlotinib, have demonstrated activity in biliary tract carcinomas. In one study, among 49 patients with unresectable biliary tract cancer treated with bevacizumab 5 mg/kg intravenously on days 1 and 15 and erlotinib 150 mg by continuous daily dosing, time to progression was 4.4 mo and 6 patients demonstrated a partial response (PR)[9]. Targeted agents, such as sorafenib, are now commonly used in HCC, another disease in which angiogenesis plays an integral role, yielding prolonged survival with acceptable toxicity[10]. Recent advances in the knowledge of molecular alterations underlying cholangiocarcinoma support the need of better patient selection for appropriate medical therapy in this disease[11]. Together, those data suggest that anti-angiogenic therapy may confer benefits in the treatment of particular subtypes of cholangiocarcinoma.
We report here three cases of patients with recurrent intrahepatic cholangiocarcinoma who showed promising results under therapy with sunitinib, in the absence of other validated therapeutic options.
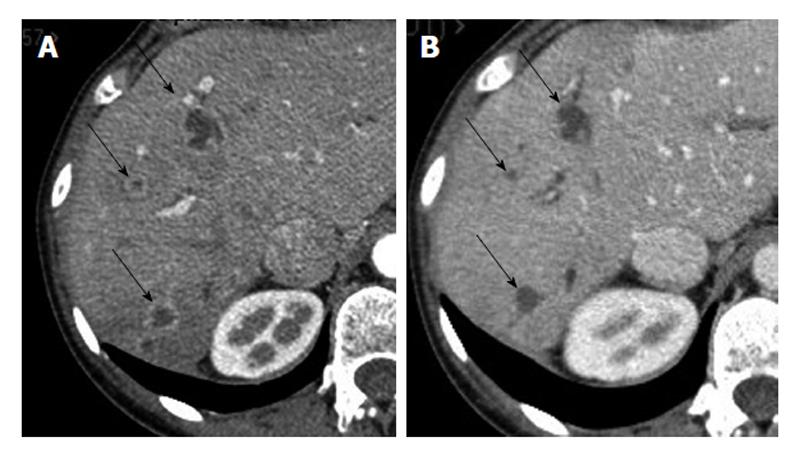
The patient, referred to our center for an intrahepatic tumor in the right lobe of the liver revealed by abdominal pain and was treated with surgical resection. Pathological examination showed a 10 cm cholangiocarcinoma, with multiple satellite nodules and vascular, perineural, and regional lymph node involvement. Given the presence of multiple risk factors for recurrence, including large tumor size, multifocal nature, and lymph node involvement, adjuvant combination chemotherapy with 6 mo of gemcitabine 1000 mg/m² and oxaliplatin 85 mg/m² every two weeks was initiated after multidisciplinary consultation and patient consent. Four months after completion of adjuvant chemotherapy, intrahepatic recurrence in liver right lobe was diagnosed on computed tomography (CT) scan, and second-line chemotherapy with irinotecan plus 5-fluorouracil (5-FU) and leucovorin (FOLFIRI) (irinotecan 180 mg/m², 5-FU 400 mg/m² bolus, leucovorin 400 mg/m² then 5-FU 2400 mg/m² as a 47-h infusion every two weeks) was initiated. After three months of treatment, the patient exhibited stable disease (SD) and therapy was continued for an additional three months. At the time of second evaluation (following 6 mo of FOLFIRI therapy; 18 mo after initial diagnosis), a CT scan showed tumor progression with peripheral rim enhancement suggesting hypervascular lesions (Figure 1A). As the patient was young with excellent performance status (PS) and with vascularized lesions on CT scans, treatment with sunitinib 50 mg/d on schedule 4/2 (4 wk on treatment followed by two weeks off treatment) was initiated. An early CT scan was performed after one month of treatment and showed the lesions to be hypodense, suggesting decrease tumor vasculature induced by anti-angiogenic treatment. After two months of treatment, the dose of sunitinib was reduced to 37.5 mg/d due to treatment-related grade 3 thrombocytopenia, asthenia and peripheral edema. Following four months of treatment, the patient had SD with disappearance of the hypervascular aspect of the tumor lesions (Figure 1B). In the context of persistent tumor stabilization in a young patient with excellent, sustained PS (0), surgical resection of the recurrent tumor was performed, consisting of repeated right hepatectomy associated with resection of segment IV. Sunitinib was stopped 3 wk before surgery. Pathological examination confirmed the diagnosis of a multifocal cholangiocarcinoma, consisting of tumor nodules with a fibro-hyaline center with multifocal vascular involvement. Sunitinib treatment was resumed three weeks after surgery at a dose of 37.5 mg/d but had to be stopped two months later due to a biliary fistula. Since the performance status of the patient further deteriorated, she was transferred to a palliative care unit in a primary center care close her home; the last news being available eight months after the second surgery.
