Published online Apr 18, 2015. doi: 10.4254/wjh.v7.i5.814
Peer-review started: October 22, 2014
First decision: December 22, 2014
Revised: January 26, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: April 18, 2015
Processing time: 179 Days and 21.3 Hours
Lymphomas may be induced by the systemic immunosuppressive therapies used to treat psoriasis, such as ciclosporin, methotrexate and tumour necrosis factor (TNF)-α blockers. The biologic agents currently used in psoriasis include alefacept, efalizumab, and the TNF-α antagonists etanercept, infliximab, and adalimumab. Infections and cancer are the main possible consequences of intended or unexpected immunosuppression. We report a 59-year-old man with a history of severe psoriasis vulgaris treated with traditional immunosuppressant drugs followed by anti-TNF-α therapy; the patient was firstly hospitalized for an acute cholestatic toxic hepatitis, which we supposed to be related to adalimumab. The first liver biopsy showed active disease with severe hepatocellular damage caused by heavy lymphocytes infiltrate in portal tracts at in the interface with a not conclusive diagnosis of lymphoproliferative disease. The correct diagnosis of T cell/histiocyte- rich large B cell lymphoma (T/HRBCL) was only reached through a gastric biopsy and a second liver biopsy. T/HRBCL is an uncommon morphologic variant of diffuse large B-cell lymphoma not described until now in psoriatic patients receiving immunosuppressive biologic agents. In psoriatic patients, treated with biologic immunosuppressive agents, the suspect of abdominal lymphoma should always be included as differential diagnosis. Abdominal ultrasound evaluation need therefore to be included in the pre-treatment screening as in the follow-up surveillance.
Core tip: We report a case of a rare T cell/histiocyte- rich large B cell lymphoma localized to liver, gastro-intestinal tract and spleen in a patient with psoriasis treated with traditional immunosuppressant drugs followed by anti-tumor necrosis factor-α therapy. Liver and spleen involvement mimicked at the beginning an inflammatory disease causing a delayed diagnosis of malignancy. We think that abdominal ultrasound evaluation need to be included in the pre-treatment screening as in the follow-up surveillance in psoriatic patients treated with biologic immunosuppressive agents.
- Citation: Nosotti L, Baiocchini A, Bonifati C, Visco-Comandini U, Mirisola C, Del Nonno F. Unusual case of B cell lymphoma after immunosuppressive treatment for psoriasis. World J Hepatol 2015; 7(5): 814-818
- URL: https://www.wjgnet.com/1948-5182/full/v7/i5/814.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i5.814
Psoriasis is a common chronic inflammatory disease of the skin and joints, which affects approximately 1%-2% of the population. It has been shown that patients with psoriasis are at higher risk of developing malignancies and this risk is greater for patients with severe disease[1,2]. The oncogenic risk is partly related to the immunologic nature of psoriasis and partly to the multiple immunosuppressants used for its treatment. Experimental evidence suggests a primarily T lymphocyte-based immunopathogenesis, with excessive Th1 and Th17 lymphocyte activity in psoriatic lesions. Chronic antigenic stimulation in psoriasis may lead, after a variable period of time, to a dominant clone in the skin and possible evolution towards a cutaneous T-cell lymphoma (CTCL)[3].
Nowadays multiple therapeutic options are available for the treatment of moderate to severe psoriasis. The process of choosing among potential treatment options requires the necessity to weigh the benefits of individual modalities of therapy against their potential risks. Systemic immunosuppressive therapies used to treat psoriasis, such as methotrexate (MTX), cyclosporine (CsA) and mycophenolate mofetil have been associated with an increased risk of lymphoma during treatment, demonstrated in clinical trials involving patients with rheumatoid arthritis and documented in case reports concerning psoriasis patients[1,4].
Furthermore, over the past several years, biologic therapies targeting T cells (e.g., efalizumab, alefacept) or cytokines such as tumor necrosis factor-α (TNF-α) (e.g., infliximab, etanercept, adalimumab) have been introduced for the treatment of moderate-severe psoriasis with a great clinical impact. However, the potential risk to induce serious infections and lymphomas by biologic agents has recently emerged[4].
We report a case of a rare extranodal diffuse large B cell lymphoma localized to liver, gastro-intestinal tract and spleen in a patient with psoriasis treated with traditional immunosuppressant drugs followed by anti-TNF-α therapy. Liver and spleen involvement mimicked an inflammatory disease causing a delayed diagnosis of the malignancy.
In February 2009 a 59-year-old man with a 39 years history of moderate to severe psoriasis vulgaris (involving 20% of the patient body surface and nails), treated in the past with several cycles of CsA and a cycle of MTX with partial improvement, was seen at a dermatological centre at the San Gallicano Dermatologic Institute.
The past medical history was consistent for bilateral degenerative maculopathy diagnosed at the age of 49 and essential hypertension diagnosed at the age of 54.
In March 2009 a treatment with etanercept (50 mg twice weekly for 12 wk, followed by 50 mg weekly) was started and stopped in September 2009 due to the complete clearing of psoriasis.
In December 2009 a new cycle of etanercept (50 mg twice weekly) was started due to a relapse of psoriasis. After 1 mo etanercept therapy was stopped because psoriasis continued to worsen. Therefore in February 2010 a therapy with adalimumab (induction dose of 80 mg) was started.
At this stage liver function tests were completely normal as well as all other routine analyses. Two weeks after starting adalimumab therapy the patient presented to the dermatologic outpatient psoriasis centre complaining of generalized malaise and weakness. At the physical examination a jaundice of the sclera was evident. At this stage adalimumab was stopped and the patient was referred to the National Institute for Infectious Diseases “L. Spallanzani”.
Liver function tests showed a grade III increase of both total bilirubin and liver enzymes. Hepatitis A, B and C and auto-antibodies were all negative.
Notwithstanding adalimumab interruption in the following days liver function values continued to rise together with the worsening of the jaundice and the patient physical condition.
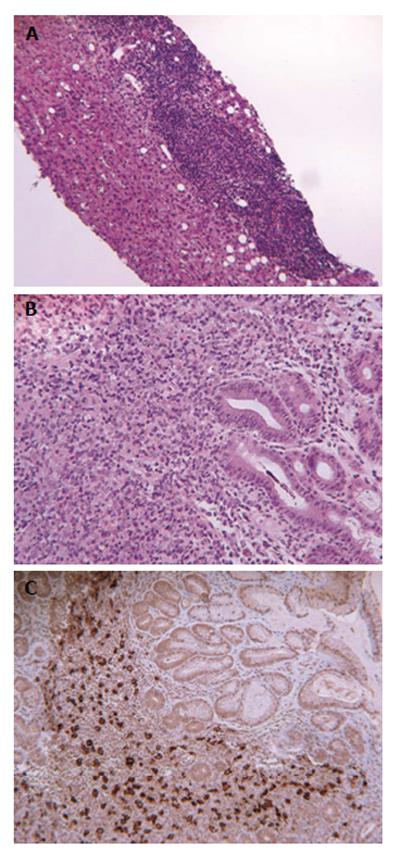
An abdominal ultrasound documented an enlarged steatotic liver, cholelithiasis with no dilatation of the bile-ducts and spleen enlargement with several hypoechogenic areas. Total body computed tomographic scan revealed multiple enlarged celiac and lumbar-aortic lymph nodes, cholangio-nuclear magnetic resonance confirmed the absence of dilatation of the bile-ducts. Total-body bone scan highlighted the presence of a small osteolytic area of uncertain nature at D10 level. Due to the worsened clinical picture (total bilirubin values reaching 20 mg/dL and severe pancytopenia) the patient underwent bone marrow and liver biopsies. The bone histology revealed a hypocellular marrow but no evidence of lymphoma. The liver biopsy showed active disease with severe hepatocellular damage caused by heavy lymphocytes infiltrate in portal tracts at in the interface (Figure 1A). Bridging necrosis was common and surviving hepatocytes often formed hepatic rosettes. Liver lymphocytes were represented mainly by T lymphocytes (CD3+, CD5+, CD56-) sometimes infiltrating the biliary epithelium and the sinusoid, with scanty B lymphocytes (CD20+, BCL-6+, BCL-2+). No large cell or blasts were observed. CD30 immunohistochemistry was negative. The morphological pattern was suggestive but not conclusive for a diagnosis of lymphoproliferative disease.
In the absence of any specific treatment, blood pancytopenia and bilirubin regressed to almost normal values. However, due to the persistence of morphological and clinical suspicion of lymphoma, the patient underwent splenectomy in July 2010. Surprisingly, spleen histology described sarcoid like granulomas without lymphomatous infiltration.
Leishmania and Bartonella serology, polymerase chain reaction for bacillus koch and atypical mycobacteria resulted negative and seric angiotensin converting enzyme was normal.
In October 2010, due to reappearance of jaundice (total bilirubin 12 mg/dL) and hepatitis the patient was treated with prednisone (1 mg/kg per die for 1 wk followed by tapered doses) which led to progressive reduction of cholestasis and cytolysis levels.
