Published online Dec 8, 2015. doi: 10.4254/wjh.v7.i28.2849
Peer-review started: July 6, 2015
First decision: September 22, 2015
Revised: October 24, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: December 8, 2015
Processing time: 156 Days and 3.1 Hours
AIM: To critically evaluate the current epidemiology data on exposures, rather than infection, to hepatitis C virus (HCV) transmission and recommend epidemiologic strategies to fill gaps.
METHODS: Standard methods for identifying and evaluating relevant epidemiologic literature and available data were used.
RESULTS: There is a large body of literature on the epidemiology of HCV transmission in Egypt that collectively identifies ongoing iatrogenic exposures as the major driver for HCV transmission due to short comings in infection control and standard procedures. Additional epidemiologic studies on HCV transmission that requires the participation of human subject is unwarranted. Alternatively, very little literature was found on the epidemiology of exposure to HCV, infection control, and safe injection practices. The information that is available on patterns of HCV exposure shows high frequencies of inadequate infection control, problems in sterilization in health care facilities, low rates of hand washing, untrained personnel, lack of stated policies in facilities, HCV contamination of instruments and very large injection frequencies with low but very significant syringe and needle reuse. There is an important need to increase the number, size, and diversity of epidemiologic studies on HCV exposures, patterns of risk factors for infection, infection control, and safe injection practices. In addition to health care facilities evaluation, relevant knowledge attitude and practice studies are recommended.
CONCLUSION: Epidemiologic methods on HCV exposure can be used to characterize the magnitude of exposures to HCV infection, target interventions to reduce exposures, and provide the best method for evaluating interventions by demonstrating the reduction of exposure to HCV infection.
Core tip: Much has been published on the epidemiology of hepatitis C virus epidemic in Egypt. The exposures that drive this epidemic are iatrogenic. This review focuses on what has been published on the epidemiology (patterns, distributions, and related factors) of the iatrogenic exposures. The review found that very little has been published on epidemiology of the exposures driving the epidemic. This is essential for developing effective interventions and evaluating prevention programs. Recommendations are given.
- Citation: Miller FD, Elzalabany MS, Hassani S, Cuadros DF. Epidemiology of hepatitis C virus exposure in Egypt: Opportunities for prevention and evaluation. World J Hepatol 2015; 7(28): 2849-2858
- URL: https://www.wjgnet.com/1948-5182/full/v7/i28/2849.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i28.2849
Kamel et al[1] reported in 1992 an unusually high prevalence of anti-hepatitis C virus (HCV) antibodies in over 2000 first time healthy Egyptian blood donors in Cairo, Egypt. The prevalence was 10.1%, five to ten times higher than what had been reported elsewhere in the world[2]. This was a population based study of first time blood donors in urban Egypt and likely underestimated anti-HCV antibodies prevalence in the general population.
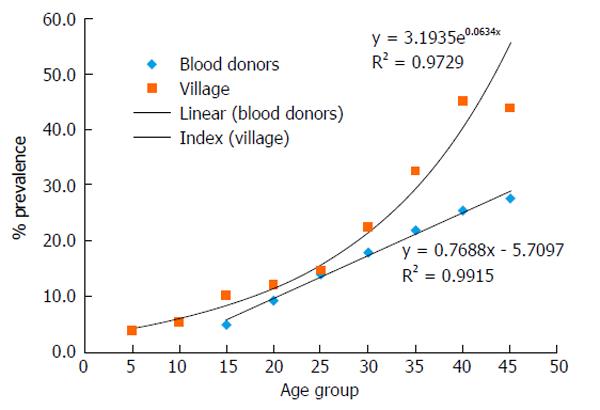
A follow up to the blood donor study, Kamel et al[3] completed a rural population based community study of anti-HCV antibodies in 1994. The study included the entire population of a remote village in the northern Nile Delta. The overall anti-HCV antibodies prevalence in the village was 17.6%. In both of these population based epidemiologic studies, prevalence of anti-HCV antibodies increased strongly with age as shown in Figure 1. In the blood donors and the village study, the prevalence of anti-HCV antibodies was similar in both sexes.
These were the first two population based studies reported in Egypt providing the initial evidence that there was an extraordinary HCV epidemic unlike anywhere else in the world. These and many similar studies that followed[4-9], including two national studies[10,11], reinforced these observations. The 2008 national estimate of anti-HCV antibodies prevalence was 14.7% and the estimate for HCV RNA prevalence was 9.7%[10]. Given a national population of about 80 million persons, 7.8 million were estimated to be asymptomatically infected with HCV comprising a large reservoir of HCV in the population. It is now well established that Egypt has a HCV epidemic, which is the largest HCV epidemic in the world, and the epidemic is ongoing[2,9,12-14].
Epidemiologic tools are needed in Egypt to evaluate the magnitude and patterns of exposure to HCV transmission. Exposure to HCV is similar in concept to risk factors or independent variables associated with HCV transmission. The distribution and determinates of HCV exposures and related factors are fundamental for rationale allocations of resources for intervention by reducing exposure to HCV infection. Secondly, an extension of these epidemiologic tools is needed to evaluate intervention programs by demonstrating a reduction in the magnitude and patterns of HCV exposure.
The aim of our study was to first briefly summarize the epidemiology of HCV transmission (HCV T), identify all epidemiologic studies completed in Egypt to date that were designed to describe the magnitude, patterns and determinates of exposures (predominately iatrogenic exposures) to HCV transmission (HCV E), characterize gaps in HCV E and finally demonstrate and provide examples for the application of HCV E for the evaluation of public health interventions to reduce exposure to HCV.