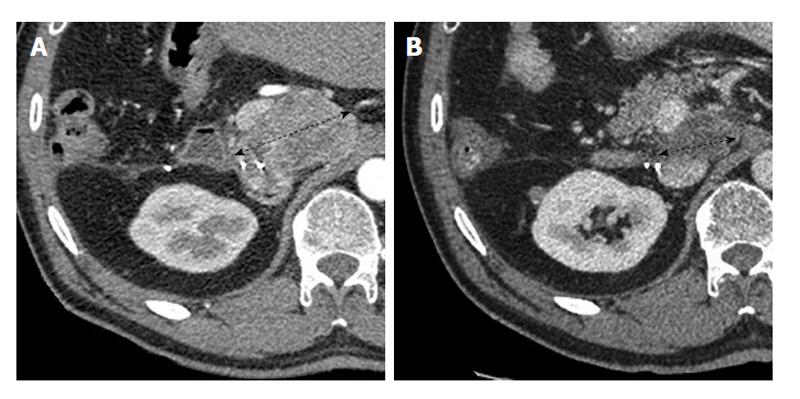
The patient complained for 6 mo of intermittent abdominal pain; imaging including CT-scan and MRI revealed a 6 cm tumor of the segment 6 of liver. A biopsy was performed and showed a low-differentiated carcinoma proliferation but immunohistochemistry staining was inconclusive. A right hepatectomy was performed. Final pathological examination and immunoassaying of the surgical specimen confirmed the diagnosis of an 8 cm intrahepatic cholangiocarcinoma with satellite nodules, vascular embolism and perineural involvement. Given the risk factors of recurrence, 6 mo of adjuvant combination chemotherapy with gemcitabine 1000 mg/m² and oxaliplatin 85 mg/m² (GEMOX) every 15 d was administered. One month after completion of adjuvant chemotherapy, a 6 cm retroperitoneal lymphadenopathy was detected by CT scan (Figure 2A). As this was a unique recurrence in a patient with excellent PS (0), lymph node dissection was scheduled. However during the explorative laparotomy, the lesion was deemed unresectable due to regional tumor adherences. After multidisciplinary discussion and patient consent, systemic therapy with sunitinib 50 mg/d on schedule 4/2 was initiated. After one month of treatment, CT scan showed extensive hypodensity of the lesion suggesting tumor necrosis, although sunitinib dose reduction to 37.5 mg/d was required due to treatment-related grade 3 thrombocytopenia. No further major toxicity was observed following dose reduction of sunitinib. After three months of treatment, CT scan showed a 25% reduction in tumor size, and sunitinib was recommenced at 37.5 mg/d with good tolerability. After four months of treatment, a PR, according to the response evaluation criteria in solid tumors (RECIST) criteria, was observed with increased necrosis of the tumor (Figure 2B). Sunitinib treatment was maintained for a total of 18 mo before disease progression was observed. The patient further developed jaundiced due to biliary compression requiring biliary stents. Gradually his general condition deteriorated and he died 7 mo after sunitinib interruption.
The third patient was diagnosed with an intrahepatic cholangiocarcinoma, associated with epigastric pain. Ultrasound examination revealed a 6 cm tumor in the left lobe of the liver, and a 9 mm satellite nodule in the right lobe. The patient was treated with six cycles of GEMOX (gemcitabine 1000 mg/m², oxaliplatin 85 mg/m²), which was well tolerated. CT scan demonstrated stable disease in the left lobe of the liver but the occurrence of new lesions in the right lobe. Because of this tumor progression, treatment was changed to a combination of cisplatin and epirubicin. After nine treatment cycles, a further CT scan revealed again tumor progression. As the patient had a good PS (0), treatment was initiated with sunitinib 50 mg/d on Schedule 4/2 after multidisciplinary discussion and patient consent. A CT scan performed three weeks after initiating sunitinib treatment demonstrated tumor hypodensity and a 15% reduction in lesion size. Following three months of treatment, the lesions were stable and the patient experienced moderate toxicity with grade 1 asthenia, grade 1 hypertension, and grade 3 thrombocytopenia. Subsequently, sunitinib treatment was continued at a dose of 37.5 mg/d. After a total of eight months of sunitinib treatment with good tolerability, progression of the right liver lesions was observed, and the treatment was interrupted. The patient general condition further deteriorated, therefore she returned to her original country for palliative care management; the last news being available at the time of the end of sunitinib treatment.
Here we report the cases of three patients treated with sunitinib for intrahepatic cholangiocarcinoma with progressive disease following surgery (two patients) and chemotherapy. Sunitinib-associated toxicity was acceptable after dose reduction from 50 mg/d to 37.5 mg/d. Treatment duration was prolonged (6-12 mo), and was associated with a PR in one patient, and SD with tumor hypodensity on CT scans in two patients. These results are promising in a setting where few therapeutic options are available. Our results support the investigation of sunitinib in clinical trials in patients with good PS who are not responsive to chemotherapy, and especially in those with an intrahepatic type of cholangiocarcinoma displaying hypervascular features.
Cholangiocarcinoma represents a tumor type with many unmet medical needs[12]. Recent data have demonstrated the efficacy of chemotherapy in other related tumors for which limited treatment options are available. As a reference for first-line treatment, the ABC-02 randomized trial of 410 patients with locally advanced or metastatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer, showed that cisplatin combined with gemcitabine was associated with a significant survival advantage, vs gemcitabine alone, for up to 24 wk [median overall survival (OS) 11.7 mo vs 8.1 mo, respectively; hazard ratio = 0.64; 95%CI: 0.52-0.80; P < 0.001][2].
Angiogenesis plays an important role in tumor growth and survival. The negative prognostic value of angiogenesis has been shown in cholangiocarcinoma, although vascular density is lower in this disease than in HCC[13]. In a study in 22 patients undergoing surgical resection for intrahepatic cholangiocarcinoma, increased microvessel density was associated with both poor prognosis and the presence of intra-hepatic metastases[14]. Moreover, there was a correlation between microvessel density and the levels of both VEGF and angiopoietin-2[13]. In addition, VEGF expression is especially important in intrahepatic cholangiocarcinoma, suggesting a potential benefit of anti-angiogenic agents in this particular subtype of tumors[7].
Thrombospondin 1 (TSP1) is also implicated in angiogenesis, although its role remains controversial. TSP1 was found to be overexpressed in tumor cells when compared with normal cells[11]. However, the risk of intrahepatic metastasis was found to be higher in cholangiocarcinomas with low levels of TSP1[15]. Moreover, a correlation was shown between levels of VEGF and lymph node involvement in a series of 36 intrahepatic cholangiocarcinomas[16].
Sunitinib is an oral multi-tyrosine kinases inhibitor targeting VEGFR, PDGF receptor, stem-cell factor receptor and fetal liver tyrosine kinase receptor 3. Sunitinib has shown potent antitumor and antiangiogenic activities with acceptable safety profile in patients with advanced solid tumors[17,18]. Adverse events related to sunitinib are generally manageable, the most common side effects including asthenia, hand-foot syndrome, and hematological toxicities. Taken together, there is a strong rational to evaluate antiangiogenics, including sunitinib, in patients with intrahepatic forms of cholangiocarcinoma.
Three previous phase II trials in unselected subtypes of cholangiocarcinoma report disparate results. In the first study, where 31 eligible patients were treated in the first-line setting with sorafenib[19], median OS was nine months and median PFS was 3 mo. Two patients achieved an unconfirmed PR and ten patients demonstrated SD. In another trial of 46 patients treated with sorafenib, one patient achieved PR and nine exhibited SD[20]. The third study investigated combination therapy with bevacizumab and erlotinib as first-line therapy[6]. Of 20 evaluable patients, four achieved PR and seven achieved SD lasting more than four months.
Of note, evaluation of tumor response is difficult in patients treated with anti-angiogenic agents for primary liver tumors. The most common evaluation criteria (RECIST) are based exclusively on tumor size. Other composite criteria, such as the Choi criteria, provide an alternative as they consider both tumor size and tumor density, particularly as anti-angiogenic agents may render a tumor hypodense without size modification[21,22]. However, these criteria require validation in large patient cohorts.
Based on the rationale to target VEGF pathways and those encouraging results in selected cases, our team has launched a prospective multicenter phase II trial to investigate the activity of sunitinib in second line for patients with advanced intrahepatic cholangiocarcinoma previously treated with chemotherapy (SUN-CK trial; NCT01718327).
Three cases of recurrent/advanced intrahepatic cholangiocarcinoma; all cases presented with no specific symptoms including abdominal pain.
The initial clinical presentation of cases had low clinical relevance; in all cases positive diagnosis was confirmed histologically.
Hepatocellular carcinoma was the main differential diagnosis. Imaging features were not typical of hepatocellular carcinoma but suggested cholangiocarcinoma imaging diagnosis. Differential diagnosis was excluded by histological examination in all 3 patients.
Computed tomography scan showed lesions in the liver (cases 1 and 3) or lymph nodes (case 2) with contrast update at the arterial phase suggesting hypervascularisation of tumor lesions. Upon sunitinib treatment all target lesions showed decrease in density compatible with sunitinib antiangiogenic effects.
For all cases, histological examination showed phenotypical features of cholangiocarcinoma; immunohistochemical staining confirmed the diagnosis with positivity for CK7 and CK20 but negativity for anti-hepatocyte and glypican.
All patients received sunitinib at the time of progression after conventional chemotherapy. Two cases received sunitinib as a third-line option after progression on platinum-based chemotherapy and one case as second-line treatment after progression on GEMOX regimen.
In the literature, many preclinical and clinical data have shown the rational to block angiogenesis pathways in cholangiocarcinoma. However, few data are available evaluating antiangiogenic agents in the specific subtype of intrahepatic cholangiocarcinoma.
Cholangiocarcinoma is a group of heterogeneous tumors including intrahepatic, perihilar and distal cholangiocarcinomas. It is the second most frequent primitive liver malignancy after hepatocellular carcinoma and the incidence of intrahepatic cholangiocarcinoma is increasing worldwide.
Those cases reports showed promising results of sunitinib in chemotherapy-pretreated patients with advanced cholangiocarcinoma. These results support the concept to target deregulated signaling pathways in this disease, here vascular endothelial growth factor receptor signaling pathway in the intrahepatic subtype of cholangiocarcinoma. Based on these observations, a prospective phase II study has been launched in France evaluating sunitinb as second-line treatment in patients progressive and/or intolerant to chemotherapy.
The manuscript addresses the question of second-line treatment where no standard of care is available for advanced cholangiocarcinoma. Those results support the investigation of targeted agents blocking angiogenesis in advanced intrahepatic forms of cholangiocarcinoma.
P- Reviewer: Froehner M, Neri V, Sun LM, Wakiyama S S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1381] [Article Influence: 125.5] [Reference Citation Analysis (1)] |
| 2. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3169] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 3. | Fava G, Demorrow S, Gaudio E, Franchitto A, Onori P, Carpino G, Glaser S, Francis H, Coufal M, Marucci L. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int. 2009;29:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Glaser SS, Gaudio E, Alpini G. Vascular factors, angiogenesis and biliary tract disease. Curr Opin Gastroenterol. 2010;26:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Möbius C, Demuth C, Aigner T, Wiedmann M, Wittekind C, Mössner J, Hauss J, Witzigmann H. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2007;33:1025-1029. [PubMed] |
| 6. | Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, Benckert C, Jonas S. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Guedj N, Zhan Q, Perigny M, Rautou PE, Degos F, Belghiti J, Farges O, Bedossa P, Paradis V. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009;51:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425. [PubMed] |
| 9. | Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, Philip PA, Picus J, Yong WP, Horvath L. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28:3491-3497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 11. | Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32:4861-4870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1076] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 13. | Kawahara N, Ono M, Taguchi K, Okamoto M, Shimada M, Takenaka K, Hayashi K, Mosher DF, Sugimachi K, Tsuneyoshi M. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512-1517. [PubMed] |
| 14. | Shirabe K, Shimada M, Tsujita E, Aishima S, Maehara S, Tanaka S, Takenaka K, Maehara Y. Prognostic factors in node-negative intrahepatic cholangiocarcinoma with special reference to angiogenesis. Am J Surg. 2004;187:538-542. [PubMed] |
| 15. | Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, Kondo M, Ota H, Yoshioka S, Kato H, Damdinsuren B. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525-532. [PubMed] |
| 16. | Park BK, Paik YH, Park JY, Park KH, Bang S, Park SW, Chung JB, Park YN, Song SY. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol. 2006;29:138-142. [PubMed] |
| 17. | Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25-35. [PubMed] |
| 18. | Sweeney CJ, Chiorean EG, Verschraegen CF, Lee FC, Jones S, Royce M, Tye L, Liau KF, Bello A, Chao R. A phase I study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol. 2010;28:4513-4520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | El-Khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, Govindarajan R, Eng C, Blanke CD. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30:1646-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Faivre SJ, Bouattour M, Dreyer C, Raymond E. Sunitinib in hepatocellular carcinoma: redefining appropriate dosing, schedule, and activity end points. J Clin Oncol. 2009;27:e248-e250; author reply e251-e252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (1)] |