Published online Sep 28, 2015. doi: 10.4254/wjh.v7.i21.2358
Peer-review started: June 20, 2015
First decision: July 27, 2015
Revised: August 5, 2015
Accepted: September 7, 2015
Article in press: September 8, 2015
Published online: September 28, 2015
Processing time: 95 Days and 15.8 Hours
AIM: To compare the nutritional status between alcoholic compensated cirrhotic patients and hepatitis C virus (HCV)-related cirrhotic patients with portal hypertension.
METHODS: A total of 21 patients with compensated cirrhosis (14 with HCV-related cirrhosis and seven with alcoholic cirrhosis) who had risky esophageal varices were investigated. In addition to physical variables, including the body mass index, triceps skinfold thickness, and arm-muscle circumference, the nutritional status was also assessed using the levels of pre-albumin (pre-ALB), retinol-binding protein (RBP) and non-protein respiratory quotient (NPRQ) measured with an indirect calorimeter.
RESULTS: A general assessment for the nutritional status with physical examinations did not show a significant difference between HCV-related cirrhosis and alcoholic cirrhosis. However, the levels of pre-ALB and RBP in alcoholic compensated cirrhotic patients were significantly higher than those in HCV-related compensated cirrhotic patients. In addition, the frequency of having a normal nutritional status (NPRQ ≥ 0.85 and ALB value > 3.5 g/dL) in alcoholic compensated cirrhotic patients was significantly higher than that in HCV-related compensated cirrhotic patients.
CONCLUSION: According to our small scale study, alcoholic compensated cirrhotic patients can develop severe portal hypertension even with a relatively well-maintained liver function and nutritional status compared with HCV-related cirrhosis.
Core tip: We compared the nutritional status between alcoholic compensated cirrhotic patients and hepatitis C virus (HCV)-related cirrhotic compensated patients. The levels of rapid-turnover proteins in alcoholic compensated cirrhotic patients were significantly higher than those in HCV-related compensated cirrhotic patients. When the nutritional status was determined using the albumin level and non-protein respiratory quotient, the frequency of having a normal nutritional status in alcoholic compensated cirrhotic patients was significantly higher than that in HCV-related compensated cirrhotic patients. These findings suggest that alcoholic compensated cirrhotic patients can develop severe portal hypertension even with a relatively well-maintained liver function and nutritional status.
- Citation: Enomoto H, Sakai Y, Iwata Y, Takata R, Aizawa N, Ikeda N, Hasegawa K, Nakano C, Nishimura T, Yoh K, Ishii A, Takashima T, Nishikawa H, Iijima H, Nishiguchi S. Development of risky varices in alcoholic cirrhosis with a well-maintained nutritional status. World J Hepatol 2015; 7(21): 2358-2362
- URL: https://www.wjgnet.com/1948-5182/full/v7/i21/2358.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i21.2358
Chronic liver diseases (CLDs), such as hepatitis virus-related liver diseases and alcoholic liver disease (ALD), cause liver fibrosis and portal hypertension, and the development of gastroesophageal varices is a major complication in patients with advanced liver diseases[1,2]. However, ALD is suggested to have several specific mechanisms which vary from viral hepatitis-related liver diseases and contribute to the progression of liver fibrosis[3,4]. In addition, alcohol intake increases the portal vein pressure by several causes which are independent of the progression of liver fibrosis[5]. For instance, the enlargement of hepatocytes with ballooning was reported to mechanically compress the sinusoid and contribute to increased pressure of the portal vein[6-8]. Therefore, the severity of portal hypertension in alcoholic liver cirrhosis tends to be more remarkable than that in hepatitis virus-related cirrhosis, and patients with alcoholic liver cirrhosis are suggested to develop large varices even with a relatively well-maintained liver function and general clinical conditions[9-11].
Protein energy malnutrition is a major complication of cirrhotic patients, and the presence of energy malnutrition is determined by a low non-protein respiratory quotient (NPRQ) level (< 0.85) which is measured with an indirect calorimeter, and the presence of protein malnutrition was determined by a low level of serological albumin (ALB) (≤ 3.5 g/dL)[12]. Although many cirrhotic patients have nutritional problems, the differences in the nutritional status between alcoholic cirrhotic patients and hepatitis virus-related cirrhotic patients have not yet been investigated in detail.
We previously evaluated cirrhotic patients with high-risk varices and reported the importance of nutritional supporting therapy during the endoscopic treatment for gastroesophageal varices[13]. We herein performed a sub-analysis and investigated clinical variables regarding the nutritional status in compensated cirrhotic patients (Child-Pugh class A) who have portal hypertension and compared those with alcoholic cirrhosis and hepatitis C virus (HCV)-related cirrhosis.
Of the patients enrolled in our previous study[13] (Clinical Trial Registration: UMIN000001534, https://upload.umin.ac.jp/), a total of 21 patients with compensated cirrhosis (14 with HCV-related cirrhosis and seven with alcoholic cirrhosis with Child-Pugh class A), who were admitted to our department for the treatment of esophageal varices with a high bleeding risk, were analyzed in the present study. Liver cirrhosis as the cause of portal hypertension was diagnosed according to the clinical findings, such as the laboratory data, ultrasonographic findings and endoscopic findings. The characteristics of the study population are summarized in Table 1. All clinical values were obtained on the day of the first-time endoscopic treatment for esophageal varices during hospitalization. The following physical variables were used to evaluate the nutritional status of the patients: body mass index, triceps skinfold thickness (%TSF), and arm-muscle circumference (%AMC). In addition to routine blood tests, pre-albumin (pre-ALB) and retinol-binding protein (RBP) levels were also measured as indicators which correlate the liver synthesis capacity and nutritional status.
| Age (yr) | 66.0 ± 11.5 |
| Gender (male/female) | 18/3 |
| Child-Pugh score | 5.4 ± 0.5 |
| AST (IU/L) | 43 (16-99) |
| ALT (IU/L) | 26 (10-86) |
| γ-GTP (IU/L) | 41 (12-821) |
| ALP (IU/L) | 289 (191-726) |
| Total bilirubin (mg/dL) | 1.1 ± 0.5 |
| ALB (g/dL) | 3.6 ± 0.3 |
| Hemoglobin (g/dL) | 11.4 ± 1.7 |
| Platelet count (× 103/μL) | 110 ± 68 |
| Prothrombin time (%) | 83.3 ± 9.1 |
| BCAA treatment (present/absent) | 9/12 |
The parameters measured by indirect calorimetry were carbon dioxide production per minute and oxygen consumption per minute[12]. The total urinary excretion of nitrogen was measured according to the methods previously reported[14]. According to the study by Tajika et al[12], the presence of energy malnutrition and protein malnutrition was determined as a low NPRQ level (< 0.85) and a low ALB level (≤ 3.5 g/dL), respectively. All clinical data were obtained under the fasting condition. The study was reviewed and approved by Hyogo College of Medicine Ethics Committee (Approval No. 650). Written informed consent about personal and medical data collection was obtained from all patients.
The data between two groups were compared using Student’s t-test (normally distributed data) or the Mann-Whitney U test (non-normally distributed data). The frequency of having a normal nutritional status between alcoholic compensated cirrhotic patients and HCV-related compensated cirrhotic patients were analyzed using the χ2 test. A value of P < 0.05 was considered to be significant.
First, we compared the clinical variables between patients with alcoholic compensated cirrhosis and HCV-related compensated cirrhosis. Since all enrolled patients had a well-maintained liver function (Child-Pugh A), most of the common clinical variables (except for γ-glutamyl transpeptidase), including PT percentage, total bilirubin level, ALB level and platelet count, did not differ between the two groups (Table 2). In addition, the general assessment for nutritional status with physical examinations, such as %AMC and %TSF, did not show any significant differences between the two groups. However, when we compared the levels of pre-ALB and RBP, which are more sensitive indicators for liver synthesis capacity and nutritional status (referred to as “rapid-turnover proteins”), these protein levels were significantly higher in alcoholic compensated cirrhotic patients compared with those in HCV-related compensated cirrhotic patients, suggesting a better maintained liver condition of alcoholic compensated cirrhosis with severe portal hypertension than that of HCV-related compensated cirrhosis (Table 3).
| Alcoholic cirrhosis(n = 7) | HCV-relatedcirrhosis (n = 14) | P value | |
| Age (yr) | 63.7 ± 6.8 | 67.2 ± 13.2 | NS |
| Gender (male/female) | 6/1 | 12/2 | NS |
| Child-Pugh score | 5.3 ± 0.5 | 5.4 ± 0.9 | NS |
| AST (IU/L) | 30 (16-99) | 47.5 (27-68) | NS |
| ALT (IU/L) | 24 (10-36) | 35.5 (18-86) | NS |
| γ-GTP (IU/L) | 113 (24-821) | 29.5 (12-159) | < 0.01 |
| ALP (IU/L) | 311 (205-726) | 281 (191-462) | NS |
| Total bilirubin (mg/dL) | 1.2 ± 0.3 | 1.0 ± 0.3 | NS |
| ALB (g/dL) | 3.7 ± 0.3 | 3.6 ± 0.3 | NS |
| Prothrombin time (%) | 84.5 ± 9.6 | 82.7 ± 9.1 | NS |
| Platelet (× 103/μL) | 104 ± 51 | 112 ± 77 | NS |
| BCAA treatment (+/-) | 4/3 | 5/9 | NS |
| Alcoholic cirrhosis(n = 7) | HCV-related cirrhosis(n = 14) | P value | |
| BMI | 25.0 ± 5.8 | 22.7 ± 3.1 | NS |
| %AMC | 102.4 ± 2.3 | 105.0 ± 12.0 | NS |
| %TSF | 142.7 ± 44.3 | 190.5 ± 75.2 | NS |
| REE/BMR | 1.06 ± 0.13 | 1.02 ± 0.13 | NS |
| FPG (mg/dL) | 124 ± 56 | 105 ± 16 | NS |
| IRI (μU/mL) | 9.1 ± 2.6 | 13.8 ± 8.2 | NS |
| HOMA-IR | 2.8 ± 1.7 | 3.7 ± 2.5 | NS |
| Pre-ALB (mg/dL) | 16.3 ± 7.2 | 9.7 ± 2.7 | < 0.01 |
| RBP (mg/dL) | 2.4 ± 1.3 | 1.4 ± 0.3 | < 0.05 |
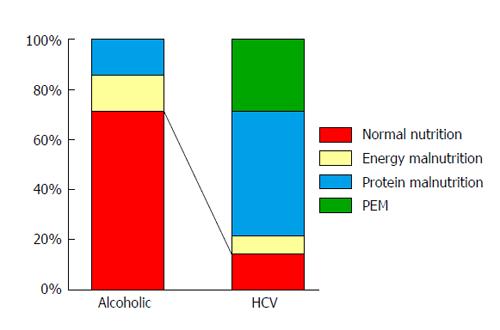
Using the indirect calorimetry in combination with the blood test, we determined the nutritional status of each patient in detail. The frequency of having a normal nutritional status (NPRQ ≥ 0.85 and ALB value > 3.5 g/dL) in patients with alcoholic compensated cirrhosis (5/7: 71.4%) was significantly higher than that in patients with HCV-related compensated cirrhosis (2/14: 14.2%) (Figure 1). These findings suggest that patients with alcoholic cirrhosis can develop severe portal hypertension even with a relatively well- maintained liver function and nutritional status when compared to patients with HCV-related cirrhosis.
ALD leads to an increased intrahepatic and portal pressure and portal hypertension depending on several conditions which vary from hepatitis virus-related CLDs, such as compression of the hepatic sinusoid by enlarged hepatocytes in the form of ballooning[6-8]. In addition, perivenular fibrosis, one of histological characteristics of ALD, is also suggested to contribute to the development of portal hypertension[15-17]. We herein compared several clinical parameters between Child-Pugh grade A patients with alcoholic compensated cirrhosis and those with HCV-related compensated cirrhosis. Although there were no significant differences in the general clinical variables, patients with alcoholic cirrhosis had better liver synthesis capacity and/or nutritional status. These findings suggest that alcoholic cirrhotic patients are prone to develop portal hypertension even under the condition of a well-maintained liver function and nutritional status.