In November 2010, gastroscopy was performed for dyspepsia and hematemesis, showing a large ulcerated lesion in the middle portion of the stomach, near the greater curvature. Microscopic examination revealed a diffuse proliferation of large cells with round irregular nuclei, with distinct nucleoli and a narrow rim of cytoplasm (Figure 1B). Immunophenotyping revealed CD20+ B cells (Figure 1C) co-expressing CD43 and B-cell lymphoma 2 (BCL-2). Cells were negative for EBV- LMP1 and CD30. A diagnosis of gastric diffuse large B cell lymphoma was made.
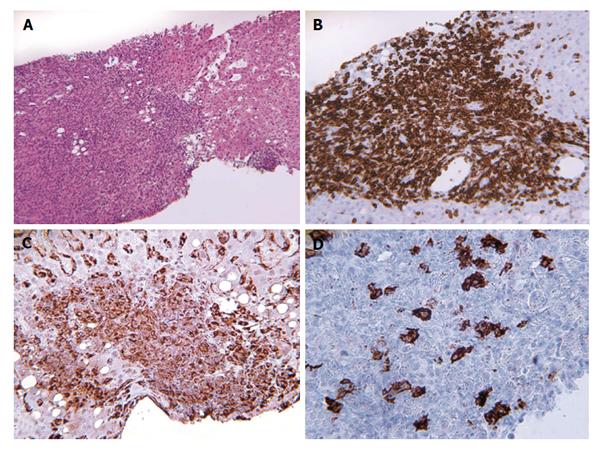
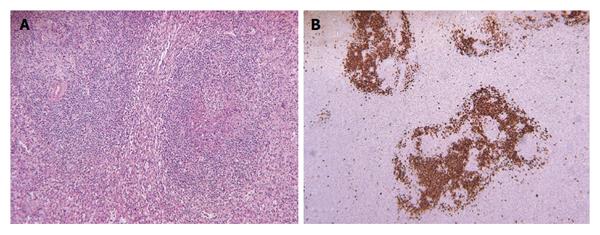
A second liver biopsy was performed, showing a diffuse lymphocytic infiltrate (Figure 2A) composed of predominantly small, mature T lymphocytes (CD3+) (Figure 2B) and histiocytes (CD68+) (Figure 2C) with scattered large neoplastic B lymphocytes, consisting of less than 10% of total cells, containing vescicular nuclei, prominent nucleoli and moderate amount of cytoplasm. These neoplastic cells expressed CD20 (Figure 2D), CD43, BCL-6, BCL-2, but not EBV-LMP1, CD10, CD138 or CD23, allowing further characterization of the lymphoma as “T cell/histiocyte- rich large B cell lymphoma”. These findings prompt pathologists to revaluate with immunohistochemical stain of the previously collected splenic specimens, revealing focal scattered large neoplastic lymphocytes in the red pulp, highlighted by CD20 stain (Figure 3). Sarcoid granulomas composed of clusters of epithelioid histiocytes with proliferating lymphocytes hided the neoplastic cells causing the first misdiagnosis.
The described lymphoma was assigned, according to the Ann Arbor Staging System, at group 4 with gastric, splenic, hepatic, abdominal lymph node and vertebral (dorsal column) localizations. The patient immediately started systemic chemotherapy.
Six cycles of CHOP-R have been administered until now with clinical remission and reduction of cholestasis.
All patients with psoriasis faced an increased risk of lymphoma with higher relative risks for Hodgkin’s lymphoma and CTCL[5].
In addition to skin cancer, the incidence of lymphoma in those patients employing PUVA therapy in combination with MTX for at least 36 mo was more than 7 times higher than that of cohort members earlier in the study who had less exposure to MTX[6].
A higher incidence of lymphoma with the use of the two monoclonal antibody agents (adalimumab and infliximab) than with the soluble-receptor agent (etanercept) was found in a large case-control study[7].
The etiology of non-Hodgkin lymphoma (NHL) remains largely unexplained, despite its dramatic worldwide rise in incidence in recent decades[8,9]. The heterogeneity of this group of malignancies is well established[10], whereas etiologic variation among subtypes has only recently been recognized. Classical risk factors for NHL include conditions of severe immunosuppression[10]. However, the role for chronic immune stimulation is also suggested from studies showing the occurrence of specific NHL subtypes in inflammatory and infectious conditions[11].
T-cell/histiocyte-rich B-cell lymphoma (T/HRBCL) is an uncommon morphologic variant of diffuse large B-cell lymphoma, accounting for about 40% of all NHL. T/HRBCL has not been described until now in psoriatic patients receiving immunosuppressive biologic agents. Pathologically, it is distinguished by < 10% malignant B cells amid a majority population of reactive T lymphocytes and histiocytes. The large amount of surrounding inflammatory T lymphocytes may mask the lymphomatous cells, mimicking an hepatitis, or sarcoid like granulomas in the spleen. Accurate diagnosis therefore rests on careful immunohistochemical analysis of the tumour cells and the inflammatory microenvironment[12].
In our case, the patient was firstly hospitalized for an acute cholestatic toxic hepatitis, that we supposed to be related to adalimumab, and the liver findings were unclear. Our patient underwent splenectomy, but also in this occasion the histology interpretation (performed by a different group in another hospital) was misleading. The correct diagnosis was only reached through a gastric biopsy and a second liver biopsy.
In psoriatic patients, treated with biologic immunosuppressive agents, the suspect of abdominal lymphoma should always be included as differential diagnosis. Abdominal ultrasound evaluation need therefore to be included in the pre-treatment screening as in the follow-up surveillance.
Two weeks after starting adalimumab therapy the patient presented to the dermatologic outpatient psoriasis centre complaining of generalized malaise and weakness. At the physical examination a jaundice of the sclera was evident.
The worsening of jaundice despite adalimumab interruption excluded liver drug toxicity.
The authors considered in the differential diagnosis the following conditions: Intra or extrahepatic cholestatic disorders.
Liver function tests showed a grade III increase of both total bilirubin and liver enzymes. Hepatitis A, B and C and auto-antibodies were all negative.
An abdominal ultrasound documented an enlarged steatotic liver, cholelithiasis with no dilatation of the bile-ducts and spleen enlargement with several hypoechogenic areas. Total body computed tomographic scan revealed multiple enlarged celiac and lumbar-aortic lymph nodes, cholangio-nuclear magnetic resonance confirmed the absence of dilatation of the bile-ducts.
The second liver biopsy performed, showed a diffuse lymphocytic infiltrate composed of predominantly small, mature T lymphocytes (CD3+) and histiocytes (CD68+) with scattered large neoplastic B lymphocytes, consisting of less than 10% of total cells, containing vescicular nuclei, prominent nucleoli and moderate amount of cytoplasm. These neoplastic cells expressed CD20, CD43, B-cell lymphoma 6 (BCL-6), BCL-2, but not EBV-LMP1, CD10, CD138 or CD23, allowing further characterization of the lymphoma as “T cell/histiocyte-rich large B cell lymphoma”.
The patient immediately started systemic chemotherapy. Six cycles of CHOP-R have been administered until now with clinical remission and reduction of cholestasis.
T-cell/histiocyte-rich B-cell lymphoma (T/HRBCL) is an uncommon morphologic variant of diffuse large B-cell lymphoma (DLBCL), accounting for about 40% of all non-Hodgkin lymphomas (NHL). T/HRBCL has not been described until now in psoriatic patients receiving immunosuppressive biologic agents.
Tumour necrosis factor (TNF)-α inhibitors are immunosuppressive agents with a profound effect on the immune system, including decreased T-cell-mediated responses; infliximab and other TNF-α antagonists have been associated with lymphoproliferative disorders of varied types in patients with autoimmune diseases. T/HRBCL is an uncommon morphologic variant of DLBCL, accounting for about 40% of all NHL.
In this case, the patient was firstly hospitalized for an acute cholestatic toxic hepatitis, that the authors supposed to be related to adalimumab, and the liver findings were unclear. The patient underwent splenectomy, but also in this occasion the histology interpretation (performed by a different group in another hospital) was misleading. The correct diagnosis was only reached through a gastric biopsy and a second liver biopsy. In psoriatic patients, treated with biologic immunosuppressive agents, the suspect of abdominal lymphoma should always be included as differential diagnosis. Abdominal ultrasound evaluation need therefore to be included in the pre-treatment screening as in the follow-up surveillance.
Useful for the scientific community that uses the immunosuppressive drugs.
P- Reviewer: Ayroldi E S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Quéreux G, Renaut JJ, Peuvrel L, Knol AC, Brocard A, Dréno B. Sudden onset of an aggressive cutaneous lymphoma in a young patient with psoriasis: role of immunosuppressants. Acta Derm Venereol. 2010;90:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Margolis D, Bilker W, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137:778-783. [PubMed] |
| 3. | Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Patel RV, Clark LN, Lebwohl M, Weinberg JM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol. 2009;60:1001-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429. [PubMed] |
| 6. | Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142:1132-1135. [PubMed] |
| 7. | Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, Heresbach D, Martin A, Schaeverbeke T, Salmon D. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis. 2010;69:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 9. | Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann Glob Health. 2014;80:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Smith MT, Skibola CF, Allan JM, Morgan GJ. Causal models of leukaemia and lymphoma. IARC Sci Publ. 2004;373-392. [PubMed] |
| 11. | Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |