Published online Dec 8, 2015. doi: 10.4254/wjh.v7.i28.2792
Peer-review started: April 24, 2015
First decision: July 21, 2015
Revised: September 24, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: December 8, 2015
Processing time: 225 Days and 15.7 Hours
Hepatitis C virus (HCV) genotype (GT) 4 represents 12%-15% (15-18 million) of total global HCV infection. It is prevalent in Northern and Equatorial Africa and the Middle East, and is also present in some countries in Europe. GT-4 (and subtype 4a in particular) dominates the HCV epidemic in Egypt. In underdeveloped countries, risk factors associated with HCV infection may be due to unsafe medical practices or other factors such as familial transmission, mother’s HCV status, or illiteracy. HCV prevention and control programs should include health education, increased community awareness towards the disease, controlling infection distribution in health-care centers, proper sterilization of medical and dental instruments, and ensuring safe supply of blood and blood-products. Response rates to a 48-wk combined pegylated-interferon (PEG-IFN) and ribavirin (RBV) treatment range from 40%-69%, and HCV-GT-4 has been considered better than GT-1 but worse than GT-2 and GT-3 in treatment with PEG-IFN/RBV. However, with the introduction of the HCV-GT-1 effective protease inhibitors boceprevir and telaprevir in 2011, HCV-GT-4 became the “most difficult (GT) to treat”. Recently, the direct-acting antivirals (DAAs) with pan- genotypic activities simeprevir, sofosbuvir, and daclatasvir have been recommended in triple regimens with PEG-IFN/RBV for the treatment of HCV-GT-4. An IFN-free regimen will be available for treatment of all genotypes of HCV in the near future. To date, several DAAs have been developed and are currently being evaluated in various combinations in clinical trials. As new regimens and new agents are being approved by the Food and Drug Administration, we can expect the guidelines for HCV treatment to be changed. The availability of shorter, simpler, and more tolerable treatment regimens can reduce the morbidity and mortality associated with HCV infection. With such a large number of therapeutic agents available, we can end up with a range of choices that we can select from to treat patients.
Core tip: Hepatitis C virus (HCV) genotype (GT) 4 represents 12%-15% of total global HCV infection. It is higher in limited resource countries. Response rates to a 48-wk peg-interferon/ribavirin combination ranges from 40%-69% for HCV-GT-4. Direct-acting antivirals may significantly improve treatment outcomes in HCV- GT-4, but use of these agents in countries endemic for HCV-GT-4 is currently precluded by the very high costs. A new hepatitis C vaccine from GlaxoSmithKline has shown promise in early clinical tests, prompting strong and broad immune responses. Another Egyptian clinical trial in the field of HCV vaccination: Clinical Trials phases I and II, started on March 2011. ClinicalTrials.gov Identifier NCT01718834.
- Citation: Abdel-Ghaffar TY, Sira MM, El Naghi S. Hepatitis C genotype 4: The past, present, and future. World J Hepatol 2015; 7(28): 2792-2810
- URL: https://www.wjgnet.com/1948-5182/full/v7/i28/2792.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i28.2792
Harvey J Alter is an American Virologist, Medical Researcher, and Physician. In mid-1970s, he was the first person to prove a new type of hepatitis virus, initially called non-A, non-B hepatitis (NANBH). During the following years, medical researchers Michael Houghton, George Kuo, and Qui-Lim Choo from Chiron Corporation (an American multinational biotechnology firm) and Dr. Daniel W Bradley from the Centers for Disease Control and Prevention of America (CDC) collaborated in identifying the virus: Using the molecular cloning process to identify an unknown organism, and confirming that the organism is a virus after finding an NANBH specimen in the organism in 1988. Two articles were published on it, and the name was changed from NANBH to hepatitis C virus (HCV) in April 1989. Later on, HCV was shown to be the principal cause of parenterally transmitted NANBH worldwide[1]. After the discovery of this virus, a flurry of international studies was conducted to document its distribution and prevalence in humans[2]. It is now well established that HCV is a global health challenge, with an estimated 2%-3% of the global population having chronic HCV infection[3]. Estimates over the last 15 years show HCV affection to have increased to 2.8%, which means > 185 million infections worldwide[4].
HCV is a small single-stranded ribonucleic acid (RNA) of positive polarity, and is an enveloped virus belonging to the Hepacivirus genus within the Flaviviridae family[5]. It consists of approximately 9600 nucleotides in length, which encode three structural proteins (core, E1, and E2), the ion channel protein p7, and six nonstructural (NS) proteins (NS2, NS3, NS4A, NS4B, NS5A, and NS5B)[6]. Because each protein is involved in HCV entry, infection, replication, or maturation, they are potential antiviral targets. Hepatitis C virus replication takes place entirely within the cytoplasm, therefore it does not establish latency making it easier to cure[7].
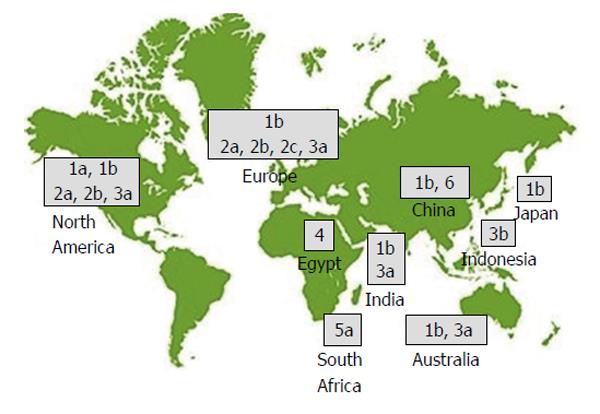
All RNA viruses show a high degree of genome-sequence heterogeneity. HCV RNA is characterized by three tiers of variability: Genotype (GT), subtype, and quasispecies, i.e., the sequence variation within a single patient. The most recent classification includes seven GTs (numbered 1-7), 67 confirmed and 20 provisional subtypes, which differ in sequence by approximately 30% to 35%, and approximately 20% to 25%, respectively. All GTs except 5 and 7 are subdivided into numerous subtypes (1a, 1b, 1c, 2a, 2b, etc.)[8]. There is a clear geographic pattern in the distribution of HCV genetic diversity (Figure 1). Highly divergent “endemic” strains that belong to the same GT are typically found in a restricted geographic area, indicating the presence of the GT in that location for hundreds or even thousands of years[9]. GT-1 dominates worldwide (83.4 million cases, 46.2% of all HCV cases), approximately one-third of which are in East Asia. GT-3 comes second (54.3 million, 30.1%). GT-2, GT-4, and GT-6 compromise a total 22.8% of all cases, and GT-5 comprises the remaining < 1%. Subtypes, specifically 1a, 1b, 2a, and 3a, are distributed worldwide, and are especially dominant in high income countries[10]. Within the United States, 75% of isolates are HCV GT-1a or GT-1b and the remainder are generally GT-2 or GT-3[11]. In Europe, HCV-GT-1a and GT-1b are the commonest subtypes. GT-2 dominates in West Africa, and GT-3 dominates in South Asia and parts of Scandinavia. While GT-1 and GT-3 dominate in most countries, GT-4 and GT-5 are mostly found in lower-income countries[10].
HCV GT-4 is prevalent in Northern and Equatorial Africa and the Middle East, while GT-5 and GT-6 have been identified in South Africa and Hong Kong, respectively[12]. GT-4 represents 12%-15% (15-18 million) of total global HCV infection (Table 1)[13], and its distribution is restricted to Egypt, Central Africa, and the Middle East regions[10], although it has recently been reported in the Caribbean region and in India[14-16]. GT-4 (and subtype 4a in particular) dominates the HCV epidemic in Egypt[10,17].
| Country or region | GT-4 % of the total HCV infected patients in the country |
| Southwestern France | 7.4% |
| Germany | 3.6% |
| Southern Italy | 1.4% |
| Northern Italy | 3.1% |
| Southern Spain | 14% |
| Saudi Arabia | 60% |
| Lebanon | 30% |
| Syria | 30% |
| Cameroon | 76% |
| Nigeria | 60% |
| Egypt | 91% |
| Gabon | 71% |
| Southern India | 6.2% |
An anomaly in the distribution of HCV infection, however, was discovered in Egypt, where the prevalence was approximately 10-fold higher than in other countries[18]. The prevalence is higher in Egypt than in industrialized countries (ranging from 0.5% to 2.3%)[2], as well as in limited-resource countries, even Pakistan (high prevalence rate of 6.5)[19].
In 2008, an Egyptian Demographic Health Survey (EDHS) was carried out in Egypt on 11126 women and men aged 15-59 years, and interviews were obtained for each individual. It was the first nationwide representative sample for HCV antibody testing done in Egypt. The blood samples were tested by a third generation enzyme-linked immunosorbent assay to detect the HCV antibody (Adaltis EIAgen HCV Ab, Adaltis Italia, Casalecchio di Reno, Italy) at the Central Laboratory in Cairo. HCV antibodies that were Sera positive were tested for HCV RNA[20]. Results showed HCV antibody prevalence was 14.7% (95%CI: 13.9%-15.5%). Most (> 90%) HCV isolates were found to belong to GT-4 with the remaining belonging to GT-1[21].
Prevalence was highest in Lower Egypt (Nile Delta), followed by Upper Egypt, then by Urban governorates (Cairo, Alexandria, Port-Said, and Suez), and then by frontier governorates (17.5%, 14.7%, 9.5% and 3.8% respectively). Increased HCV antibody prevalence was shown with increasing age, in males (P < 0.001), and in rural areas (P < 0.001). Two thirds of the participants positive for HCV antibody were viremic, and viremia was more prevalent in males, but there was no difference in prevalence according to age or type of exposure. Decreased HCV antibody prevalence was shown with increasing educational level and wealth, but the prevalence was increased with increasing number of people in the same household. Previous history of blood transfusion, parenteral anti-schistosomiasis treatment (PAT), contaminated syringes, and female circumcision were all associated with HCV infection in univariate analysis[20].
The prevalence of HCV antibody positivity in 2008 (after adjusting for the younger than 15 years and the older than 59 years individuals) was estimated at 12%. However, only two thirds of the infected population were viremic in the EDHS, resulting in an all age group viremic prevalence of 8.5% in 2008[22]. Mass campaigns of PAT were blamed for the HCV epidemic in Egypt[23,24]. Between 1964 and 1982, 2 million Egyptians, most of whom were children above five years of age and young adults who lived in areas where schistosomiasis was prevalent, received intravenous weekly injections of antimony salts for 12-16 wk. Insufficient sterilization of the syringes was considered the cause of the HCV transmission at that time[23]. EDHS, performed 30 years after the treatment campaigns, showed PAT to be related to 7.8% and 11.0% of infected people in rural and urban areas, respectively, whereas other means of transmission attributed to the rest of the patients. The introduction of Praziquantel in 1982, an oral drug to treat schistosomiasis[24], did not stop the transmission of HCV due to multifactorial mechanisms including blood transfusion[25-27], contaminated syringes[26,28], dental intervention[26-28], surgical and invasive medical procedures[26-30].
An attempt to determine HCV prevalence in special populations estimated a vertical transmission rate among 1224 pregnant women. Presence of maternal positive HCV antibodies was in 105 of the women (8.6%, 95%CI: 7.05-10.17) with only 83 (6.8%) positive for HCV-RNA. Tests on infants during their first month showed that 43 out of 53 infants (81%) were positive for HCV antibodies and 7 of them (13%) were HCV-RNA positive. Six months later, only two infants (3.8%) remained HCV-RNA positive[31].
In another cohort study[32] to detect mother-to-infant infection, 1863 pregnant women were included and tested for HCV infection. Among those pregnant women, 15.7% and 10.9% tested positive for HCV antibodies and HCV-RNA, respectively. Out of 33 (10%) infants positive for both antibodies and RNA, 29 had RNA-positive mothers and four had only antibody positive mothers who underwent clearing of infection. Fifteen (4.6%) had detectable HCV-RNA at 12 mo and 14 had cleared infection early. At 2-3 years of age, only eight (2.4%) had persistent HCV-RNA while seven had late clearance of infection. The rate of HCV vertical transmission can be increased in the presence of human immunodeficiency virus (HIV) coinfection or elevated maternal HCV viral load, but it is not affected by breastfeeding or HCV GTs[33].
Prevalence ranged among blood donors between 5%-25%, among blood-transfusion dependent patients between 10%-55%, and among dialyzed patients between 50%-90%[34]. Many studies were conducted in the pediatric population. Among hemophilic children, HCV-antibodies were positive in 40% of patients[35], 47.5% of them were positive for HCV-RNA. In 2011, Barakat et al[36] screened 500 school children from 10 schools and found 5.8% HCV seroprevalence with viremia in 75% of them. In 2010, El-Karaksy et al[37] reported that the prevalence of HCV in diabetic and non-diabetic children aged below nine years was 2.5% vs 1.4% (P = 0.25).
In the last estimate of HCV incidence in Egypt it was found that the number of new infections annually is < 150000[38]. In 2008, the EDHS estimated the incidence in Egypt at a rate of 6.9/1000 (95%CI: 5.5-7.4 per person per year), indicative of possibly ongoing hyper epidemic transmission[18].
Another study was conducted on 2852 uninfected infants who were followed from birth up to 5.5 years, to detect incidence and risk factors for acquired HCV in rural Egyptian children. Fifteen infants (0.53%) seroconverted to either HCV antibodies and/or HCV-RNA: 10 of them had both antibody and HCV-RNA positive, 4 of them had only antibody positive, whereas the last one had only HCV-RNA positive. The incidence rate at all ages was 2.7/1000 person-years (PY): Higher during infancy than between 1-5 years of age (3.8/1000 PY and 2.0/1000 PY respectively). It was shown that prolonged hospitalization and low birth weight increased the risk of infection, whereas maternal HCV was the source of infection in only two older children. By the end of the follow-up period, six children (40%) had natural clearance[39]. A four-year population-based cohort study was conducted on seronegative villagers to calculate the incidence of new HCV cases. Out of 10578 participants, 25 (11 females and 14 males) caught the infection (incidence rate of 2.4/1000 PY; 95%CI: 1.6-3.5)[40].
Using systematic literature review methods, Reker et al[41] selected 11 articles published between 2008 and 2013 which met the study selection criteria aiming to determine risk factors responsible for the high incidence and prevalence of HCV in Egypt. They categorized the risk factors into two major groups: “Unsafe medical practices and other risk factors”. Unsafe medical practices included surgery, intravenous injections, PAT, dental intervention, stitches, and catheterization. The other risk factors included illiteracy, maternal HCV, and familial transmission[41].
Primary prevention is the best strategy. Health education and increased community awareness towards the disease is needed. Development of new approaches to learn more about HCV transmission is required. Effort should be invested towards making HCV vaccine and direct-acting antivirals (DAAs) accessible for patients during infancy and early childhood[33].
The Egyptian Ministry of Health and Population implemented a program in 2001 to decrease HCV transmission through medical practices, and launched a comprehensive national viral hepatitis control program in 2008 for treatment. This led to a decrease in the incidence of infection among dialyzed patients from 28% to 6%[42]. But Egypt still continues to suffer from an ongoing HCV epidemic. Therefore, a comprehensive plan is required to control the infection, which includes increase in community awareness and health education, proper sterilization of medical and dental instruments, a safe blood supply, monitoring the effect of programs, and providing proper treatment[41-48].
Primary infection is mostly asymptomatic which makes early detection of the disease difficult, and this leads to underestimation of its true incidence rate. Its diagnosis may be confirmed via a documented seroconversion to anti-HCV in a person who was previously negative. Primary HCV infection has no specific markers, and it may be diagnosed with or without an increase in alanine aminotransferase (ALT) levels[49,50].
In primary exposure, serum HCV RNA cannot be detected before a window of 1-3 wk. Symptoms are mild and non-specific, so patients often do not seek medical assistance. Elevated ALT levels indicating the first signs of liver injury can be detected 4-12 wk after infection, and wide fluctuations are common. Severe liver inflammation is uncommon, and fulminant hepatitis is rare. Seroconversion may occur between 4 and 10 wk after exposure[51].
When HCV viremia persists more than 6 mo, it is defined as chronic. The risk of chronic HCV developing from acute infection is high, ranging between 55% and 85%[52,53]. Natural clearance is more likely to occur in symptomatic cases[54]. On the other hand, asymptomatic cases are more likely to progress to chronicity. Spontaneous clearance in adults with chronic HCV is rarely seen[55], but is more observed in children (8% to 45%). Spontaneous viral clearance is unlikely beyond four years of age, and viral infection that does not get cleared in the first years will progress to chronicity[56-59]. We can expect natural clearance mostly to occur within the first three months after exposure[60]. Other factors may also increase the chance of viral clearance include interferon L3 gene, formerly known as interleukin 28B (IL28B polymorphisms)[61] and the intensity of the cellular immune response[51]. El-Awady et al[62] conducted a study in 2012 on a total of 404 subjects divided into patients infected with HCV-GT-4a (n = 304) and a healthy group (n = 100). They found a significant increase (P < 0.0005) in frequencies of IL28B rs12979860 C/C GTs in the healthy population than in the other group. On the other hand, the C/C GT was significantly higher (P < 0.0005) in spontaneous resolvers cases (n = 84) than in healthy subjects, so they reported that GT C/C was associated with viral clearance during acute infection and suggested a central role of this GT against HCV disease progression[62].
Higher rates of spontaneous resolution have been found in infants with the rs12979860 CC GT for the IL28B polymorphism. Infants, in particular, may have defense mechanisms that explain the inefficiency of HCV perinatal transmission, including the placenta, which has an immunoprotective role, and human leukocyte antigen DR13, where they are less likely to experience chronic HCV from vertical transmission[63]. Co-infection with HIV seems to hinder resolution. The role of type of exposure, viral load, age, sex, and previous recovery following HCV exposure is debatable. Patients, especially those with higher risk of transmission, should be educated that HCV re-infection is possible even after viral eradication[64,65].
HCV can progress to cirrhosis after decades. It can also lead to liver cell failure or hepatocellular carcinoma (HCC) (approximately 2% to 4% yearly). Several factors influence the progression of the disease, the most important of which is the extent of intrahepatic inflammation elicited by HCV[66]. Persistent normal ALT level indicates slow progression of the disease[67]. Unlike the case with other viral infections, serum HCV RNA is not an indicator for disease progression[68]. GT-3 HCV is associated with accelerated fibrogenesis and an increased risk of developing HCC relative to other GTs[69-71]. Host factors are important in influencing disease progression, and those include gender, race and age. Liver fibrogenesis and HCC incidence are more seen in males[68,72], whereas mild liver fibrosis and normal liver enzymes are more observed in females. Spontaneous eradication of the virus is also more seen in females[73]. A study on black and white Americans comparing disease progression showed no significant difference, making the impact of race unclear[74]. Egyptians infected with HCV GT-4 have high rates of advanced fibrosis. An Egyptian study reported strong correlation between subtypes 4a and 4o with HCC[75].
Age at infection affects prognosis[76,77]. Slower progression of the disease, at least during the first 1-2 decades, is observed in children[78] and females when infected at a young age[79]. A study by Yosry et al[80] was conducted on Egyptian children aged 3-17 years with chronic HCV on the relationship between HLA class II with clinical chemical and histopathological state in that special population. The most frequent alleles were DRB1*03, DRB1*04, and DRB1*13 (45.6%, 39.1% and 26.1%), respectively. Nearly half of the patients had DRB1*03, and it was associated with a minimal amount of liver affection. Low serum albumin (P = 0.04) was shown in patients with DRB1*04, while high aspartate aminotransferase (AST) level (P = 0.05) was shown in patients with DRB1*13. In comparison to controls, DRB1*15 was significantly reduced among cases.
Environmental factors are important in influencing disease progression, and those include alcohol consumption, tobacco inhalation and coffee consumption. Excessive alcohol intake affects disease progression and HCC risk[77,81]. Tobacco inhalation is associated with liver affection, and consequently with increased fibrosis score[82], whereas coffee has a protective role against fibrosis[83] and HCC[84]. Steatosis[66,85], insulin resistance[86,87], and type 2 diabetes[88,89] are associated with disease progression and the possibility of HCC. Iron overload is related to severe fibrosis[90]. HCV and HBV coinfection increases the incidence of HCC[81]. Large meta-analyses on patients co-infected with HIV have also shown accelerated disease progression[91,92]. Liver transplantation almost always leads to HCV re-infection, as well as accelerated disease progression (compared to non-transplant patients) which may be related to a variety of factors: Chronic HCV infection is observed in liver biopsy after 1 year post-transplantation in 50%-90% of patients, whereas cirrhosis is seen in around 20% within 5 years[93,94].
Several extrahepatic manifestations have been associated with HCV infection that may significantly affect its morbidity and mortality. Between 38% and 76% of chronic HCV patients develop at least one extrahepatic manifestation, the presence of which when clinically significant may sometimes represent a sufficient indication for treatment, even in the presence of mild liver disease[95]. HCV infection can lead to various extrahepatic manifestations, including diseases that affect the small vessels, skin, kidneys, salivary gland, eyes, thyroid, and immune system. The majority of these manifestations are immune mediated[96].
The most common extrahepatic manifestation associated with HCV infection is mixed cryoglobulinemia, or type II or III cryoglobulinemia, a lymphoproliferative disorder characterized by the production and tissue deposition of immune complexes formed by monoclonal and polyclonal immunoglobulins[97,98]. Anti-HCV antibodies and HCV RNA tend to concentrate in the cryoprecipitate[97]. The vast majority (up to approximately 95% in some studies) of patients with essential mixed cryoglobulinemia have HCV infection[98]. Approximately one half of HCV-infected patients have circulating cryoglobulins. Complexes tend to accumulate in small- to medium-sized blood vessels: Leukocytoclastic vasculitis is the typical histopathologic finding and can be found in the skin as well as in various organs and tissues, including the brain, gut, and peripheral nerves[99]. Deposition of immune complexes may affect the kidneys, resulting in glomerulonephritis, mostly of the membranoproliferative type, that may lead to renal insufficiency. A long-term consequence of the syndrome is the establishment of B-cell non-Hodgkin’s lymphoma[100]. Successful treatment of HCV infection with antivirals leads to a decrease of cryoglobulin levels in serum and to the remission of cryoglobulin - related symptoms and pathologic lesions[101].
Type 2 diabetes is more frequent in HCV infection than in HBV[102], affecting patients who are already at risk for glucose metabolism disturbances. In such patients, overt diabetes may develop earlier than in HCV-negative persons[103]. Glucose metabolism is altered by HCV at early stages of the infection, leading to insulin resistance[86] which, when combined with type 2 diabetes, accelerates liver disease progression[86,88] and reduces the response to combined pegylated interferon (PEG-IFN) with Ribavirin (RBV)[104,105], although the response to regimens containing direct-acting antiviral (DAA) does not seem to be affected by glucose metabolism alterations[106]. Other extrahepatic-associated diseases include porphyria cutanea tarda, lichen planus, necrolytic acral erythema, sialadenitis, sicca syndrome, and autoimmune thyroiditis[96].
HCV screening is necessary for all individuals at high risk of HCV infection due to a history of illicit injection drug use, history of hemodialysis, history of tattooing, healthcare workers upon accidental exposure, infants born to HCV-positive mothers, history of transfusion with blood or organ transplantation, HIV infection, or chronic liver disease/hepatitis with unknown cause including elevated liver enzymes[107,108].
Two types of assays for detecting the presence of HCV infection include serologic assays to test for antibody to HCV and molecular assays to test for HCV RNA. Identifying patient GT is important to establish prognostic risk and to guide management. HCV GT helps predict the degree of response to treatment: Patients with GT-1 or GT-4 are less likely to be cured than those with GT-2 or GT-3 with combined therapy[109].
The American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend that persons for whom HCV testing is advised should initially be tested for HCV antibodies (Management Guidelines)[108]. HCV antibody testing is sensitive and inexpensive, and it detects antibodies against the core and the NS3, NS4, and NS5 proteins. Current serologic assays are highly specific (> 99%). In children born to HCV-positive mothers, testing for anti-HCV antibodies is unreliable before 18 mo of age because their detection may be related to the passive transfer of maternal antibodies and not active infection[110].
In 2011, the Food and Drug Administration (FDA) granted approval to the OraQuick HCV Rapid Antibody test (OraSure Technologies, Bethlehem, PA), for detection of HCV antibody in finger stick capillary blood and venipuncture whole blood. Its sensitivity and specificity are similar to those of FDA-approved, laboratory-conducted HCV antibody assays[111]. Because the test is rapid, it can be performed for a larger population at risk[110].
The CDC has recommended that positive HCV antibody results can be confirmed with a HCV RNA test that detects HCV viremia[112]. It is recommended to perform quantitative HCV RNA testing on all patients candidate for HCV treatment. HCV RNA testing is also recommended for patients with negative HCV antibody test results if they are immunocompromised. In special situations in which acute HCV infection is suspected, it is important to remember that HCV antibodies may not be present. Although the seronegative window of acute infection has diminished with improved sensitivity of HCV antibody testing, HCV RNA testing is recommended as early as 1-2 wk after the initial exposure in individuals in whom early detection is desired[108,112,113].
The AASLD, IDSA, and European Association for the Study of the Liver (EASL) guidelines recommend that the fibrosis stage be initially determined either by liver biopsy (LB) or validated noninvasive techniques in all patients with HCV infection[108,113]. A LB provides the grade and stage of liver disease and may reveal unsuspected cirrhosis, necessitating surveillance for HCC. In children with HCV infection there is great variability in the degree of inflammation and fibrosis reported. Badizadegan et al[114] found periportal fibrosis in 78% and cirrhosis in 8% of 40 children younger than 18 years of age treated at Boston Children’s Hospital. A study in Cairo, Egypt, found fibrosis in 72.1% of children with HCV GT-4 (46.5% with mild and 25.6% with moderate to severe fibrosis stages), with a median age for progression of fibrosis at 5.5 years[115].
A major effort has been devoted to identifying alternative noninvasive or minimally invasive procedures for assessing liver disease by detecting the liver fibrosis stage and establishing antiviral therapy. Several algorithms have been created using clinical information or serologic markers commonly gathered at the time of a routine diagnostic workup, such as AST, ALT, and platelet count[116] or more specific substances, such as α-2-macroglobulin or hyaluronate. Combining more than one noninvasive assay appears to increase the diagnostic accuracy and may eliminate the need for LB. Transient elastography is another noninvasive approach that defines liver fibrosis using ultrasound and low-frequency elastic waves[117]. A study was conducted on chronic HCV Egyptian patients to evaluate different new noninvasive methods for assessment of liver fibrosis. The aim of that study was to evaluate whether GT-4, increased body mass index, and co-infection with schistosomiasis can interfere with liver fibrosis assessment. Egyptian HCV chronic patients (n = 312) with GT-4 underwent a LB, an elastometry measurement (Fibroscan©), and serum markers: AST-to-platelet ratio index, fibrosis-4 score (Fib4), and Fibrotest©. The researchers found that the algorithm using the Fib4 for identifying patients with F2 stage or more reduced by nearly 90% the number of LBs, and reported that noninvasive techniques were feasible in Egypt for HCV GT-4-infected patients and that Fib4 may be used to assess the F2 threshold, which decides whether treatment should be proposed or delayed[118].
At the start of the millennium, two major advances in the management of HCV took place: one, the approval by the FDA of PEG-IFN for the treatment of HCV infection, which allowed weekly subcutaneous injections instead of the previous daily or thrice weekly injections with standard IFN; and the use of weight-based RBV. By the mid-2000s, it was established that PEG-IFN-α2a or PEG-IFN-α2b could be combined with weight-based RBV for GT-1 infected patients, or with flat-dosed RBV for GT-2 or GT-3 infected patients, and that combination was better than treatment with standard IFN and RBV[119]. HCV-GT-4 response to treatment with PEG-IFN/RBV has been considered better than GT-1, and worse than GT-2 and GT-3[120,121].
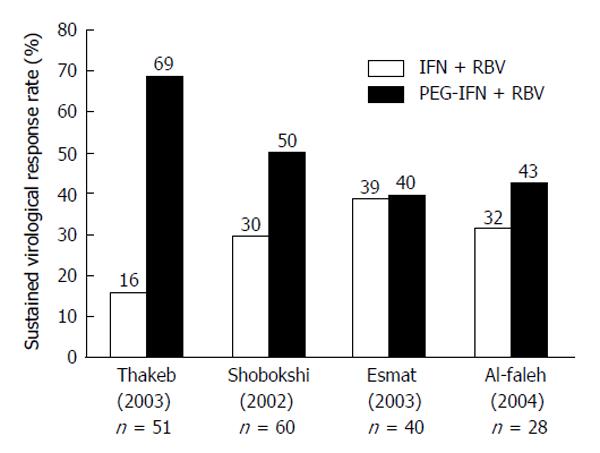
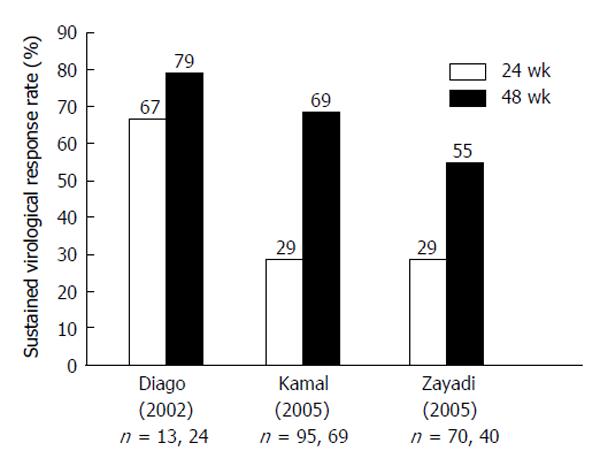
With the introduction of the HCV-GT-1 effective protease inhibitors boceprevir (BOC)[122,123] and telaprevir[122,124-126] in 2011, HCV-GT-4 became the “most difficult (GT) to treat”, as both protease inhibitors are not indicated for treatment of GT-4[127]. Many studies used 48 wk duration of combined therapy to treat HCV-GT-4, and a few of them compared responses between 24-wk treatment and 48-wk treatment response rates. Figures 2 and 3 summarize the results of those studies after using standard-dose PEG-IFN and RBV. The results show sustained virologic response (SVR) rates with 24 wk of therapy to be far less than rates with 48-wk, making the longer duration the standard of care[13].
Drawbacks for the IFN/RBV treatment include its long course duration, severe side effects, and cost, as it is not as affordable for patients in limited-resource countries[135-137].
A major indicator of good response to PEG-IFN/RBV therapy in patients with HCV GT-4 is the IL28B GT[138-140]. The favorable CC phenotype is found in 20%-30% of Egyptian patients with chronic HCV[141,142]. In Europe, SVR rates for HCV GT-4 infected patients who are IL28B CC is > 80%[139,140,143]. In Egypt, CC patients had response rates between 67% and 87%[141,142]. Mutations in the NS5A region, particularly in patients with more than 6 aa mutations in the Interferon RBV resistance - determining region (IRRDR) are highly associated with good response to PEG-IFN and RBV combination therapy, while a less diverse (≤ 5) IRRDR sequence is associated with non-response[144-146]. Another predictor of response to PEG-IFN/RBV therapy was found to be insulin resistance, which impairs response rates to PEG-IFN/RBV therapy in HCV GT-4 patients, and patients with Homeostasis Model Assessment for Insulin Resistance scores > 2 had lower SVR rates than those with scores < 2 (36% vs 72%)[147].
Abu-Mouch et al[148] found that adding vitamin-D to the standard of care with PEG-IFN/RBV therapy for HCV-GT-1 infected patients increases SVR rates from 42% to 82%. Esmat et al[149] reported that vitamin D supplementation, despite its role in other GTs, has no positive impact on treatment outcome in HCV-GT-4 patients where SVR was achieved in 51.2% in group 2, [who received the standard of care therapy (SOC therapy) plus vitamin D3 (Cholecalciferol) in a dose of 15000 IU/wk during the treatment course] and 71.4% in group 1 (who received the SOC therapy consisting of PEG-IFN-α2b plus RBV) by per-protocol analysis and in 44% in group 2 and in 68.6% in group 1 by intention to treat analysis (P value 0.22 and 0.220, respectively)[149].
The Ministry of Health in Egypt has embarked on a national treatment program since 2006, where all eligible patients are treated with PEG-IFN/RBV for 48 wk. Annually, 40000-50000 patients have been treated, and 350000 patients had received therapy in this program by 2013[150]. SVR rates for patients treated with the original PEG-IFN-α2a and alfa-2b were 54%-59%[151,152] and response rates to a locally produced biosimilar PEG-IFN were reported at 52%[153].
Infected children are generally asymptomatic and often have normal ALT levels. However, children are likely to clear the virus. Given the typically slow histologic progression of liver disease in children, some argue that treatment should be postponed until adulthood. In addition, response rates with currently available antiviral therapies approved by the FDA for the pediatric population remain suboptimal, and are associated with high costs and potential toxicities[154].
On the other hand, early eradication of HCV is likely to reduce the social stigma associated with viral infection, a source of significant caregiver stress[155], and improve the psychosocial status of patients and their families. Children also possess multiple characteristics that make them ideal candidates for treatment. Shorter duration of infection and a lesser degree of hepatic fibrosis are associated with improved response to antiviral therapy for HCV. In addition, children have fewer co-morbidities than adults and parental motivation enhances adherence to treatment. Children also tolerate currently available therapies better than adults, with mild adverse effects[156]. Economically, the cost of treatment is less than that in adults (fewer drugs used). It is also expected that eradicating the infection sooner will decrease the risk of transmission to the population at large[157-164].
The decision to initiate treatment should be individualized to each patient. However, for children with GT-1 and GT-4 who have mild disease at the time of biopsy, a watch and wait approach is acceptable, anticipating the availability of more efficacious drugs. According to the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the AASLD guidelines for management of HCV infection, considerations for treating children aged 3-17 years follow the same guidelines as adults[109].
The results of the first randomized, placebo-controlled trial of chronic pediatric HCV treatment in the United States have been published. They show that 21% of children treated with PEG-IFN alone in contrast with 53% of those treated with PEG-IFN in combination with RBV achieved a SVR, demonstrating the superiority of the combination over single therapy[165]. An international multicenter study of 107 children published by Wirth et al[166] demonstrated a SVR in 53% of patients with GT-1, 93% of those with GT-2 and GT-3, and 80% of those with GT-4.
In 2008, the results of these two trials led to FDA approval of combination therapy with PEG-IFN-α2b and RBV for use in children three years of age or older infected with HCV. The established duration of treatment at present is 48 wk for GT-1 and GT-4[154]. A study was conducted on 118 Egyptian children with chronic HCV, including both naïve patients and previous non-responders. All received PEG-IFN-α2b plus RBV. Early virological response was achieved in 69.5%, end of treatment response (ETR) was 51.7%, and SVR was achieved in 50% of the patients. Children with previously failed treatment achieved a SVR rate of 28.6%[167]. A pilot study was conducted on a small sample of Egyptian chronic HCV children (seven cases). ETR was 42.9% and SVR was 28.6%. Children with SVR were the youngest; their mean duration of infection was 4.5 years vs 12.7 years in the others. Side effects of both IFN and RBV were mild, and required no reduction in doses[168].
In another study on chronic HCV children, the novel Hansenula-derived PEG-IFN: IFN-α2a (Reiferon Retard) was used in the infected patients, whether naïve or previously treated. At week 12, patients were divided according to polymerase chain reaction (PCR) results into two groups: The first group included patients who continued treatment on a weekly basis (7-d schedule) and the second group included patients who continued treatment on a 5-d schedule. Patients from either group who were PCR-negative at week 48, but had at least one PCR positive test during therapy, were assigned to have an extended treatment course of up to 72 wk. The study was registered at http://www.clinicaltrials.gov (NCT02027493). The 5-d schedule did not affect the response rate. Treatment duration (whether 48 wk or extended 72 wk) gave similar response rates (P = 0.49). Type of previous treatment (short acting IFN vs PEG-IFN) did not affect the response to retreatment. On the other hand, SVR was significantly higher in previous relapsers than in previous non-responders (P = 0.039). It was safe, but the customized regimen did not improve response rate as SVR was detected in 23.9% of children, breakthrough was seen in 39.1% of patients, and 30.4% were non-responders. Despite recent success after the introduction of combination therapy with IFN-alpha and RBV, GT-4 is considered difficult to treat. Approximately 60% of patients fail to respond. Resistance to antiviral therapy remains a serious problem in the management of chronic HCV[169].
Currently, research is orientated to the development and approval of DAA agents regulated within the Center for Drug Evaluation and Research at the FDA for the treatment of chronic HCV. DAA agents inhibit specific stages in the HCV replication cycle through targeting the HCV polyprotein and its cleavage products[170].
The HCV life cycle: The HCV genome is composed of approximately 9600 nucleotides and generates structural and non structural (NS) proteins. The structural proteins are used to assemble new viral particles and the NS proteins support viral RNA replication. The NS3/4A, a serine protease (NS3) and cofactor (NS4A) catalyze the post-translational processing of NS proteins from the polyprotein. The products released go on to form a replicative complex (NS5A) responsible for producing viral RNA using the RNA dependent/RNA polymerase (NS5B). Finally, virions are assembled, packaged, and released[118].
Targeting this protease may inhibit viral replication. Telaprevir (TPV), BOC, and simeprevir (SMV) are all examples of HCV protease inhibitors[120,122,171].
The HCV polymerase inhibitors are another promising DAA class. These molecules are divided into nucleoside/nucleotide competitive polymerase [nucleoside inhibitors (NIs)] and allosteric inhibitors of RNA polymerase [non-NIs (NNIs)]. NS5B NIs as sofosbuvir (SOF) are structural analogues to the natural substrates of the polymerase and are incorporated into the RNA chain. This causes direct chain termination[172]. Since the active site of NS5B is highly conserved, NIs are effective against all GTs, and resistance to NIs is usually very low. Allosteric polymerase inhibitors inhibit the NS5B by binding to one of several discrete sites on the HCV polymerase, resulting in a conformational protein change. They are less potent than NIs, resistance occurs more frequently[173] and they appear to be GT specific. SOF is a nucleotide analogue inhibitor of the HCV NS5B RNA-dependent RNA polymerase[174].
Inhibition of NS5A is associated with significant reductions in HCV RNA levels, which makes these agents among the most potent antiviral molecules yet developed. NS5A inhibitors have pan-genotypic activity, i.e., they suppress replication of all HCV GTs. Daclatasvir (DCV) and ledipasvir (LDV) are examples of NS5A inhibitors[171].
With the advent and improvement of DAAs, drugs now can target almost all steps of the HCV life cycle, including entry, translation, RNA replication, assembly, and export of progeny viruses[175]. This has facilitated the designing of highly potent oral drugs characterized by shorter treatment duration, simplified dosing, a high genetic barrier to resistance, and improved safety profiles[176].
Selective inhibitors of HCV NS3/4 serine protease, BOC and TPV were developed and found to be effective in treating HCV-infected GT-1 patients in combination with PEG-IFN and RBV. Both agents are not indicated for use in GT-2 and GT-3 patients. Combination therapy using TPV or BOC with PEG-IFN and RBV resulted in higher rates of SVR for the treatment of naïve HCV GT-1 patients (range 61%-75%) compared with treatment with PEG-IFN and RBV (range 38%-49%). However, the addition of these drugs resulted in increased adverse effects such as anemia, neutropenia, fatigue, pyrexia, and insomnia[120,122]. Telaprevir may cause skin rash, in up to 5% of cases it can be severe, and it may cause Stevens-Johnson Syndrome (SJS)[177]. On December 2012; FDA Drug Safety Communication reported severe skin affection after combination treatment of PEG-IFN and RBV with Incivek (Telaprevir). These types of skin reactions (toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and SJS) lie along a continuum of serious skin reactions that can be difficult to distinguish from each other. FDA added instructions on the drug label to stop Incivek combination treatment whenever symptoms of severe skin rash appear[178].
The rates of stoppage of treatment containing TPV or BOC were higher than treatment of PEG-IFN and RBV alone. This affected compliance and the ability to complete treatment duration[179].
SMV, a HCV NS3/4 serine protease inhibitor, was recently developed, approved by the FDA in November 2013, and is now used to treat HCV GT-1 patients in combination with PEG-IFN and RBV[171,180]. The response rate is much higher than what was obtained with the use of first-generation protease inhibitors, BOC and TPV. Recently, DAAs with pan-genotypic activities SMV, SOF and DCV have been recommended in triple regimens with PEG-IFN/RBV for the treatment of HCV genotypes 4[181]. SMV is active against genotypes 1, 2, 4, 5 and 6. It is administered as a once-daily tablet orally and has demonstrated a favorable safety profile and limited drug-drug interactions[182]. RESTORE, a phase III, multicenter, single-arm, open-label study, conducted in France and Belgium, evaluated SMV (150 mg once-daily for 12 wk in combination with PegIFN/RBV, followed by 12-36 wk of Peg-IFN/RBV only) in 107 patients with HCV 4, either naïve or treatment-experienced[183]. Recent European guidelines have included a 24-48 wk SMV plus PEG-IFN/RBV combination as an option for HCV 4-related compensated liver disease (including cirrhotics), suggesting interruption of treatment if HCV-RNA levels are ≥ 25 IU/mL at week 4, 12 or 24[123].
Recently (in December 2013), the FDA approved SOF as a new component of combined HCV antiviral treatment. Phase III clinical trial using combination therapy with PEG-IFN, weight-based RBV, and SOF for 12 wk resulted in high SVR rates in GT-1, GT-4, GT-5, and GT-6 patients (89%-90%)[174,184,185]. The addition of SOF to PEG-IFN and RBV reduces the total duration of treatment.
The recent approval of SOF for treatment of HCV-GT-4 promises significant improvement in the outcome of therapy. When given as part of triple therapy with PEG-IFN/RBV for 12 wk in the phase III Neutrino trial, SOF resulted in a SVR rate of 96% in HCV-GT-4 patients[185]. When it was given for 12 wk without IFN as a dual therapy with RBV to HCV-GT-4 infected patients of Egyptian origin living in the United States, SVR rates of 79% and 59% were found in naïve and treatment experienced patients, respectively. Extending the treatment for 24 wk yielded higher SVR rates: 100% and 93%[186].
Another compound with promising efficacy against HCV-GT-4 is DCV (a NS5A inhibitor) in combination with PEG-IFN-α2a and RBV. Hézode et al[187] reported that this was generally well tolerated and achieved higher SVR rates at week 24 compared with placebo/PEG-IFN alfa/RBV among patients infected with HCV-GT-1 or GT-4.
Over the past year, IFN-sparing regimens for treating chronic HCV have become available and more superior, as those are characterized by high rates of SVR, short duration of treatment, increased tolerability, as well as room to tailor treatment according to the patients’ individual needs due to the presence of multiple agents that can interrupt several stages of the HCV lifecycle. IFN-sparing treatment is currently the new approach for both treatment-naïve and treatment-experienced patients, including even cirrhotic patients. Most treatment-experienced patients can now achieve high SVR rates (above 90%). Cirrhotic patients can reach high SVR with longer duration of treatment and/or addition of RBV therapy[188].
The first attempt to use an IFN-free regimen for HCV involved a combination of SOF and RBV. SOF is active against all GTs[185] and has an excellent safety profile and high barrier to resistance. All oral combination therapies with SOF and RBV for 12-24 wk in HCV GT-1 were evaluated[189,190]. SVR rates using weight-based dosing of RBV with SOF were higher in GT-1 treatment-naïve patients (SVR 68%-84%)[184,190]. In patients with HCV GT-2 or GT-3, a study showed higher rates of SVR after SOF plus RBV for 12 wk in comparison with PEG plus RBV for 24 wk. For HCV GT-2-infected patients, SVR rates were 97% vs 78% for each treatment group, respectively. However, for HCV GT-3-infected patients, the improvement in SVR rates in the SOF group was not observed (SVR 56% vs 63%, respectively)[185]. Another study confirmed the high SVR rate for SOF plus RBV in treatment-naïve and treatment-experienced GT-2 patients (SVR 93%) and showed an improved SVR rate when this combination is used for 24 wk in patients with HCV GT-3 (SVR 80%)[191].
A randomized, open-label study was conducted at three centers in Egypt (ClinicalTrials, NCT01838590) using SOF plus RBV for 12 wk (52 patients) or SOF plus RBV for 24 wk (51 patients). Treatment-naïve (TN) and treatment-experienced (TE) patients with chronic HCV-GT-4 (up to 20% with compensated cirrhosis) were included, 74 were GT-4a while 11 were GT- 4l, 4n, 4o, 4p, and 4u. SOF plus RBV for 24 wk resulted in a 90% SVR12 rate in patients regardless of prior treatment experience. SVR12 rates with SOF plus RBV for 12 wk were higher in TN vs TE patients. No SOF-resistance mutation S282T was found in any patient with virologic failure. Combined SOF plus RBV for 12 or 24 wk were well tolerated. The authors concluded that SOF plus RBV for 24 wk provides a simple, effective, IFN-free regimen for patients with HCV-GT-4[192].
Phase 2 trials with SOF and RBV in adolescents and children (3-17 years) with GT-2 and GT-3 began in 2014 with an estimated study completion date in May 2018 (ClinicalTrials, Identifier: NCT02175758). HCV infected children may soon realize the benefits from the tremendous research in anti-HCV therapy in the last five years[185,190,193].
Dual therapy tends to be more effective than monotherapy in regards to viral eradication and decreasing the risk of viral resistance[194]. These targets include NS3/4A protease inhibitors (PI), NS5A inhibitors, and NS5B polymerase inhibitors [both NI/tide inhibitors and NNI]. More recently, combinations of these DAAs have been effectively used without the use of IFN and RBV to achieve high rates of SVR. In an open-label study, oral SOF and DCV taken once daily were associated with high SVR in patients infected with HCV GT-1, GT-2, and GT-3, including patients with no response to prior therapy with TPV or BOC[195].
Phase 3 is a multicenter, open-label, single-arm study conducted at multiple sites in Spain, to investigate the efficacy and safety of a 12-wk regimen of SMV in combination with SOF in TN or TE subjects (age: 18-70 years) with chronic GT-4 HCV infection (ClinicalTrials, Identifier: NCT02250807, started January 2015 with estimated study completion date January 2016). The FDA approved SMV to be used with SOF as a combination therapy in GT-1 in November 2014. Another Egyptian study (NCT 02278419) to investigate the efficacy and safety of an 8- or 12-wk treatment regimen of SMV in combination with SOF in TN and TE adult participants with chronic HCV-GT-4 is ongoing.
For children and adolescents with chronic HCV infection, a Phase 2 study will be conducted to investigate the safety and efficacy of LDV/SOF fixed dose combination in this particular age group (ClinicalTrials, Identifier: NCT02249182). The FDA approved the first combination pill-LDV/SOF (Harvoni) Gilead, for treatment of HCV GT-1 in October 2014.
The FDA approved a three drug regimen called the AbbVie Viekira Pak (Ombitasvir, Paritaprevir, and Ritonavir tablets co-packed with Dasabuvir tablets) to treat patients with chronic HCV-GT-1 infection including cirrhotic cases in December 2014. In a randomized, open-label trial of Faldaprevir (a NS3/4A protease inhibitor) and Deleobuvir (a nonnucleoside NS5B polymerase inhibitor) with or without RBV (phase IIB), used by 362 TN patients infected with HCV GT-1, the SVR at 12 wk was 52%-59% among patients who received IFN-free treatment with Faldaprevir plus Deleobuvir plus RBV[196].
The AASLD announced detailed results from The PEARL-I study [ABT-450/R (Protease inhibitor and Ritonavir) + ABT-267 (NS5A inhibitor) ± RBV] (open- label Phase 2b), which demonstrated that 100% of GT-4 patients who were new to therapy (n = 42/42) or who had failed previous treatment with PEG-IFN and RBV (n = 49/49) achieved SVR rate at 12 wk post-treatment after taking the AbbVie investigational treatment with RBV. Additionally, 90.9% of patients who were new to therapy achieved SVR 12 (n = 40/44) after taking the treatment without RBV[197].
Another open-label Egyptian study (phase 3) began in November 2014 to evaluate the safety and efficacy of the co-administration of ABT-450/Ritonavir/ABT-267 (ABT-450/r/ABT-267) with RBV in adults with chronic HCV-GT-4 in Egypt. It includes 160 patients and has an estimated study completion date of August 2016 (ClinicalTrials, Identifier: NCT02247401). Currently, Egypt is conducting a national mass treatment program for HCV GT-4 patients based on the EASL’s 2014 practice guidelines for HCV-GT-4, which (based on two studies: Neutrino study for IFN-based therapy and Ruane study for IFN-free regimens) involves triple therapy using PEG-IFN/weight-based RBV/SOF 400 mg for 12 wk for IFN-eligible patients or RBV/SOF 400 mg for 24 wk for patients who are unable to tolerate IFN. Advanced liver fibrosis patients are included in the IFN-free regimen.
Due to advancements in HCV therapy, the AASLD and IDSA designed a new protocol for the use of new agents[108]. The EASL also announced new protocols on HCV therapy in 2014, including the use of all-oral combinations of SOF/SMV and SOF/DCV[113]. The AASLD, IDSA, and EASL will release updated guidelines as new therapeutics and regimens become approved by the FDA and European Medicines Agency.
The field of IFN-free HCV therapy is under constant development. Currently, DAA’s provide patients with high SVR rates (above 90%) with short duration treatment and increased tolerable adverse effect profiles. Various oral therapy combinations can be used to improve SVR outcomes in the TE patient, according to HCV GT/subtype, type of prior regimen, and presence of cirrhosis[188].
The next steps in the clinical development of anti-HCV therapy are expected in late 2015 and early 2016 with the availability of pangenotypic ultrarapid (4-8 wk) single pill regimens such as Grazoprevir/MK8742, SOF/GS5816, and BMS791325/DAC/Asunaprevir[198].
The most common and tolerable adverse effects of DAA combination therapy are nasopharyngitis, headache and malaise[199]. However The FDA warned on March 2015 that serious slowing of the heart rate can occur when the antiarrhythmic drug amiodarone is taken together with either Harvoni (Ledipasvir/SOF) or with SOF taken in combination with another direct acting antiviral for the treatment of hepatitis C infection. They recommended that health care professionals should not prescribe either Harvoni or SOF combined with another direct acting antiviral, such as DCV or Olysio (SMV), with amiodarone[200].
A new HCV vaccine from GlaxoSmithKline has shown promise in early clinical tests, prompting strong and broad immune responses. Researchers have evaluated the vaccine in humans, and it is now ready for phase 2 efficacy studies[201].
Another Egyptian clinical trial in the field of HCV vaccination is promising: Safety and efficacy of a novel candidate peptide vaccine against HCV infection in healthy volunteers and in treated (Non-responders/responders) chronic HCV patients. Clinical Trials (phases I and II) started in March 2011 (Clinical Trial, Identifier: NCT01718834; National Liver Institute, Menofyia University, Egypt).
HCV therapy is steadily moving to an all oral, well-tolerated, DAA, short-term, and more efficacious regimen. An IFN-free regimen will be available for treatment of all GTs of HCV in the near future. To date, several DAAs have been developed and are currently being evaluated in various combinations in clinical trials (for current management strategies of chronic HCV see http://www.aasld.org). We can expect changes in treatment recommendations of HCV as new regimens are developed and new agents are approved by the FDA. The availability of shorter, simpler, well-tolerated treatment regimens can have a major impact in reducing the morbidity and mortality associated with HCV infection. With such a large number of therapeutic agents we can end up with a world of choices that we can select from to treat patients. We hope not just to treat some patients with HCV infection but also treat all patients to achieve a cure regardless of their fibrosis state.
P- Reviewer: Basu PP, Kim SR S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Houghton M. Discovery of the hepatitis C virus. Liver Int. 2009;29 Suppl 1:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 2. | Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1931] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 3. | WHO. Hepatitis C Fact sheet No. 164, 2014. [Accessed 2015 May 1]. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 4. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 5. | Preciado MV, Valva P, Escobar-Gutierrez A, Rahal P, Ruiz-Tovar K, Yamasaki L, Vazquez-Chacon C, Martinez-Guarneros A, Carpio-Pedroza JC, Fonseca-Coronado S. Hepatitis C virus molecular evolution: transmission, disease progression and antiviral therapy. World J Gastroenterol. 2014;20:15992-16013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (5)] |
| 6. | Tang H, Grisé H. Cellular and molecular biology of HCV infection and hepatitis. Clin Sci (Lond). 2009;117:49-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Lambers FA, Prins M, Thomas X, Molenkamp R, Kwa D, Brinkman K, van der Meer JT, Schinkel J. Alarming incidence of hepatitis C virus re-infection after treatment of sexually acquired acute hepatitis C virus infection in HIV-infected MSM. AIDS. 2011;25:F21-F27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 965] [Cited by in RCA: 982] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 9. | Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85:3173-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 10. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1146] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 11. | Zein NN, Rakela J, Krawitt EL, Reddy KR, Tominaga T, Persing DH. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Collaborative Study Group. Ann Intern Med. 1996;125:634-639. [PubMed] |
| 12. | Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, Beall E, Yap PL, Kolberg J, Urdea MS. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74:2391-2399. [PubMed] |
| 13. | Wantuck JM, Ahmed A, Nguyen MH. Review article: the epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment Pharmacol Ther. 2014;39:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Martial J, Morice Y, Abel S, Cabié A, Rat C, Lombard F, Edouard A, Pierre-Louis S, Garsaud P, Béra O. Hepatitis C virus (HCV) genotypes in the Caribbean island of Martinique: evidence for a large radiation of HCV-2 and for a recent introduction from Europe of HCV-4. J Clin Microbiol. 2004;42:784-791. [PubMed] |
| 15. | Raghuraman S, Abraham P, Sridharan G, Daniel HD, Ramakrishna BS, Shaji RV. HCV genotype 4--an emerging threat as a cause of chronic liver disease in Indian (south) patients. J Clin Virol. 2004;31:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Singh S, Malhotra V, Sarin SK. Distribution of hepatitis C virus genotypes in patients with chronic hepatitis C infection in India. Indian J Med Res. 2004;119:145-148. [PubMed] |
| 17. | Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1363] [Article Influence: 123.9] [Reference Citation Analysis (0)] |
| 18. | El-Zanaty F, Way A. Demographic and Health Survey. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El-Zanaty and Associates, and Macro International 2009; Available from: http://dhsprogram.com/pubs/pdf/FR220/ FR220.pdf. |
| 19. | Baatarkhuu O, Kim do Y, Ahn SH, Nymadawa P, Dahgwahdorj Y, Shagdarsuren M, Park JY, Choi JW, Oyunbileg J, Oyunsuren T. Prevalence and genotype distribution of hepatitis C virus among apparently healthy individuals in Mongolia: a population-based nationwide study. Liver Int. 2008;28:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. Genetic epidemiology of hepatitis C virus throughout egypt. J Infect Dis. 2000;182:698-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hézode C, Lázaro P. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [PubMed] |
| 24. | Rao MR, Naficy AB, Darwish MA, Darwish NM, Schisterman E, Clemens JD, Edelman R. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect Dis. 2002;2:29. [PubMed] |
| 25. | El Sherbini A, Mohsen SA, Hasan W, Mostafa S, El Gohary K, Moneib A, Abaza AH. The peak impact of an Egyptain outbreak of hepatitis C virus: has it passed or has not yet occurred? Liver Int. 2007;27:876-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 195] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Medhat A, Shehata M, Magder LS, Mikhail N, Abdel-Baki L, Nafeh M, Abdel-Hamid M, Strickland GT, Fix AD. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg. 2002;66:633-638. [PubMed] |
| 28. | Arafa N, El Hoseiny M, Rekacewicz C, Bakr I, El-Kafrawy S, El Daly M, Aoun S, Marzouk D, Mohamed MK, Fontanet A. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol. 2005;43:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Darwish MA, Faris R, Darwish N, Shouman A, Gadallah M, El-Sharkawy MS, Edelman R, Grumbach K, Rao MR, Clemens JD. Hepatitis c and cirrhotic liver disease in the Nile delta of Egypt: a community-based study. Am J Trop Med Hyg. 2001;64:147-153. [PubMed] |
| 30. | Mohamed MK, Magder LS, Abdel-Hamid M, El-Daly M, Mikhail NN, Abdel-Aziz F, Medhat A, Thiers V, Strickland GT. Transmission of hepatitis C virus between parents and children. Am J Trop Med Hyg. 2006;75:16-20. [PubMed] |
| 31. | AbdulQawi K, Youssef A, Metwally MA, Ragih I, AbdulHamid M, Shaheen A. Prospective study of prevalence and risk factors for hepatitis C in pregnant Egyptian women and its transmission to their infants. Croat Med J. 2010;51:219-228. [PubMed] |
| 32. | Shebl FM, El-Kamary SS, Saleh DA, Abdel-Hamid M, Mikhail N, Allam A, El-Arabi H, Elhenawy I, El-Kafrawy S, El-Daly M. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J Med Virol. 2009;81:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, Chuang CK. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J Hepatol. 2014;6:643-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 35. | Abdelwahab MS, El-Raziky MS, Kaddah NA, Abou-Elew HH. Prevalence of hepatitis C virus infection and human immunodeficiency virus in a cohort of Egyptian hemophiliac children. Ann Saudi Med. 2012;32:200-202. [PubMed] |
| 36. | Barakat SH, El-Bashir N. Hepatitis C virus infection among healthy Egyptian children: prevalence and risk factors. J Viral Hepat. 2011;18:779-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | El-Karaksy H, Anwar GH, El-Raziky MS, El-Hawary M, Hashem M, El-Sayed R, El-Shabrawi M, Mohsen N, Fouad H, Esmat G. Anti-HCV prevalence among diabetic and non-diabetic Egyptian children. Curr Diabetes Rev. 2010;6:388-392. [PubMed] |
| 38. | Breban R, Doss W, Esmat G, Elsayed M, Hellard M, Ayscue P, Albert M, Fontanet A, Mohamed MK. Towards realistic estimates of HCV incidence in Egypt. J Viral Hepat. 2013;20:294-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Saleh DA, Shebl FM, El-Kamary SS, Magder LS, Allam A, Abdel-Hamid M, Mikhail N, Hashem M, Sharaf S, Stoszek SK. Incidence and risk factors for community-acquired hepatitis C infection from birth to 5 years of age in rural Egyptian children. Trans R Soc Trop Med Hyg. 2010;104:357-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Mostafa A, Taylor SM, el-Daly M, el-Hoseiny M, Bakr I, Arafa N, Thiers V, Rimlinger F, Abdel-Hamid M, Fontanet A. Is the hepatitis C virus epidemic over in Egypt? Incidence and risk factors of new hepatitis C virus infections. Liver Int. 2010;30:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Reker C, Islam KM. Risk factors associated with high prevalence rates of hepatitis C infection in Egypt. Int J Infect Dis. 2014;25:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Centers for Disease Control and Prevention (CDC). Progress toward prevention and control of hepatitis C virus infection--Egypt, 2001-2012. MMWR Morb Mortal Wkly Rep. 2012;61:545-549. [PubMed] |
| 43. | Esmat G, Hashem M, El-Raziky M, El-Akel W, El-Naghy S, El-Koofy N, El-Sayed R, Ahmed R, Atta-Allah M, Hamid MA. Risk factors for hepatitis C virus acquisition and predictors of persistence among Egyptian children. Liver Int. 2012;32:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Kandeel AM, Talaat M, Afifi SA, El-Sayed NM, Abdel Fadeel MA, Hajjeh RA, Mahoney FJ. Case control study to identify risk factors for acute hepatitis C virus infection in Egypt. BMC Infect Dis. 2012;12:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 45. | Paez Jimenez A, Sharaf Eldin N, Rimlinger F, El-Daly M, El-Hariri H, El-Hoseiny M, Mohsen A, Mostafa A, Delarocque-Astagneau E, Abdel-Hamid M. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59:1554-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Plancoulaine S, Mohamed MK, Arafa N, Bakr I, Rekacewicz C, Trégouët DA, Obach D, El Daly M, Thiers V, Féray C. Dissection of familial correlations in hepatitis C virus (HCV) seroprevalence suggests intrafamilial viral transmission and genetic predisposition to infection. Gut. 2008;57:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Saleh DA, Shebl F, Abdel-Hamid M, Narooz S, Mikhail N, El-Batanony M, El-Kafrawy S, El-Daly M, Sharaf S, Hashem M. Incidence and risk factors for hepatitis C infection in a cohort of women in rural Egypt. Trans R Soc Trop Med Hyg. 2008;102:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Zahran KM, Badary MS, Agban MN, Abdel Aziz NH. Pattern of hepatitis virus infection among pregnant women and their newborns at the Women’s Health Center of Assiut University, Upper Egypt. Int J Gynaecol Obstet. 2010;111:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 50. | Centers for Disease Contro and Provention. Disease Burden from Viral Hepatitis A, B, and C in the United States. 2015; Available from: http://www.cdc.gov/hepatitis/Statistics/index.htm. |
| 51. | Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | McHutchison JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21-S29. [PubMed] |
| 53. | Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 417] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 54. | Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80-88. [PubMed] |
| 55. | Yoshikawa M, Morimoto Y, Shiroi A, Yoshiji H, Kuriyama S, Fukui H. Spontaneous elimination of serum HCV-RNA after total gastrectomy for early gastric cancer in a patient with chronic hepatitis C. Am J Gastroenterol. 2001;96:922-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Fujisawa T, Komatsu H, Inui A, Miyagawa Y, Onoue M, Sekine I, Yokota S, Hanada R, Yamamoto K, Inui M. Spontaneous remission of chronic hepatitis C in children. Eur J Pediatr. 1997;156:773-776. [PubMed] |
| 57. | Iorio R, Giannattasio A, Sepe A, Terracciano LM, Vecchione R, Vegnente A. Chronic hepatitis C in childhood: an 18-year experience. Clin Infect Dis. 2005;41:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Vogt M, Lang T, Frösner G, Klingler C, Sendl AF, Zeller A, Wiebecke B, Langer B, Meisner H, Hess J. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 325] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 59. | Wirth S. Current treatment options and response rates in children with chronic hepatitis C. World J Gastroenterol. 2012;18:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Santantonio T, Medda E, Ferrari C, Fabris P, Cariti G, Massari M, Babudieri S, Toti M, Francavilla R, Ancarani F. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 62. | El-Awady MK, Mostafa L, Tabll AA, Abdelhafez TH, Bader El Din NG, Zayed N, Shenawy RE, El Abd Y, Hasan RM, Zaghlol H. Association of IL28B SNP With Progression of Egyptian HCV Genotype 4 Patients to End Stage Liver Disease. Hepat Mon. 2012;12:271-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | Ruiz-Extremera A, Muñoz-Gámez JA, Salmerón-Ruiz MA, de Rueda PM, Quiles-Pérez R, Gila-Medina A, Casado J, Belén Martín A, Sanjuan-Nuñez L, Carazo A. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology. 2011;53:1830-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Danta M, Semmo N, Fabris P, Brown D, Pybus OG, Sabin CA, Bhagani S, Emery VC, Dusheiko GM, Klenerman P. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis. 2008;197:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Schnuriger A, Dominguez S, Guiguet M, Harfouch S, Samri A, Ouazene Z, Slama L, Simon A, Valantin MA, Thibault V. Acute hepatitis C in HIV-infected patients: rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS. 2009;23:2079-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 67. | Mathurin P, Moussalli J, Cadranel JF, Thibault V, Charlotte F, Dumouchel P, Cazier A, Huraux JM, Devergie B, Vidaud M. Slow progression rate of fibrosis in hepatitis C virus patients with persistently normal alanine transaminase activity. Hepatology. 1998;27:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Müllhaupt B, Borovicka J, Heim M, Moradpour D, Cerny A. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 69. | Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 70. | Nkontchou G, Ziol M, Aout M, Lhabadie M, Baazia Y, Mahmoudi A, Roulot D, Ganne-Carrie N, Grando-Lemaire V, Trinchet JC. HCV genotype 3 is associated with a higher hepatocellular carcinoma incidence in patients with ongoing viral C cirrhosis. J Viral Hepat. 2011;18:e516-e522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 72. | Chiba T, Matsuzaki Y, Abei M, Shoda J, Aikawa T, Tanaka N, Osuga T. Multivariate analysis of risk factors for hepatocellular carcinoma in patients with hepatitis C virus-related liver cirrhosis. J Gastroenterol. 1996;31:552-558. [PubMed] |
| 73. | Narciso-Schiavon JL, Schiavon LL, Carvalho-Filho RJ, Freire FC, Cardoso JR, Bordin JO, Silva AE, Ferraz ML. Anti-hepatitis C virus-positive blood donors: are women any different? Transfus Med. 2008;18:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Terrault NA, Im K, Boylan R, Bacchetti P, Kleiner DE, Fontana RJ, Hoofnagle JH, Belle SH. Fibrosis progression in African Americans and Caucasian Americans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:1403-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Abdel-Hamid M, El-Daly M, Molnegren V, El-Kafrawy S, Abdel-Latif S, Esmat G, Strickland GT, Loffredo C, Albert J, Widell A. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J Gen Virol. 2007;88:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, Paggi S, Conte D. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [PubMed] |
| 78. | Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol. 2004;19:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Wiese M, Grüngreiff K, Güthoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany--a 25-year multicenter study. J Hepatol. 2005;43:590-598. [PubMed] |
| 80. | Yosry A, Fouad R, Mahmoud S, El-Raziky MS, El-Hennawy A, Ghoneim MA. The association of HLA class II DR B1 alleles with HCV infection in Egyptian children. Arab J Gastroenterol. 2011;12:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695-699. [PubMed] |
| 82. | Hézode C, Lonjon I, Roudot-Thoraval F, Mavier JP, Pawlotsky JM, Zafrani ES, Dhumeaux D. Impact of smoking on histological liver lesions in chronic hepatitis C. Gut. 2003;52:126-129. [PubMed] |
| 83. | Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, Lee WM, Lok AS, Di Bisceglie AM, Bonkovsky HL. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50:1360-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 84. | Ohishi W, Fujiwara S, Cologne JB, Suzuki G, Akahoshi M, Nishi N, Takahashi I, Chayama K. Risk factors for hepatocellular carcinoma in a Japanese population: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 86. | Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected]. Gastroenterology. 2003;125:1695-1704. [PubMed] |
| 87. | Hung CH, Wang JH, Hu TH, Chen CH, Chang KC, Yen YH, Kuo YH, Tsai MC, Lu SN, Lee CM. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265-2271. [PubMed] |
| 88. | Hu SX, Kyulo NL, Xia VW, Hillebrand DJ, Hu KQ. Factors associated with hepatic fibrosis in patients with chronic hepatitis C: a retrospective study of a large cohort of U.S. patients. J Clin Gastroenterol. 2009;43:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Veldt BJ, Chen W, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, de Knegt RJ, Zeuzem S, Manns MP, Hansen BE. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 90. | Bonkovsky HL, Lambrecht RW, Shan Y. Iron as a co-morbid factor in nonhemochromatotic liver disease. Alcohol. 2003;30:137-144. [PubMed] |
| 91. | Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. World J Gastroenterol. 2009;15:996-1003. [PubMed] |
| 92. | Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 93. | Germani G, Tsochatzis E, Papastergiou V, Burroughs AK. HCV in liver transplantation. Semin Immunopathol. 2013;35:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Meriden Z, Forde KA, Pasha TL, Hui JJ, Reddy KR, Furth EE, Wells RG. Histologic predictors of fibrosis progression in liver allografts in patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8:289-296, 296.e1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Himoto T, Masaki T. Extrahepatic manifestations and autoantibodies in patients with hepatitis C virus infection. Clin Dev Immunol. 2012;2012:871401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Ko HM, Hernandez-Prera JC, Zhu H, Dikman SH, Sidhu HK, Ward SC, Thung SN. Morphologic features of extrahepatic manifestations of hepatitis C virus infection. Clin Dev Immunol. 2012;2012:740138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 97. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 861] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 98. | Sène D, Limal N, Cacoub P. Hepatitis C virus-associated extrahepatic manifestations: a review. Metab Brain Dis. 2004;19:357-381. [PubMed] |
| 99. | Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, Sulfaro S, Franzin F, Tulissi P, Moretti M. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047-3053. [PubMed] |
| 101. | Saadoun D, Delluc A, Piette JC, Cacoub P. Treatment of hepatitis C-associated mixed cryoglobulinemia vasculitis. Curr Opin Rheumatol. 2008;20:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 103. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 104. | Deltenre P, Louvet A, Lemoine M, Mourad A, Fartoux L, Moreno C, Henrion J, Mathurin P, Serfaty L. Impact of insulin resistance on sustained response in HCV patients treated with pegylated interferon and ribavirin: a meta-analysis. J Hepatol. 2011;55:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 105. | Elgouhari HM, Zein CO, Hanouneh I, Feldstein AE, Zein NN. Diabetes mellitus is associated with impaired response to antiviral therapy in chronic hepatitis C infection. Dig Dis Sci. 2009;54:2699-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Younossi Z, Negro F, Serfaty L, Pol S, Diago M, Zeuzem S, Andreone P, Lawitz EJ, Roberts S, Focaccia R. Homeostasis model assessment of insulin resistance does not seem to predict response to telaprevir in chronic hepatitis C in the REALIZE trial. Hepatology. 2013;58:1897-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-39. [PubMed] |
| 108. | AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C. [Accessed 2015 Apr 29]. Available from: http://hcvguidelines.org/full-report-view. |
| 109. | Rose R, Markov PV, Lam TT, Pybus OG. Viral evolution explains the associations among hepatitis C virus genotype, clinical outcomes, and human genetic variation. Infect Genet Evol. 2013;20:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 111. | Shivkumar S, Peeling R, Jafari Y, Joseph L, Pant Pai N. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 112. | Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-365. [PubMed] |
| 113. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 114. | Badizadegan K, Jonas MM, Ott MJ, Nelson SP, Perez-Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology. 1998;28:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | El-Hawary MA, El-Raziky MS, Esmat G, Soliman H, Abouzied A, El-Raziky M, El-Akel W, El-Sayed R, Shebl F, Shaheen AA. Assessment of hepatic fibrosis in pediatric cases with hepatitis C virus in Egypt. World J Gastroenterol. 2007;13:2846-2851. [PubMed] |
| 116. | Castera L. Transient elastography and other noninvasive tests to assess hepatic fibrosis in patients with viral hepatitis. J Viral Hepat. 2009;16:300-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 117. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 118. | Bonnard P, Elsharkawy A, Zalata K, Delarocque-Astagneau E, Biard L, Le Fouler L, Hassan AB, Abdel-Hamid M, El-Daly M, Gamal ME. Comparison of liver biopsy and noninvasive techniques for liver fibrosis assessment in patients infected with HCV-genotype 4 in Egypt. J Viral Hepat. 2015;22:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Cortez KJ, Kottilil S. Beyond interferon: rationale and prospects for newer treatment paradigms for chronic hepatitis C. Ther Adv Chronic Dis. 2015;6:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Irshad M, Ansari MA, Singh A, Nag P, Raghvendra L, Singh S, Badhal SS. HCV-genotypes: a review on their origin, global status, assay system, pathogenecity and response to treatment. Hepatogastroenterology. 2010;57:1529-1538. [PubMed] |
| 121. | Varghese R, Al-Khaldi J, Asker H, Fadili AA, Al Ali J, Hassan FA. Treatment of chronic hepatitis C genotype 4 with peginterferon alpha-2a plus ribavirin. Hepatogastroenterology. 2009;56:218-222. [PubMed] |
| 122. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 123. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 124. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1854] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 125. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 126. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1199] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 127. | Benhamou Y, Moussalli J, Ratziu V, Lebray P, De Backer K, De Meyer S, Ghys A, Luo D, Picchio GR, Beumont M. Telaprevir activity in treatment-naive patients infected hepatitis C virus genotype 4: a randomized trial. J Infect Dis. 2013;208:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Alfaleh FZ, Hadad Q, Khuroo MS, Aljumah A, Algamedi A, Alashgar H, Al-Ahdal MN, Mayet I, Khan MQ, Kessie G. Peginterferon alpha-2b plus ribavirin compared with interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C in Saudi patients commonly infected with genotype 4. Liver Int. 2004;24:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 129. | Esmat G, Mohamed MK, Abdel Hamid M, Zalata K, Khatab H, El Batanony M, Abouzied AM, El Raziky M, Shaheen AM, Ismail A. The impact of steatosis on baseline characteristic and end of treatment response for chronic hepatitis (C) genotype 4 patients treated with interferon. J Hepato. 2003;38:139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 130. | Shobokshi O, Serebour F, Skakni L, Tantawi A, Dinish T, Al Quaiz M, Sandokji A, Al-Kayyal B, Al Momen S, Akbar H. Efficacy of pegylated (40 KDA) IFN alfa-2a (PEGASYS) plus ribavirin in the treatment of hepatitis C genotype 4 chronic active patients in Saudi Arabia. J Hepato. 2002;36 Supplement 1:129. [DOI] [Full Text] |
| 131. | Thakeb F, Omar M, Bilharz T, Awady M, Isshak S. Randomized controlled trial of peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus-genotype 4 among Egyptian patients. Hepatology. 2003;38:278A. [DOI] [Full Text] |
| 132. | Diago M, Hadziayannus S, Bodenheimer H, Hassanein T, Uchman S, Marcellin P, Ramadori G, Delwaide J, Sedarati F. Optimized virological response in genotype 4 chrnoic hepatitis C patients treated with peginterferon alfa-2a (PEGASYS) in combination with ribavirin (RBV). Hepatology. 2002;36:364A. |
| 133. | El-Zayadi AR, Attia M, Barakat EM, Badran HM, Hamdy H, El-Tawil A, El-Nakeeb A, Selim O, Saied A. Response of hepatitis C genotype-4 naïve patients to 24 weeks of Peg-interferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Kamal SM, El Tawil AA, Nakano T, He Q, Rasenack J, Hakam SA, Saleh WA, Ismail A, Aziz AA, Madwar MA. Peginterferon {alpha}-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut. 2005;54:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Davies A, Singh KP, Shubber Z, Ducros P, Mills EJ, Cooke G, Ford N. Treatment outcomes of treatment-naïve Hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PLoS One. 2013;8:e55373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Ford N, Kirby C, Singh K, Mills EJ, Cooke G, Kamarulzaman A, duCros P. Chronic hepatitis C treatment outcomes in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ. 2012;90:540-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Ford N, Singh K, Cooke GS, Mills EJ, von Schoen-Angerer T, Kamarulzaman A, du Cros P. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 138. | Antaki N, Bibert S, Kebbewar K, Asaad F, Baroudi O, Alideeb S, Hadad M, Abboud D, Sabah H, Bochud PY. IL28B polymorphisms predict response to therapy among chronic hepatitis C patients with HCV genotype 4. J Viral Hepat. 2013;20:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 139. | De Nicola S, Aghemo A, Rumi MG, Galmozzi E, Valenti L, Soffredini R, De Francesco R, Prati GM, D’Ambrosio R, Cheroni C. Interleukin 28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis C genotype 4. Hepatology. 2012;55:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 140. | Stättermayer AF, Strassl R, Maieron A, Rutter K, Stauber R, Strasser M, Beinhardt S, Datz C, Scherzer TM, Steindl-Munda P. Polymorphisms of interferon-λ4 and IL28B - effects on treatment response to interferon/ribavirin in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2014;39:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 141. | El Awady MK, Bader El Din NG, Tabll A, El Hosary Y, Abdel Aziz AO, El Khayat H, Salama M, Abdelhafez TH. IL28B polymorphism and cytomegalovirus predict response to treatment in Egyptian HCV type 4 patients. World J Gastroenterol. 2013;19:290-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Ragheb MM, Nemr NA, Kishk RM, Mandour MF, Abdou MM, Matsuura K, Watanabe T, Tanaka Y. Strong prediction of virological response to combination therapy by IL28B gene variants rs12979860 and rs8099917 in chronic hepatitis C genotype 4. Liver Int. 2014;34:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 143. | Asselah T, De Muynck S, Broët P, Masliah-Planchon J, Blanluet M, Bièche I, Lapalus M, Martinot-Peignoux M, Lada O, Estrabaud E. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol. 2012;56:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 144. | Kim SR, El-Shamy A, Imoto S, Kim KI, Ide YH, Deng L, Shoji I, Tanaka Y, Hasegawa Y, Ota M. Prediction of response to pegylated interferon/ribavirin combination therapy for chronic hepatitis C genotype 1b and high viral load. J Gastroenterol. 2012;47:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 145. | El-Shamy A, Nagano-Fujii M, Sasase N, Imoto S, Kim SR, Hotta H. Sequence variation in hepatitis C virus nonstructural protein 5A predicts clinical outcome of pegylated interferon/ribavirin combination therapy. Hepatology. 2008;48:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 146. | Yano Y, Seo Y, Miki A, Saito M, Kato H, Hamano K, Oya M, Ouchi S, Fujisawa T, Yamada H. Mutations in non-structural 5A and rapid viral response to pegylated interferon-α-2b plus ribavirin therapy are associated with therapeutic efficacy in patients with genotype 1b chronic hepatitis C. Int J Mol Med. 2012;30:1048-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Moucari R, Ripault MP, Martinot-Peignoux M, Voitot H, Cardoso AC, Stern C, Boyer N, Maylin S, Nicolas-Chanoine MH, Vidaud M. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut. 2009;58:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184-5190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 149. | Esmat G, El Raziky M, Elsharkawy A, Sabry D, Hassany M, Ahmed A, Assem N, El Kassas M, Doss W. Impact of vitamin D supplementation on sustained virological response in chronic hepatitis C genotype 4 patients treated by pegylated interferon/ribavirin. J Interferon Cytokine Res. 2015;35:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Doss W, Mohamed MK, Esmat G, El Sayed M, Fontanet A, Cooper S, El Sayed N. Arab Republic of Egypt, Ministry of Health and Population National Committee for the Control of Viral Hepatitis 2008. Available from: http://www.hepnile.org/images/stories/doc/NSP_10_April_2008_final2.pdf. |
| 151. | El Raziky M, Fathalah WF, El-Akel WA, Salama A, Esmat G, Mabrouk M, Salama RM, Khatab HM. The Effect of Peginterferon Alpha-2a vs. Peginterferon Alpha-2b in Treatment of Naive Chronic HCV Genotype-4 Patients: A Single Centre Egyptian Study. Hepat Mon. 2013;13:e10069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 152. | Esmat G, El Kassas M, Hassany M, Gamil M, El Raziky M. Optimizing treatment for HCV genotype 4: PEG-IFN alfa 2a vs. PEG-IFN alfa 2b; the debate continues. Liver Int. 2014;34 Suppl 1:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 153. | Taha AA, El-Ray A, El-Ghannam M, Mounir B. Efficacy and safety of a novel pegylated interferon alpha-2a in Egyptian patients with genotype 4 chronic hepatitis C. Can J Gastroenterol. 2010;24:597-602. [PubMed] |
| 154. | Fifi A, Barreto A, Delgado-Borrego A. Optimal management of pediatric hepatitis C infection: a review. Pediatric Health Med Ther. 2014;5:173-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 155. | Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, Narkewicz MR, Rosenthal P, Smith LJ. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48:341-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 156. | Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, Wintermeyer P, Jenke A. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology. 2005;41:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 157. | Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. J Pediatr Gastroenterol Nutr. 2001;33:570-575. [PubMed] |
| 158. | Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 216] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 159. | Delgado-Borrego A, Smith L, Jonas MM, Hall CA, Negre B, Jordan SH, Ogrodowicz M, Raza R, Ludwig DA, Miller T. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. J Pediatr. 2012;161:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 160. | González-Peralta RP, Langham MR, Andres JM, Mohan P, Colombani PM, Alford MK, Schwarz KB. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2009;48:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 161. | Karnsakul W, Alford MK, Schwarz KB. Managing pediatric hepatitis C: current and emerging treatment options. Ther Clin Risk Manag. 2009;5:651-660. [PubMed] |
| 162. | Mohan N, González-Peralta RP, Fujisawa T, Chang MH, Heller S, Jara P, Kelly D, Mieli-Vergani G, Shah U, Murray KF. Chronic hepatitis C virus infection in children. J Pediatr Gastroenterol Nutr. 2010;50:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 163. | Shiraki K, Ohto H, Inaba N, Fujisawa T, Tajiri H, Kanzaki S, Matsui A, Morishima T, Goto K, Kimura A. Guidelines for care of pregnant women carrying hepatitis C virus and their infants. Pediatr Int. 2008;50:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 164. | Tovo PA, Palomba E, Ferraris G, Principi N, Ruga E, Dallacasa P, Maccabruni A. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124. [PubMed] |
| 165. | Schwarz KB, Gonzalez-Peralta RP, Murray KF, Molleston JP, Haber B, Jonas MM, Mohan P, Balistreri WF, Rosenthal P, Narkewicz MR. Peginterferon with or without ribavirin for chronic hepatitis C in children and adolescents: final results of the Peds-C trial. Hepatology. 2008;48:418A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 166. | Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, Shelton M, Kerkar N, Galoppo M, Pedreira A. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010;52:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 167. | El Naghi S, Mahmoud F. Study of outcome of naïve and previous nonresponder hepatitis C virus Egyptian children treated with combined therapy of pegylated interferon plus ribavirin. Egyptian Liver Journal. 2014;4:87-93. |
| 168. | Ghaffar TY, El Naghy S, El Sebaie H, El Monaiery M, Ghaffar AY. Pegylated alpha interferon 2B plus ribavirin in the treatment of HCV genotype 4 infection. Indian J Pediatr. 2009;76:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 169. | El Naghi S, Abdel-Ghaffar TY, El-Karaksy H, Abdel-Aty EF, El-Raziky MS, Allam AA, Helmy H, El-Araby HA, Behairy BE, El-Guindi MA. Safety and efficacy of Hansenula-derived PEGylated-interferon alpha-2a and ribavirin combination in chronic hepatitis C Egyptian children. World J Gastroenterol. 2014;20:4681-4691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 170. | (CDER) USDoHaHSFaDACfDEaR. Guidance for Industry Chronic Hepatitis C Virus Infection: Developing Direct-Acting Antiviral Drugs for Treatment 2013. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm225333.pdf. |
| 171. | Hayashi N, Seto C, Kato M, Komada Y, Goto S. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol. 2014;49:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 172. | Asselah T, Marcellin P. Direct acting antivirals for the treatment of chronic hepatitis C: one pill a day for tomorrow. Liver Int. 2012;32 Suppl 1:88-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 173. | Schneider MD, Sarrazin C. Antiviral therapy of hepatitis C in 2014: do we need resistance testing? Antiviral Res. 2014;105:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 174. | Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, Bernstein DE, Afdhal N, Vierling JM, Gordon SC. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 175. | Schmidt WN, Nelson DR, Pawlotsky JM, Sherman KE, Thomas DL, Chung RT. Direct-acting antiviral agents and the path to interferon independence. Clin Gastroenterol Hepatol. 2014;12:728-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 176. | Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34 Suppl 1:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 177. | Pellicer Corbí M, Matoses Asensio S, Garcia Muñoz C, Ortiz Campos M, Herranz Muñoz C, Fernandez-Pacheco M, Garcia-Valdecasas , Luque Infantes M. PS-071 Telaprevir-induced Stevens-Johnson Syndrome. A case report. Eur J Hosp Pharm-S P. 2014;21:A172-A173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 178. | FDA Drug Safety Communication. Serious skin reactions after combination treatment with the Hepatitis C drugs Incivek (telaprevir), peginterferon alfa, and ribavirin. 2012; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm332731.htm. |
| 179. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 180. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 181. | Papastergiou V, Karatapanis S. Current status and emerging challenges in the treatment of hepatitis C virus genotypes 4 to 6. World J Clin Cases. 2015;3:210-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 182. | Gaetano JN. Benefit-risk assessment of new and emerging treatments for hepatitis C: focus on simeprevir and sofosbuvir. Drug Healthc Patient Saf. 2014;6:37-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 183. | Moreno C, Hezode C, Marcellin P, Bourgeois S, Francque S, Samuel D, Zoulim F, Grange JD, Lenz O, Ouwerkerk-Mahadevan S. P1319 once-daily simeprevir (tmc435) with peginterferon/ribavirin in treatment-naive or treatment-experienced chronic hcv genotype 4-infected patients: final results of a phase iii trial. J Hepatol. 2014;60:S535. [DOI] [Full Text] |
| 184. | Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, Dejesus E, Freilich B. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 185. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1325] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 186. | Ruane PJ, Ain D, Stryker R, Meshrekey R, Soliman M, Wolfe PR, Riad J, Mikhail S, Kersey K, Jiang D. Sofosbuvir plus ribavirin for the treatment of chronic genotype 4 hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol. 2015;62:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 187. | Hézode C, Hirschfield GM, Ghesquiere W, Sievert W, Rodriguez-Torres M, Shafran SD, Thuluvath PJ, Tatum HA, Waked I, Esmat G. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2015;64:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 188. | Peter J, Nelson DR. Optimal interferon-free therapy in treatment-experienced chronic hepatitis C patients. Liver Int. 2015;35 Suppl 1:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 189. | Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 564] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 190. | Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 191. | SOVALDI US full Prescribing Information. CA: Gilead Sciences, Inc. Foster City. [Accessed 2015 May 1]. Available from: URL: http://hcp.sovaldi.com/. |
| 192. | Esmat GE, Shiha G, Omar RF, Hassany M, Hammad R, Khairy M, Samir W, Soliman R, Brainard DM, Jiang D. Sofosbuvir plus Ribavirin in the treatment of Egyptian patients with chronic genotype 4 HCV infection. The Liver Meeting®, November 7-11, Boston, MA (Poster presentation) no.: 959. 2014;. |
| 193. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 838] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 194. | Rong L, Dahari H, Ribeiro RM, Perelson AS. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med. 2010;2:30ra32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 195. | Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 911] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 196. | Zeuzem S, Soriano V, Asselah T, Bronowicki JP, Lohse AW, Müllhaupt B, Schuchmann M, Bourlière M, Buti M, Roberts SK. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 197. | Hezode C, Marcellin P, Pol S, Hassanein T, Fleischer-Stepniewska K, Baykal T, Wang T, Lovell SS, Pilot-Matias T, Vilchez RA. O58 results from the phase 2 pearl-i study: interferon-free regimens of abt-450/r abt-267 with or without ribavirin in patients with hcv genotype 4 infection. J Hepatol. 2014;60:S24. [DOI] [Full Text] |
| 198. | Petta S, Craxì A. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int. 2015;35 Suppl 1:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 199. | Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F, Yanase M. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 200. | Hepatitis C Treatments Containing Sofosbuvir in Combination With Another Direct Acting Antiviral Drug: Drug Safety Communication - Serious Slowing of Heart Rate When Used With Antiarrhythmic Drug Amiodarone. 2015. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm439662.htm. |
| 201. | Franciscus A. HCV Advocate Eblast: November 15, 2015. Feds to Medicaid: Stop HCV Treatment Restrictions. The latest news on hepatitis C and coinfection, research, drugs in development, treatment side effects and interferon-free direct antiviral combinations. [Accessed 2015; May 1] Available from: http://hcvadvocate.org/hcv-advocate-eblast-october-15-2015-2/. |