A literature review was conducted to identify publications on the epidemiology of HCV transmission and on the epidemiology of iatrogenic exposures in Egypt using methods previously published by us[14]. Briefly, a search of all published peer-reviewed literature (English language) from 1992 to 2015 on HCV and Egypt was made using the National Library of Medicine, PubMed, Google Scholar, Web of Science, Biological Abstracts, manual review of citations in search-identified publications and in Egypt for reports available only locally. Studies that: (1) reported HCV prevalence or incidence; (2) described the serologic methods; (3) were of cross-sectional or prospective epidemiologic design; and (4) could be abstracted for the purposes of the study were included. Studies on infection control were included as proxy for iatrogenic exposures.
One of our objectives was to build a complete bibliographic database on iatrogenic exposures and infection control practices in Egypt. From this, an assessment of epidemiologic methods for investigations on iatrogenic exposures or HCV E could be evaluated. Methodologically sound studies were sought as examples for investigation as well as methods for evaluation of intervention programs to reduce and prevent iatrogenic transmission of HCV in Egypt. Additional data were based on personal onsite visits in Egypt and anecdotal observation as some potentially iatrogenic practices have not been formally published and appear to be unique to Egypt. An example is the widely practiced re-use of latex gloves or not using latex gloves when indicated.
Following the pioneering work of Kamel et al[1,3], many similar studies in rural communities and selected health clinics followed without adding any significant new findings. Beyond the national blood donor screening program, no national program for the prevention of HCV emerged in the first decade after discovery. In part, this may have been due to the natural history of HCV infection. In a primary HCV infection, there is rarely an acute phase. Manifestations of liver dysfunction and disease usually do not occur until one or more decades later. HCV became a silent epidemic.
The origins of the HCV epidemic in Egypt are not clear but thought to be due to past and ongoing iatrogenic exposures[15,16]. Iatrogenic exposures and failure in infection control could be frequently seen on visits to health care facilities throughout the large Egyptian health care system.
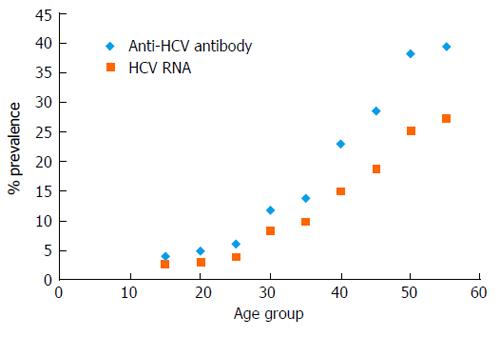
A report in 1997 showed an association between anti-HCV antibodies and a history of parenteral therapy for schistosomiasis and surgery[17]. In 2000, a report in the Lancet suggested that the epidemic was due in part to the previous wide spread rural campaigns of parenteral anti-schistosomiasis therapy (PAT)[15]. The Egyptian medical care system embraced this report as the cause of the epidemic. More importantly, Egypt’s physicians concluded that since these campaigns had ended more than three decades ago, the cause of the epidemic, PAT, had ended and therefore transmission had ended as well. If this was true, then epidemiologically the prevalence of anti-HCV antibodies in Egyptians 30 years old and younger should be similar to other countries. That is from 1% to 3% or less. As shown in Figures 1 and 2, anti-HCV antibodies prevalence increase from the earliest age. There is now abundant evidence that there is an ongoing HCV epidemic in Egypt.
The first formal epidemiologic study on infection control and iatrogenic exposures (HCV E) to HCV transmission in Egypt was in 2006[18]. This was a survey of large and small health care facilities. The study showed a complete lack of infection control practices in all facilities. In this study the presence or absence of infection control programs was an index of iatrogenic exposure to HCV. The American Centers for Disease Control and Prevention reported in 2012 that Egypt continued to face an ongoing HCV epidemic and that a comprehensive prevention plan was needed[13].
HCV is a blood borne pathogen and the transmission and epidemiology of HCV is well established[16]. The virus is inherently liable with exponential die off in 24 h under laboratory conditions[19,20], is less infectious than hepatitis B virus (HBV) and slightly more infectious than human immunodeficiency virus (HIV)[21].
The probability of HCV transmission varies by different routes of exposure as shown in Table 1. Note that the significance relative to the ongoing epidemic in Egypt of a given exposure route of transmission has been included in this table. For example, the probability of HCV transmission by contaminated blood or organ donation results in all recipients becoming infected. In this case the probability of transmission and infection is one (100%). Contaminated blood and organ donation have the highest HCV transmission. Nationally mandated HCV blood donor screening program in 1994 reduced exposure frequency to the point that blood transfusion, once a significant route of transmission in Egypt and elsewhere, no longer plays a significant role in regard to the general population[22].
| Exposure route | Exposure frequency | Transmission probability | Population exposed | Significance1 | Ref. |
| Blood transfusion | Very low | 1 (100%) | 2300 k/yr | Zero | [2,16] |
| Organ donation | Very low | 1 | < 100/yr | Zero | [2] |
| Injection drug users | High | ≥ 0.8 | Very small | Very low | [23,24] |
| Hemodialysis | High | ≥ 0.75 | Small | Very low | [26-28] |
| Sexual | High | Unk3 | Adults | Zero | [29] |
| Intrafamilial | Unknown | Unk | General | Very low | [15,16] |
| Needle stick | High | ≤ 0.02 | Occupational | Low | [31] |
| Injections | 44.1/p per year | ≤ 0.02 | General | High | [17,30,53,60] |
| Maternal | High | 0.02 | New born | Low | [33,43] |
| Dental | High | ≤ 0.02 | General | High | [62-65] |
| Iatrogenic | High | ≤ 0.02 | General | High | [14,42,43,45,49,50,54] |
Injection drug users (IDU) were found to have very high prevalence of HCV infection presumably due to the reuse and sharing of drug injection equipment[23,24]. Contaminated drug injection equipment has a high probability of transmission although there is no exact probability estimate available. The exact population of IDU in Egypt is not well defined although considered small. Moreover, HCV transmission with IDU groups, unlike HIV, poses a very low probability of exposure to the general population[25].
It has been long recognized that HCV infection, like HIV, was a risk for hemodialysis patients[26-28]. In Egypt, this became a national scandal. From 46.1% to 100% of HCV negative dialysis patients would convert to HCV positive within a year in dialysis centers throughout the country[16,26,28]. Considerable efforts both in the public and private sector have reduced HCV transmission in local dialysis centers. However, the general population exposed to this risk is small and the significance of HCV positive dialysis patients to the overall epidemic is very low.
Sexual and intra-familial transmission of HCV remains to be unequivocally established in Egypt or elsewhere. Sexual transmission of HCV is controversial. HCV discordant monogamous couples showed almost no transmission for long periods and recovery of HCV from semen or other genital fluids has proved to be difficult[29]. Sexual transmission does not play a significant role in Egypt. The studies of intra-familial transmission of HCV in Egypt[30-32] have validity issues (small numbers, confounding, selection biases) and have not been replicated. No specific intra-familial exposure to HCV transmission has been identified. Familial sharing of any medical equipment such as syringe and needles or diabetic testing equipment could result in exposure to HCV transmission, but this remains to be established.
Confirmed occupationally related accidental needle sticks from HCV positive patients have a probability of infection slightly greater than HIV but much lower relative to the probability of HBV infection. The probability of transmission has been estimated to be approximately 0.01 to 0.02[21]. Accidental occupational needle sticks in Egypt is a significant exposure[33-36]. However, the extent that this exposure contributes to transmission in the general population is not known but not likely to be significant.
Transmission of HCV infection from mother to new born however is well established. In Egypt, we have estimated that there are 5000 newborns infected with HCV every year[37].
Iatrogenic transmission of HCV has been documented globally[2,24]. Iatrogenic transmission can be complex due to the many possible routes of exposure from contaminated medical and dental instruments, sharps, needles, invasive procedures, contaminated multi-dose vials, blood, or blood product transfusion, organ transplantation or any of many kinds of medical/dental percutaneous exposures.
A key element of iatrogenic transmission is patient to patient exposure where the first patient is knowingly or more likely unknowingly asymptomatically infected. This patient is a key to the exposure and contamination of medical or dental instruments, sharps, or needles. Failure in infection control or standard procedures to prevent a second patient to be percutaneouslly or parenterally exposed to a contaminated instrument or sharp has a relatively low probability of HCV transmission and infection[21].
Iatrogenic transmission of HCV in Egypt is well documented[7,17,38-54]. These reports identify iatrogenic transmission as the principal driver of the HCV epidemic in Egypt. Accordingly, in Egypt, if the probability of iatrogenic exposure is the same across a health care system, then the probability of transmission will be greater in patient populations who have a higher prevalence of chronic HCV RNA infection, symptomatic or not. This reservoir of chronic HCV infected patients is known to be high in Egypt. Overall, 10% of the Egyptian population is HCV RNA positive[10]. This varies with rural populations having a higher prevalence of HCV RNA relative to urban populations. In Egypt, like anti-HCV antibodies, HCV RNA positivity increases directly with age as shown in Figure 2. The reservoir of HCV infection is therefore higher in older patients relative to younger patients.
The epidemiology of HCV T in Egypt is defined as reports which estimate patterns of HCV infection in people and associations with a possible exposure to HCV infection. For example the report by el-Sayed et al[17] showed an odds ratio (OR = 7.9) with a history of PAT exposure. It is important to note that, as shown in this study, there is an abundance of similar HCV T literature on the prevalence, incidence, and risk factors for infection. Additional HCV T studies are unwarranted. There is no continued justification to test individuals for HCV antibodies or HCV RNA for the purposes of HCV T studies. Conversely, the epidemiology of exposure to HCV transmission (HCV E) would provide data on the patterns and frequency of exposures. Examples of HCV E are given below.
Injections: Reuse of inadequately sterilized needles and syringes during the PAT campaigns in rural Egypt over 30 years ago is cited as a major factor in the origin of the Egyptian HCV epidemic[15,21,33,34,55-60]. The PAT hypothesis has been challenged on the bases that there was a greater concurrent abundance of iatrogenic exposures throughout Egypt in addition to PAT exposures[49]. Accordingly, HCV E studies would characterize the patterns and frequency of injections and identify the magnitude and determinates of safe and unsafe injection practices.
Talaat et al[60] has estimated a high rate of injections in Egypt at 281 million per year. These injections were administrated by public and private sector physicians, pharmacists, barbers, doctor assistants, housekeepers, relatives and friends and 8.4% reported that the syringe was not taken from a closed packet[60]. Many in rural areas resort to informal nonprofessionals for injections[56].
Safe injection practices were directly observed in a cross sectional study among 1100 healthcare workers located in 25 healthcare facilities in the Gharbiya governorate in the Nile Delta[33,34]. Noted was a lack of supplies needed for safe injections and safe practice was infrequent. Important policies were lacking including an infection control committee and dedicated infection control personal. Most importantly, there were an estimated 13.2% of syringes and needles reused. The important data on exposure in this study included lack of supplies, safe injection practices, essential policies, lack of infection control committee and personal and syringe and needle reuse.
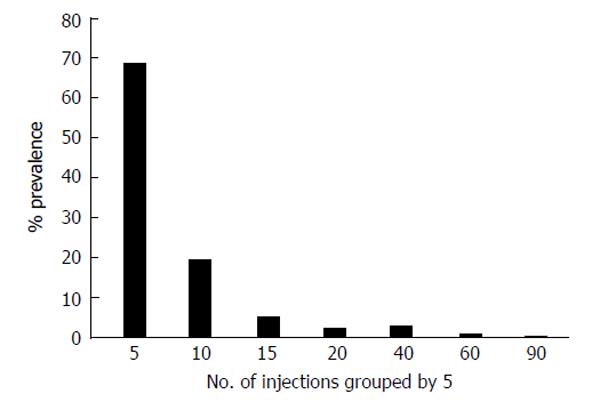
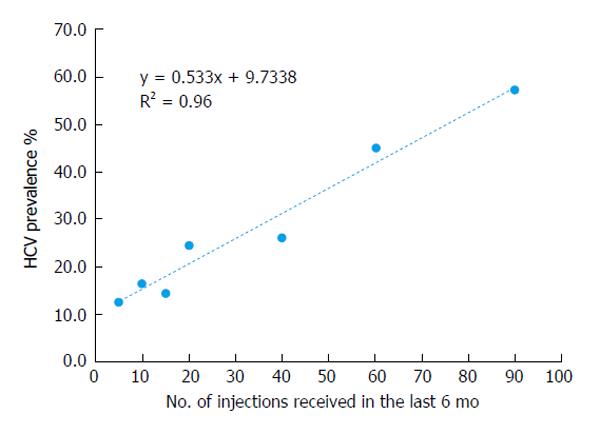
Shown here for the first time is an analysis of injection data collected by the 2008 Egyptian Demographic Health Survey[10]. Shown in Table 2 is the frequency of injections reported by participants in the 2008 nationally representative study. Almost 19% (18.5%) received one or more injections in the last six months. Women reported receiving twice as many injections as men (OR = 2.3; 95%CI: 2.0-2.5). Figure 3 shows the pattern of participants reporting multiple injections in groups by 5. By far, the largest group received 1 to 5 injections in the last 6 mo followed by a sharp decline. Figure 4 shows the relationship between HCV antibodies prevalence and the pattern of multiple injections. A strong (R² = 0.96) and direct relationship of increasing HCV antibody prevalence and the number of injections is shown. These results suggest that increased frequency of injections has a direct relation with increased HCV antibodies positivity. An alternate interpretation is that individuals who are receiving multiple frequent injections have serious medical conditions and more likely have additional iatrogenic exposures.
| Gender | Yes | No | Total | Prevalence % | 95%CI (lower-upper) |
| Female | 1493 | 4661 | 6154 | 24.3 | 23.2-25.4 |
| Male | 625 | 4403 | 5028 | 12.4 | 11.5-13.3 |
| Total | 2118 | 9064 | 11182 | 18.9 | 18.2-19.6 |
| OR = 2.3 | 2.0-2.5 |
The measure of exposure to HCV was the following question: “The last time you had an injection from a health worker, did the person who gave you that injection take the syringe and needle from a new, unopened package?”. Of those that received injections, 14.8% answered “No”. Data are shown in Table 3. There may be concerns about how this question was interpreted by the participant. The injection may have been prepared in a separate area from the patient. The patient may not know or be able to confirm that the syringe and needle came from a new unopened package. However, this estimate is similar in magnitude to that from the study conducted in Gharbiya[33,34], in which Talaat et al[60] estimated about 8.4% in an answer to a similar question.
| Question1 | Yes | No | Total | Prevalence % | 95%CI (lower-upper) |
| New | 1273 | 221 | 1494 | 14.80 | 13.0-16.6 |
| Unopened | |||||
| Package |
Dental health care: Dental health care has a significant potential for iatrogenic HCV transmission[61-63]. Only a single report of HCV iatrogenic exposure in Egyptian dental clinics was found published by Hashish et al[64] in 2012. The study measured the presence of HCV RNA by reverse transcription polymerase chain reaction (RT-PCR) on various dental instruments in selected dental clinics in Alexandria. The study found that 18% of the dental instruments in the dental clinics visited were positive for HCV RNA indicating the presence of the virus. This study demonstrates a method for measuring iatrogenic exposure to HCV infection from dental health care by showing the presence of HCV contaminated dental instruments.
The exact reason for the contamination of these instruments was not given. The authors did suggest that the lack of sterilization equipment for instruments was a short coming. What was not provided was the number of sets of instruments, patient load, hand washing practices, use of latex gloves, how instruments were cleaned and disinfected, the presence of operating sterilization equipment, and if there had been specific efforts and policies present for training and preventing HCV transmission.
The prevalence of HCV is lowest in Alexandria most likely due to better infection control than most other areas of Egypt, especially rural areas. The observed results are therefore most likely an under estimate.
The correct method to expand this epidemiologic approach to HCV exposure in dental clinics would be to include other measures of infection control mentioned above in recording data. Secondly, this study design could be used at the community level to describe the epidemiology of HCV exposure in dental clinics by obtaining a list of all dental care facilities in the community and decide on drawing a representative sample of dental care facilities or to include all facilities in the study. Results of a sample could be used to describe the magnitude of HCV contamination and provide justification for professional improvement or training programs. Results based on facility specific level would be used for compliance and licensing.
Detection of HCV RNA by RT-PCR could be resource challenging. A lower resource approach would have the same primary step of community and facility identification and sampling. Each facility would provide details on cleaning and sterilization methods and an inventory of dental equipment sets and daily patient loads. Large patient to equipment ratios would suggest a trigger for compliance or licensing issues.
Health care facilities: Egypt has a national health care system dating back to the 19th century. Health care facilities in Egypt are a complex organization of public and private sector facilities that include medical care, dental care, clinical laboratories, and pharmacies.
Pharmacies in Egypt give injections, intravenous fluids, and provide testing for glucose levels. Glucometers are an overlooked exposure to HCV transmission. Testing is carried out by pharmacy technicians who may have only secondary school education and do unsupervised home visits. No studies have been done on exposure to HCV infections related to Egyptian pharmacies.
El-Zanaty et al[65] carried out a national service provision assessment survey in 2004. A portion of this study was on “Systems for Infection Control”. Medical care facilities rather than individuals were surveyed in a nationally representative sample using very specific standardized data forms. This report has considerable details representing all regions of Egypt and covering all types of medical care facilities. Private clinics, private pharmacies, and dental services were not included.
A statement in a summary of the findings reported a significant decrease from 2002 to 2004 in almost all indicators of infection control. It was concluded that infection control practices were extremely weak. Only 4% of all facilities were adherent to all infection control measures. New disposable syringes and needles were however universal. The study could uniquely compare changes over time to their previous publication in 2002[66].
Informal health care providers: Egypt has a large undocumented sector of informal health care provides. These providers do not have formal education or training and provide services for injections, dentistry, wound treatment, and male circumcision. Traditional birth attendants were reported to oversee > 50% of all births. “Injectionists” included barbers and staff at pharmacies. A study of Informal health providers in two Egyptian villages found that these providers, “knew little about HCV” and its transmission[56].
A number of studies in Egypt have included barbers as a possible exposure to HCV[41,43,44,56,67,68]. This is based on the assumption of percutaneous exposure by shaving. Most studies found that there is no evidence for transmission. This is consistent with viral fragility and the unlikelihood of the virus remaining viable in the soap used in shaving. In a study of barbers in the Gharbia governorate[68], anti-HCV antibodies were detected in 12.3% of barbers and 12.7% of clients. Knowledge of HCV prevention was reported to be high among the majority of participating barbers and good practices during shaving and hair-cutting were observed for the majority of barbers.
The transmission of HCV is entirely preventable. Throughout the world, HCV transmission is prevented by good medical and dental care practices which reduce and eliminate iatrogenic exposure to infection. This includes following well established aseptic techniques, standard procedures and universal precautions[13]. Measures should and can be taken to reduce and eliminate all HCV transmission routes listed in Table 1.
HCV prevention in the Egyptian health care educational system: Aseptic techniques, standard procedures, and universal precautions are or should be introduced in all health care curricular in the Egyptian health care educational system and reinforced at every level of health care service. This educational intervention is not intellectually complex or challenging to teach. In fact, teaching and training could be done entirely using modern computer based social media. Moreover, the interventions to reduce HCV exposure do not require new or costly technology. There is no publicly available information on the evaluation of the Egyptian health care educational system in regard to HCV prevention curricular. Technically, evaluation of the Egyptian health care educational system is straight forward.
Recommendation: Conduct an examination on a sample of recent and past graduates from medicine, nursing, dentistry, and pharmacy on HCV prevention. This is an essential data for status on current knowledge and a benchmark to which future evaluation can be based.
Evaluation of knowledge, attitudes, and practices (KAP) of health care providers on standard procedures and infection control is necessary for the reduction of exposure to HCV. Well-designed repeated KAP studies based on representative samples stratified by health care provider categories can provide direct evidence of HCV exposures defined as incomplete knowledge and practice errors. The large Egyptian health care syndicates can provide needed sampling frames. These syndicates can also provide opportunities to spread important messages about HCV exposure prevention.
Recommendation: KAP study on health care providers on HCV prevention is needed.
In addition to curricular interventions, professional development or continued health care education should be developed for online certification or re-certification for preventing exposure to HCV infection.
Screening of blood and blood products: Globally, blood donations are screened for HCV. Egypt mandated a national blood donation HCV screening program by 1994[22]. Before this national program, approximately 9% of blood and blood products recipients would have become infected. Assuming the probability of HCV transmission at 100% among exposed recipients and 300000 recipients per year, this program has prevented to date at least half a million people from becoming infected. Maintaining and enhancing Egypt’s national blood donation HCV screening program is essential.
The number and extent of studies which test for the presence of HCV in individuals (HCV T) have been considerable dating from the first reports in 1992. Given the abundance of literature on HCV T, additional HCV T studies are unwarranted. There is no continued justification to test individuals for HCV antibodies or RNA for the purposes of HCV T studies. Aside from cost, there are additional methodological limitations for both cross sectional and prospective HCV T studies in Egypt that further support discontinuation of these investigations. Due to the natural history of HCV, there are serious validity issues for cross sectional, prospective, and case control study designs undermining hypothesis testing related inferences. Population prevalence estimates are not useful for evaluating interventions and prevention measures[69]. Generating national incidence rates requires very large samples, is very costly, requires long term follow ups, and has limited validity determining past exposures. Prospective incidence studies are strongly discouraged especially for intervention assessment and project evaluation. The difficultly of using prospective incidence studies for intervention assessment is not unique to this public health problem but is widely recognized by public health practitioners.
Successful interventions will reduce exposure to HCV infection which will decrease the incidence. It is strongly recommended to use HCV E studies, rather than prospective incidence studies to evaluate the control of the ongoing epidemic. For that, benchmark HCV E data are needed which can be obtained from cross sectional studies.
Few studies on HCV E were found. However, these studies illustrate the basic low cost low technology methodology needed to document exposures to HCV infection. The only national level study using facility surveys completed by El-Zanaty et al[65,66] in 2002 and 2004 is a good example. These facility studies could be modified to focus more on infection control evaluation. This would provide direct data on the frequency and patterns of HCV exposures. These observational based studies can be complemented by interview based KAP studies adapted to the unique Egyptian epidemic directed at infection control documentation and evaluation.
It is essential to conceptualize the methodological approach epidemiologically for HCV E. The objective is to epidemiologically describe the prevalence of a specifically defined set of health care practices and procedures which can be evaluated as being correctly or incorrectly done with regard to standard procedures[64,65] in a given health care setting. As mentioned above this should include both observational and interview based data collection. Specific modifications are needed for the different types of Egyptian health care facilities.
Methods for standardizing data collection on infection control in general and injection practices in specific for Egypt are needed. Standardized methods are also needed for direct and indirect observational data collection and data collected by interview. Standards for collecting photographic documentation would be useful. Pilot testing with independent verification is advisable. This capacity exist in Egypt as demonstrated by the studies carried out by El-Zanaty et al[10,65].
There are many unique infection control violations in Egypt which have the potential for exposure to HCV infection. These exposures unique to Egypt are poorly documented and should be thoroughly investigated and included in any comprehensive prevention program to reduce exposure to HCV transmission. There is a large unregulated informal health care system in Egypt that contributes significantly to injections and other poorly regulated procedures[18,60].
It is recommended that efforts be made to develop strong HCV E studies that generate a comprehensive inventory of all typical and unique iatrogenic exposures, where these exposures are occurring, the magnitude of these exposures (number of individuals potentially exposed), probability of transmission and create an index of iatrogenic transmission. The HCV index of transmission (HCV IT) would incorporate the magnitude of population exposed and probability of transmission. For example, HCV contaminated blood transfusion has 100% probability of transmission[2,16], a restricted population exposed (blood donor recipients) and with the current level of blood donor screening an overall low index. Given the magnitude of injections received in Egypt with much lower probability of transmission via contaminated drug vials or syringe and or needle reuse, the overall HCV IT is likely to be very significant[53].
Strong well designed HCV E studies have a dual function. The first objective is to better and more precisely document the distribution and determinates of specific iatrogenic exposures. This is essential to provide a baseline for evaluation. The second objective is evaluation of intervention programs. That is to quantitatively demonstrate a reduction in exposure to HCV transmission by an improvement in infection control measures and safe injection practices by direct and indirect measures[10,60,64,65] with follow up HCV E studies.
Egypt has the largest epidemic of hepatitis C virus (HCV) in the world. A review of the epidemiologic literature on HCV was completed.
HCV is entirely preventable. The exposure factors to HCV infection driving the epidemic in Egypt have been thoroughly identified as iatrogenic. The authors’ aim was to assess the magnitude of the epidemiologic literature on iatrogenic exposures in Egypt.
The authors found that the amount of epidemiologic information on iatrogenic exposures needed for designing the prevention of HCV transmission was very limited, especially in contrast to the epidemiology on HCV infection or transmission.
The epidemiology of exposure to HCV transmission, that is predominately iatrogenic exposures, is essential information and knowledge needed to design, guide, and evaluate interventions to reduce iatrogenic exposures and prevent HCV transmission. The application of epidemiologic investigation on exposures does not include human subjects which vastly reduces the cost and complexity of data collection. Recommendations and suggested epidemiologic approaches and designs were given.
The authors refer to classical epidemiology of HCV infection, transmission, and prevalence as HCV transmission. Individuals participating is these types of studies have to provide a specimen for HCV testing, understand the consequences of being found HCV positive, and if viremic, referred for treatment. The authors refer to the epidemiologic investigation of exposures to HCV infection as HCV E. Knowledge, attitude, practice (KAP) studies are an example of methodology that could be adapted to exposure epidemiology. The KAP of injection preparation and administration is an example.
The review is very interesting and gives a fairly comprehensive overview of the situation in Egypt.
P- Reviewer: De Paschale M, McQuillan GM, Yokota S S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Kamel MA, Ghaffar YA, Wasef MA, Wright M, Clark LC, Miller FD. High HCV prevalence in Egyptian blood donors. Lancet. 1992;340:427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 838] [Cited by in RCA: 831] [Article Influence: 46.2] [Reference Citation Analysis (2)] |
| 3. | Kamel MA, Miller FD, el Masry AG, Zakaria S, Khattab M, Essmat G, Ghaffar YA. The epidemiology of Schistosoma mansoni, hepatitis B and hepatitis C infection in Egypt. Ann Trop Med Parasitol. 1994;88:501-509. [PubMed] |
| 4. | el Gohary A, Hassan A, Nooman Z, Lavanchy D, Mayerat C, el Ayat A, Fawaz N, Gobran F, Ahmed M, Kawano F. High prevalence of hepatitis C virus among urban and rural population groups in Egypt. Acta Trop. 1995;59:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Quinti I, Renganathan E, El Ghazzawi E, Divizia M, Sawaf G, Awad S, Pana A, Rocchi G. Seroprevalence of HIV and HCV infections in Alexandria, Egypt. Zentralbl Bakteriol. 1995;283:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Darwish MA, Faris R, Clemens JD, Rao MR, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in The Nile Delta: a pilot study. Am J Trop Med Hyg. 1996;54:554-558. [PubMed] |
| 7. | el-Sayed NM, Gomatos PJ, Rodier GR, Wierzba TF, Darwish A, Khashaba S, Arthur RR. Seroprevalence survey of Egyptian tourism workers for hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and Treponema pallidum infections: association of hepatitis C virus infections with specific regions of Egypt. Am J Trop Med Hyg. 1996;55:179-184. [PubMed] |
| 8. | Mohamed MK, Rakhaa M, Shoeir S, Saber M. Viral hepatitis C infection among Egyptians the magnitude of the problem: epidemiological and laboratory approach. J Egypt Public Health Assoc. 1996;71:79-111. [PubMed] |
| 9. | Lehman EM, Wilson ML. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J Viral Hepat. 2009;16:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo: El-Zanaty and Associates, and Macro International 2009; . |
| 11. | Mohamed MK. Epidemiology of HCV in Egypt. Afro-Arab Liver J. 2004;3:41-52. |
| 12. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 945] [Article Influence: 67.5] [Reference Citation Analysis (2)] |
| 13. | Centers for Disease Control and Prevention (CDC). Progress toward prevention and control of hepatitis C virus infection--Egypt, 2001-2012. MMWR Morb Mortal Wkly Rep. 2012;61:545-549. [PubMed] |
| 14. | Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci USA. 2010;107:14757-14762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 16. | Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | el-Sayed HF, Abaza SM, Mehanna S, Winch PJ. The prevalence of hepatitis B and C infections among immigrants to a newly reclaimed area endemic for Schistosoma mansoni in Sinai, Egypt. Acta Trop. 1997;68:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Talaat M, Kandeel A, Rasslan O, Hajjeh R, Hallaj Z, El-Sayed N, Mahoney FJ. Evolution of infection control in Egypt: achievements and challenges. Am J Infect Control. 2006;34:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kamili S, Krawczynski K, McCaustland K, Li X, Alter MJ. Infectivity of hepatitis C virus in plasma after drying and storing at room temperature. Infect Control Hosp Epidemiol. 2007;28:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Song H, Li J, Shi S, Yan L, Zhuang H, Li K. Thermal stability and inactivation of hepatitis C virus grown in cell culture. Virol J. 2010;7:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77:789-800. [PubMed] |
| 22. | Moftah FM. Regionalization of the blood transfusion service in Egypt. Vox Sang. 2002;83 Suppl 1:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | el-Ghazzawi E, Drew L, Hamdy L, El-Sherbini E, Sadek Sel-D, Saleh E. Intravenous drug addicts: a high risk group for infection with human immunodeficiency virus, hepatitis viruses, cytomegalo virus and bacterial infections in Alexandria Egypt. J Egypt Public Health Assoc. 1995;70:127-150. [PubMed] |
| 24. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1931] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 25. | Bruggmann P, Berg T, Øvrehus AL, Moreno C, Brandão Mello CE, Roudot-Thoraval F, Marinho RT, Sherman M, Ryder SD, Sperl J. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl 1:5-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 26. | Hassan AA, Khalil R. Hepatitis C in dialysis patients in egypt: relationship to dialysis duration, blood transfusion, and liver disease. Saudi J Kidney Dis Transpl. 2000;11:72-73. [PubMed] |
| 27. | El Sayed NM, Gomatos PJ, Beck-Sagué CM, Dietrich U, von Briesen H, Osmanov S, Esparza J, Arthur RR, Wahdan MH, Jarvis WR. Epidemic transmission of human immunodeficiency virus in renal dialysis centers in Egypt. J Infect Dis. 2000;181:91-97. [PubMed] |
| 28. | Hassan NF, el Ghorab NM, Abdel Rehim MS, el Sharkawy MS, el Sayed NM, Emara K, Soltant Y, Sanad M, Hibbs RG, Arthur RR. HIV infection in renal dialysis patients in Egypt. AIDS. 1994;8:853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Vandelli C, Renzo F, Romanò L, Tisminetzky S, De Palma M, Stroffolini T, Ventura E, Zanetti A. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Magder LS, Fix AD, Mikhail NN, Mohamed MK, Abdel-Hamid M, Abdel-Aziz F, Medhat A, Strickland GT. Estimation of the risk of transmission of hepatitis C between spouses in Egypt based on seroprevalence data. Int J Epidemiol. 2005;34:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Mohamed MK, Abdel-Hamid M, Mikhail NN, Abdel-Aziz F, Medhat A, Magder LS, Fix AD, Strickland GT. Intrafamilial transmission of hepatitis C in Egypt. Hepatology. 2005;42:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Mohamed MK, Magder LS, Abdel-Hamid M, El-Daly M, Mikhail NN, Abdel-Aziz F, Medhat A, Thiers V, Strickland GT. Transmission of hepatitis C virus between parents and children. Am J Trop Med Hyg. 2006;75:16-20. [PubMed] |
| 33. | Ismail NA, Aboul Ftouh AM, El Shoubary WH. Safe injection practice among health care workers, Gharbiya, Egypt. J Egypt Public Health Assoc. 2005;80:563-583. [PubMed] |
| 34. | Ismail NA, Aboul Ftouh AM, El-Shoubary WH, Mahaba H. Safe injection practice among health-care workers in Gharbiya Governorate, Egypt. East Mediterr Health J. 2007;13:893-906. [PubMed] |
| 35. | Talaat M, Kandeel A, El-Shoubary W, Bodenschatz C, Khairy I, Oun S, Mahoney FJ. Occupational exposure to needlestick injuries and hepatitis B vaccination coverage among health care workers in Egypt. Am J Infect Control. 2003;31:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Washer P. Hepatitis C: transmission, treatment and occupational risk. Nurs Stand. 2001;15:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Benova L, Awad SF, Miller FD, Abu-Raddad LJ. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology. 2015;61:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Arafa N, El Hoseiny M, Rekacewicz C, Bakr I, El-Kafrawy S, El Daly M, Aoun S, Marzouk D, Mohamed MK, Fontanet A. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol. 2005;43:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | El Gaafary MM, Rekacewicz C, Abdel-Rahman AG, Allam MF, El Hosseiny M, Hamid MA, Colombani F, Sultan Y, El-Aidy S, Fontanet A. Surveillance of acute hepatitis C in Cairo, Egypt. J Med Virol. 2005;76:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | El-Raziky MS, El-Hawary M, Esmat G, Abouzied AM, El-Koofy N, Mohsen N, Mansour S, Shaheen A, Abdel Hamid M, El-Karaksy H. Prevalence and risk factors of asymptomatic hepatitis C virus infection in Egyptian children. World J Gastroenterol. 2007;13:1828-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (11)] |
| 41. | el-Sadawy M, Ragab H, el-Toukhy H, el-Mor Ael-L, Mangoud AM, Eissa MH, Afefy AF, el-Shorbagy E, Ibrahem IA, Mahrous S. Hepatitis C virus infection at Sharkia Governorate, Egypt: seroprevalence and associated risk factors. J Egypt Soc Parasitol. 2004;34:367-384. [PubMed] |
| 42. | El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Egyptian: Ministry of Health. Cairo: El-Zanaty and Associates, and Macro International 2009; 431. |
| 43. | Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Medhat A, Shehata M, Magder LS, Mikhail N, Abdel-Baki L, Nafeh M, Abdel-Hamid M, Strickland GT, Fix AD. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg. 2002;66:633-638. [PubMed] |
| 45. | Saleh DA, Shebl F, Abdel-Hamid M, Narooz S, Mikhail N, El-Batanony M, El-Kafrawy S, El-Daly M, Sharaf S, Hashem M. Incidence and risk factors for hepatitis C infection in a cohort of women in rural Egypt. Trans R Soc Trop Med Hyg. 2008;102:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Saleh DA, Shebl FM, El-Kamary SS, Magder LS, Allam A, Abdel-Hamid M, Mikhail N, Hashem M, Sharaf S, Stoszek SK. Incidence and risk factors for community-acquired hepatitis C infection from birth to 5 years of age in rural Egyptian children. Trans R Soc Trop Med Hyg. 2010;104:357-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Farghaly AG, Barakat RM. Prevalence, impact and risk factors of hepatitis C infection. J Egypt Public Health Assoc. 1993;68:63-79. [PubMed] |
| 48. | Kandeel AM, Talaat M, Afifi SA, El-Sayed NM, Abdel Fadeel MA, Hajjeh RA, Mahoney FJ. Case control study to identify risk factors for acute hepatitis C virus infection in Egypt. BMC Infect Dis. 2012;12:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 49. | Cuadros DF, Branscum AJ, Miller FD, Abu-Raddad LJ. Spatial epidemiology of hepatitis C virus infection in Egypt: analyses and implications. Hepatology. 2014;60:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Chemaitelly H, Abu-Raddad LJ, Miller FD. An apparent lack of epidemiologic association between hepatitis C virus knowledge and the prevalence of hepatitis C infection in a national survey in Egypt. PLoS One. 2013;8:e69803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Mostafa A, Taylor SM, el-Daly M, el-Hoseiny M, Bakr I, Arafa N, Thiers V, Rimlinger F, Abdel-Hamid M, Fontanet A. Is the hepatitis C virus epidemic over in Egypt? Incidence and risk factors of new hepatitis C virus infections. Liver Int. 2010;30:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Paez Jimenez A, Sharaf Eldin N, Rimlinger F, El-Daly M, El-Hariri H, El-Hoseiny M, Mohsen A, Mostafa A, Delarocque-Astagneau E, Abdel-Hamid M. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59:1554-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Breban R, Arafa N, Leroy S, Mostafa A, Bakr I, Tondeur L, Abdel-Hamid M, Doss W, Esmat G, Mohamed MK. Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Glob Health. 2014;2:e541-e549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Breban R, Fontanet A. Feasible HCV targets in Egypt - authors’ reply. Lancet Glob Health. 2014;2:e688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Drain PK, Nelson CM, Lloyd JS. Single-dose versus multi-dose vaccine vials for immunization programmes in developing countries. Bull World Health Organ. 2003;81:726-731. [PubMed] |
| 56. | El Katsha S, Labeeb S, Watts S, Younis A. Informal health providers and the transmission of hepatitis C virus: pilot study in two Egyptian villages. East Mediterr Health J. 2006;12:758-767. [PubMed] |
| 57. | Hutin YJ, Hauri AM, Armstrong GL. Use of injections in healthcare settings worldwide, 2000: literature review and regional estimates. BMJ. 2003;327:1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 58. | Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77:801-807. [PubMed] |
| 59. | Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine. 1999;17:1730-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Talaat M, el-Oun S, Kandeel A, Abu-Rabei W, Bodenschatz C, Lohiniva AL, Hallaj Z, Mahoney FJ. Overview of injection practices in two governorates in Egypt. Trop Med Int Health. 2003;8:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Garbin CA, de Souza NP, de Vasconcelos RR, Garbin AJ, Villar LM. Hepatitis C virus and dental health workers: an update. Oral Health Prev Dent. 2014;12:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Klevens RM, Moorman AC. Hepatitis C virus: an overview for dental health care providers. J Am Dent Assoc. 2013;144:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Mahboobi N, Porter SR, Karayiannis P, Alavian SM. Dental treatment as a risk factor for hepatitis B and C viral infection. A review of the recent literature. J Gastrointestin Liver Dis. 2013;22:79-86. [PubMed] |
| 64. | Hashish MH, Selim HS, Elshazly SA, Diab HH, Elsayed NM. Screening for the hepatitis C virus in some dental clinics in Alexandria, Egypt. J Egypt Public Health Assoc. 2012;87:109-115. [PubMed] [DOI] [Full Text] |
| 65. | El-Zanaty F, Population MoHa, Macro O. Egypt Service Provision Assessment Survey 2004. Calverton, Maryland, USA: Ministry of Health and Population and ORC Macro 2005; . |
| 66. | El-Zanaty F, Macro O, Ministry of Health and Population (MOHP) (Egypt) . Egypt Service Provision Assessment Survey 2002: Calverton, Maryland: Ministry of Health and Population, El-Zanaty Associates, and ORC Macro. 2003;. |
| 67. | Eassa S, Eissa M, Sharaf SM, Ibrahim MH, Hassanein OM. Prevalence of hepatitis C virus infection and evaluation of a health education program in el-ghar village in zagazig, egypt. J Egypt Public Health Assoc. 2007;82:379-404. [PubMed] |
| 68. | Shalaby S, Kabbash IA, El Saleet G, Mansour N, Omar A, El Nawawy A. Hepatitis B and C viral infection: prevalence, knowledge, attitude and practice among barbers and clients in Gharbia governorate, Egypt. East Mediterr Health J. 2010;16:10-17. [PubMed] |
| 69. | Kleinbaum D, Kupper L, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York, NY: John Wiley and Sons 1982; . |