In the present study, the general clinical variables including liver functional tests and physical examinations did not differ between patients with HCV-related compensated cirrhosis and alcoholic compensated cirrhosis. However, we found alcoholic compensated cirrhotic patients showed significantly higher levels of pre-ALB and RBP than HCV-related compensated cirrhotic patients (Table 2). Rapid-turnover proteins, such as pre-ALB and RBP, have shorter life-spans than ALB (pre-ALB: approximately 2 d, RBP: approximately 12 h, and ALB: approximately 3 wk). Therefore, these rapid turnover proteins are able to sensitively reflect the liver synthesis capacity and nutritional status[18-20]. In addition, in HCV-related compensated cirrhotic patients, the levels of ALB and the NPRQ were decreased in 35.7% (5/14) and 78.6% (11/14) of the patients, respectively. Although we did not clarify the role of HCV-infection in the development of malnutrition, our findings suggested that patients with HCV-related cirrhosis potentially had either protein or energy malnutrition, even compensated cirrhotic patients (Child-Pugh A) who did not exhibit cirrhosis-related clinical symptoms. It has been previously reported that cirrhotic patients with either energy malnutrition (NPRQ < 0.85) or protein malnutrition (ALB value ≤ 3.5) have an unfavorable prognosis[12,21]. Recent advancements in antiviral treatment are expected to lead to a significant decrease in the frequency of HCV infection[22,23]. It would be interesting to evaluate changes in the nutritional status of patients with cirrhosis after the elimination of HCV-related compensated cirrhosis.
In Table 2, the mean value of %TSF was numerically higher in HCV-related cirrhotic patients than that in alcoholic cirrhotic patients, although a statistical significance was not found between the groups. Although physical examinations are generally accepted as a method to assess the nutritional status, measurement errors can easily occur (particularly regarding the levels of TSF and AMC)[13], and therefore we should pay careful attention to the evaluation of the physical variables.
Although the present study is a novel one that focused on the data of rapid-turnover proteins and indirect calorimetry in patients with compensated cirrhosis, there are some limitations associated with our study. First, the number of patients enrolled was small. It would therefore be important to investigate a larger number of patients in order to confirm our results. Second, indirect calorimetry cannot be routinely used in every institute. However, our study is unique in that it investigated compensated cirrhotic patients with similar clinical conditions (Child-Pugh grade A) and determined the differences in the nutritional parameters between patients with different etiologies. Further studies with a greater accumulation of patients and readily available tools for measuring the protein levels are necessary.
We are grateful to Higuchi Y, Tawara N, Nakatani R, Kanazawa N, Matsushita Y, Fujii S, Kido H and Minemoto K (Hyogo College of Medicine) for their technical and secretarial assistance.
Although many cirrhotic patients have nutritional problems, the differences in the nutritional status between alcoholic cirrhotic patients and hepatitis virus-related cirrhotic patients have not yet been investigated in detail. The authors herein compared the nutritional status between alcoholic compensated cirrhosis and hepatitis C virus (HCV)-related compensated cirrhosis patients with portal hypertension.
Assessment of the nutritional statuses in patients with chronic liver diseases has been increasingly important, particularly in cirrhotic patients.
This is the first report to compare the nutritional statuses between alcoholic cirrhosis and HCV-related cirrhosis patients with risky esophageal varices.
The present study showed that alcoholic compensated cirrhotic patients can develop severe portal hypertension even with a relatively well-maintained liver function and nutritional status when compared to patients with HCV-related cirrhosis.
This is quite an interesting topic. It focuses on a more realistic field of knowledge.
P- Reviewer: He ST, Maasoumy B, Penkova-Radicheva MP S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: How changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62:S121-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 2. | Garcia-Tsao G, Lim JK. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104:1802-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Bolognesi M, Verardo A, Di Pascoli M. Peculiar characteristics of portal-hepatic hemodynamics of alcoholic cirrhosis. World J Gastroenterol. 2014;20:8005-8010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 4. | Fujii H, Kawada N. Fibrogenesis in alcoholic liver disease. World J Gastroenterol. 2014;20:8048-8054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Luca A, García-Pagán JC, Bosch J, Feu F, Caballería J, Groszmann RJ, Rodés J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology. 1997;112:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Nakano M, Maruyama K, Okuyama K, Takahashi H, Yokoyama K, Takagi S, Shiraki H, Ishii H. The characteristics of alcoholics with HCV infection: histopathologic comparison with alcoholics without HCV infection and chronic type C hepatitis. Alcohol Alcohol Suppl. 1993;1B:35-40. [PubMed] |
| 7. | Blendis LM, Orrego H, Crossley IR, Blake JE, Medline A, Isreal Y. The role of hepatocyte enlargement in hepatic pressure in cirrhotic and noncirrhotic alcoholic liver disease. Hepatology. 1982;2:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Vidins EI, Britton RS, Medline A, Blendis LM, Israel Y, Orrego H. Sinusoidal caliber in alcoholic and nonalcoholic liver disease: diagnostic and pathogenic implications. Hepatology. 1985;5:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Le Moine O, Hadengue A, Moreau R, Sogni P, Soupison T, Yang S, Hartleb M, Lebrec D. Relationship between portal pressure, esophageal varices, and variceal bleeding on the basis of the stage and cause of cirrhosis. Scand J Gastroenterol. 1997;32:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Momiyama K, Nagai H, Sumino Y. Comparison of the hemodynamics between patients with alcoholic or HCV-related cirrhosis. Hepatogastroenterology. 2011;58:2036-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Bolognesi M, Sacerdoti D, Mescoli C, Bombonato G, Cillo U, Merenda R, Giacomelli L, Merkel C, Rugge M, Gatta A. Different hemodynamic patterns of alcoholic and viral endstage cirrhosis: analysis of explanted liver weight, degree of fibrosis and splanchnic Doppler parameters. Scand J Gastroenterol. 2007;42:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Sakai Y, Iwata Y, Enomoto H, Saito M, Yoh K, Ishii A, Takashima T, Aizawa N, Ikeda N, Tanaka H. Two randomized controlled studies comparing the nutritional benefits of branched-chain amino acid (BCAA) granules and a BCAA-enriched nutrient mixture for patients with esophageal varices after endoscopic treatment. J Gastroenterol. 2015;50:109-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Dickerson RN, Tidwell AC, Minard G, Croce MA, Brown RO. Predicting total urinary nitrogen excretion from urinary urea nitrogen excretion in multiple-trauma patients receiving specialized nutritional support. Nutrition. 2005;21:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Nakano M, Worner TM, Lieber CS. Perivenular fibrosis in alcoholic liver injury: ultrastructure and histologic progression. Gastroenterology. 1982;83:777-785. [PubMed] |
| 16. | Goodman ZD, Ishak KG. Occlusive venous lesions in alcoholic liver disease. A study of 200 cases. Gastroenterology. 1982;83:786-796. [PubMed] |
| 17. | Miyakawa H, Iida S, Leo MA, Greenstein RJ, Zimmon DS, Lieber CS. Pathogenesis of precirrhotic portal hypertension in alcohol-fed baboons. Gastroenterology. 1985;88:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Smith FR, Goodman DS, Zaklama MS, Gabr MK, el-Maraghy S, Patwardhan VN. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. I. A functional defect in hepatic retinol release. Am J Clin Nutr. 1973;26:973-981. [PubMed] |
| 19. | Devoto G, Gallo F, Marchello C, Racchi O, Garbarini R, Bonassi S, Albalustri G, Haupt E. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52:2281-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Chaves GV, Peres WA, Gonçalves JC, Ramalho A. Vitamin A and retinol-binding protein deficiency among chronic liver disease patients. Nutrition. 2015;31:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Hanai T, Shiraki M, Nishimura K, Imai K, Suetsugu A, Takai K, Shimizu M, Naiki T, Moriwaki H. Free fatty acid as a marker of energy malnutrition in liver cirrhosis. Hepatol Res. 2014;44:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure. J Hepatol. 2015;62:S87-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 23. | van de Ven N, Fortunak J, Simmons B, Ford N, Cooke GS, Khoo S, Hill A. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology. 2015;61:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |