Published online Sep 8, 2015. doi: 10.4254/wjh.v7.i19.2229
Peer-review started: February 26, 2015
First decision: June 18, 2015
Revised: August 10, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: September 8, 2015
Processing time: 197 Days and 11.4 Hours
The antiphospholipid syndrome (APS) is an acquired thrombophilic disorder in which autoantibodies are produced to a variety of phospholipids determinants of cell membranes or phospholipid binding proteins. There are few reports about association between antiphospholipid antibodies and development of Budd-Chiari syndrome (BCS). We report the case of BCS development in young Russian male with primary APS. The patient underwent orthotopic liver transplantation on August 26, 2012. At present time his state is good, the blood flow in the liver restored and its function is not impaired. We report about the first time the successful use of dabigatran etexilate for prolonged anticoagulation therapy in APS patient with BCS. In addition patient is managed with immunosuppressive drugs.
Core tip: Budd-Chiari syndrome (BCS) is rare disease with a potentially dismal outcome if not treated optimally. In manuscript is reported the case report of the BCS development in young Russian male with primary antiphospholipid syndrome (APS), who was underwent orthotopic liver transplantation and now is managed with immunosupressive drugs and with prolonged anticoagulation. For the first time, it is reported the successful use of dabigatran etexilate for prolongation anticoagulation therapy in primary APS patient with BCS.
- Citation: Reshetnyak TM, Seredavkina NV, Satybaldyeva MA, Nasonov EL, Reshetnyak VI. Liver transplantation in a patient with primary antiphospholipid syndrome and Budd-Chiari syndrome. World J Hepatol 2015; 7(19): 2229-2236
- URL: https://www.wjgnet.com/1948-5182/full/v7/i19/2229.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i19.2229
The antiphospholipid syndrome (APS) is an acquired thrombophilic disorder in which autoantibodies are produced to a variety of phospholipids determinants of cell membranes or phospholipid binding proteins[1-5]. Clinical features for definite APS include vascular thrombosis (arterial and/or venous or small-vessels) that must be diagnosed on the basis of objective criteria and pregnancy morbidity[1-5]. Laboratory criteria are well defined and require anticardiolipin antibodies (aCL) of IgG and/or IgM isotypes in serum or plasma presented in medium or high levels (> 40 IgG phospholipid units or IgM phospholipid units or > 99th percentile), antibodies to β2 glycoprotein 1 (anti-β2GPI) of IgG and/or IgM isotypes in serum or plasma in medium or high levels (> 99th percentile) and lupus anticoagulant (LA) in plasma. Laboratory findings must be confirmed on repeated testing 12 wk later[2]. It helps to exclude transient positivity due to infection. Patients with APS may have other risk factors for thrombosis, which are shown in Table 1. The presence of other risk factors for thrombosis does not exclude APS and patients should be stratified according to the presence or the absence of risk factors for thrombosis.
| Age (> 55 in men, > 65 in women) |
| Risk factors for CVD |
| Hypertension |
| Diabetes mellitus |
| Elevated LDL or low HDL - cholesterol |
| Smoking |
| Family history of premature CVD |
| BMI ≥ 30 kg/m2 |
| Microalbuminuria |
| Estimated GFR < 60 mL/min |
| Inherited thrombophilias |
| Oral contraceptives |
| Nephrotic syndrome |
| Malignancy |
| Immobilization |
| Surgery |
APS is characterized by a hypercoagulable state potentially resulting in thrombosis of all segments of the vascular bed[7]. Venous thrombosis typically presents with deep vein thrombosis in the lower extremities, observed in 29% to 55% of cases over a follow-up period of less than 6 years[6]. Other thrombotic presentations include osteonecrosis and venous occlusion of solid organs, such as the liver [Budd-Chiari syndrome (BCS)][8,9], kidneys[10] and the adrenal glands with resulting in adrenal insufficiency[11]. Clinical manifestations of APS with the involvement of the abdominal cavity are various and are shown in Table 2.
| Abdominal organ | Manifestations |
| Liver | Budd-Chiari syndrome: |
| Hepatic veno-occlusive disease and occlusion of small hepatic veins | |
| Nodular regenerative hyperplasia | |
| Hepatic infarction | |
| Cirrhosis | |
| Portal hypertension | |
| Autoimmune hepatitis | |
| Biliary cirrhosis | |
| Liver transplantation | |
| Intestine | Mesenteric ischemia (acute-chronic)[13,14] |
| Peptic ulcer disease[15,16] | |
| Bowel ischemia and perforation[17] | |
| High prevalence of aPL but no increased vascular thromboses in inflammatory bowel disease | |
| Spleen | Splenic infarction |
| Autosplenectomy or functional asplenia | |
| Pancreas | Acute pancreatitis |
The aim is to describe case report of the BCS development in young Russian male with primary APS, who was underwent orthotopic liver transplantation and now is managed with immunosuppressive drugs and with prolonged anticoagulation.
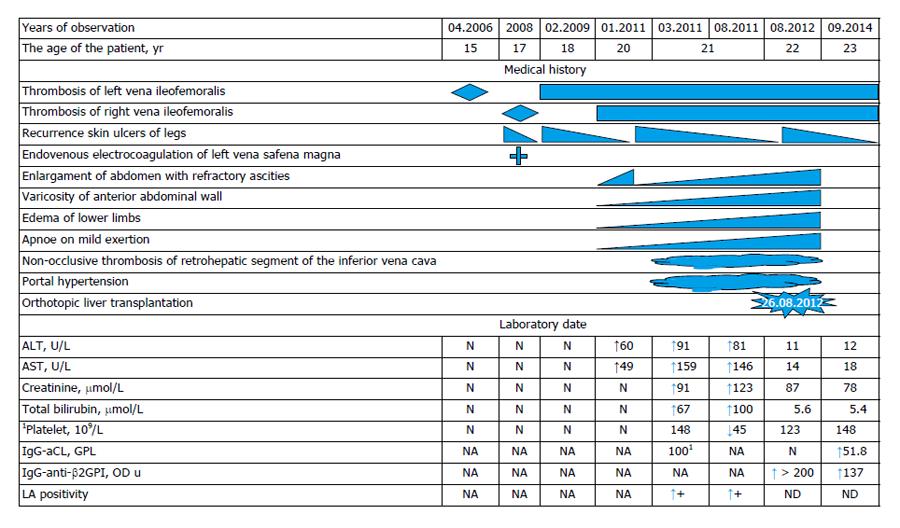
Patient S, aged 22, hospitalized at VA Nasonova Scientific Research Institute of Rheumatology in order to make more accurate diagnosis and to correct his therapy. His medical history (Figure 1) shows that he had suffered from left side ileofemoral thrombosis in April 2006 (at the age of 15), underwent a low-molecular heparins (Nadroparinum calcium) during one month then took Sulodexid for about 2 mo, till March 2008, later he did not take any anticoagulants an anti-platelet drugs. Concomitant medication was venotonics (Detralex = Hespiridine + Diosmine).
At the end of 2007 trophic ulcers appeared on the skin of the lower third of the left shank. In March 2008 (at the age of 17 years) the patient had a right-side ileofemoral thrombosis, trophic ulcers remained on the skin of the left shank. The patient was treated with anti-platelet drugs (Aspirin, Pentoxifylline), and periodically with antibiotics due to purulent discharge from the ulcers. In September 2008 varicose vein disease was detected due to which the patient underwent an endovasal electrocoagulation of the great saphenous vein of the left lower limb and subcutaneous dissection and ablation of veins on the hip, shank and foot. In February 2009 the patient had acute deep and superficial vein thromboses of the right lower limb. He underwent therapy with anticoagulants (heparin) combined with low doses of Aspirin (thrombo-ASS - 100 mg) for 2 mo. He did not take any anticoagulants afterwards. Hyperpigmentation of feet and shanks skin and recurrent trophic ulcers on shanks skin were observed. The patient’s state was qualified as post-thrombotic syndrome.
The appearance and worsening of ascites was noted in January 2011 (at the age of 20 years). On March 3, 2013, laparocentesis with evacuation of 12 L of fluid was performed. Diagnostic abdominal paracentesis showed a straw-colored ascetic fluid with protein content 28 g/L. Computed tomography angiography on March 16, 2011, showed the lumen of the inferior vena cava was visualized only above the part of confluence of renal veins. The vein's lumen from this level was constructively quite homogeneous. The lumen of the inferior vena cava at the confluence of renal veins was severely narrowed (4-5 mm) with less contrast of its lumen in venous phase of multiphase contrast protocol. Hepatic veins were not visualized. The portal vein was not expanded (at the level of the gate of the liver up to 14 mm, splenic vein to 10 mm), the lumen of these veins were homogeneous. There was slightly expressed additional network of collateral veins in the abdominal cavity, the largest of which were located along the rear bottom edge of the liver in hepatorenal area. The strong network of dilated venous vessels was visualized subcutaneously along the anterior abdominal wall; the recanalization of the umbilical vein was absent. Based on these data it was concluded of apparent stenosis of the infrarenal part of inferior vena cava due to the occlusion or obliteration. The lack of visualization of the hepatic veins, enlarged liver with abnormality of its perfusion, ascitis and the presence of venous collaterals might be due to the BCS.
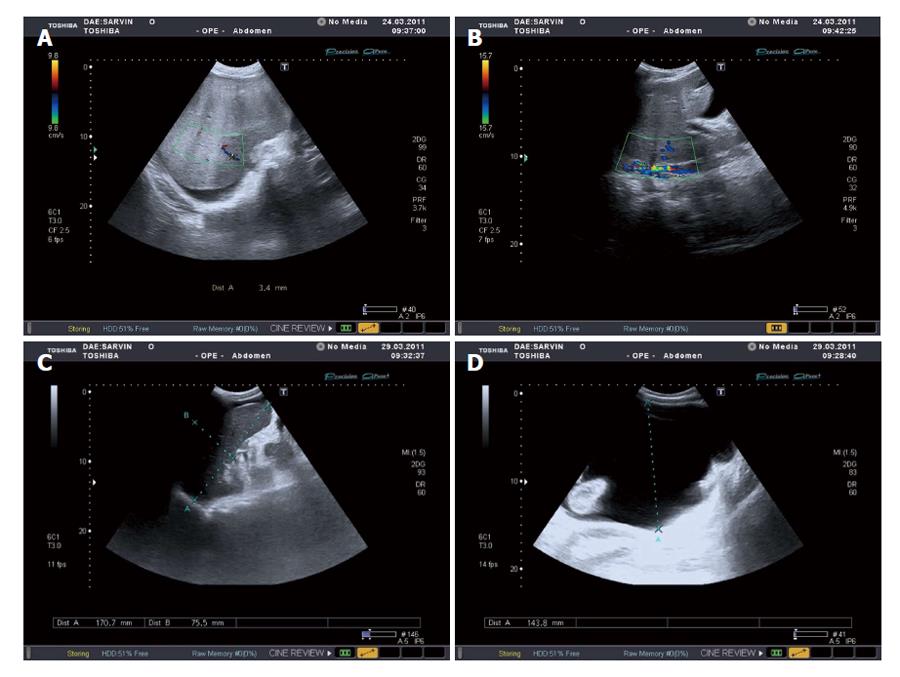
There was no evidence of infection or malignancy. The liver biopsy was not done due to prolonged prothrombin time. In March 2011 the patient underwent an in-patient treatment at regional clinical institute, where BCS, refractory ascites, hepatosplenomegaly and stenosis of lower infrarenal vena cava were diagnosed (Ultrasound Doppler examination data are shown on the Figure 2).
On March 15, 2011, laparocentesis with evacuation of 11 L of fluid was performed, Nadroparinum calcium, Soludexid, Detralex prescribed. From March 23, 2011 till May 11, 2015, the patient was hospitalized for in-patient treatment at Endotoxicoses Department of N.V. Sklifosovsky Scientific Research Institute of Emergency Medical Aid; a drainage of abdominal cavity, fractional evacuation of ascetic fluid, anticoagulant (Nadroparinum calcium). Symptomatic therapy was performed at in-patient facility. From July 25, 2011 till August 20, 2011 the patient underwent in-patient treatment at the Scientific Haematology Center of Russian Academy of Sciences. A research of hypercogulation causes was carried out. An abdominal ultrasound scan showed non-occlusive thrombosis of retrohepatic segment of the inferior vena cava (Figures 1 and 2). Doppler scan and computer tomography (CT) scan of abdominal cavity showed the same findings.
At the same period the patient underwent tests for antiphospholipid antibodies (aPL) that showed high levels of total aPL > 100 (normal range: 0-20). Kaolin clotting time (KCT), activated partial thromboplastin time, prothrombin time, diluted Russel’s viper venom time (dRVVT) were prolonged and were positive for LA. At the clinic Nadroparinum calcium was substituted with Dabigitran etexilate, plasmapheresis number of 9 was performed. The patient was put in the waiting list for liver transplantation. On August 26, 2012, orthotopic liver transplantation was made at N.V. Sklifosofsky Scientific Research Institute of Emergency Medical Aid as well as biliary reconstruction: choledoho-choledoho end-to-end anasthomosis. In postoperative period (on September 16, 2012) clinical findings showed transient cerebral circulation disturbances. Magnetic resonance tomography of the brain showed the signs of glial changes of the right frontal lobe, the attack was stopped by the patient himself and had no relapses, the patient was examined by neurologist and psychiatrist. Toxical hyperkinesis with asthenic syndrome was diagnosed. At the same time an episode of herpetic infection recurrence was noticed. Immunosuppressive therapy included: Mycophenolate mofetil, Tarcrolimus, Methylprednisolone therapy. In March 2013 Mycophenolate mofetil was discontinued. Patient was screened for a hypercoagulable state. At the same time the testing showed the levels of IgG- and IgM-aCL within the norm, anti-β2GPI > 200 optical density units (OD u) (Normal range < 20). Coagulation time remained to be extended in KCT, dRVVT tests. However, screening for IgG-aCL, IgM-aCL by ELISA technique showed that aCL were negative but IgG-anti-β2GPI were high positive and LA was also positive. This led to the diagnosis of primary APS as the underlying cause of the extensive venous thrombosis.
In addition to APS, the patient was tested for the presence of inherent thrombophilia (Table 3). The next mutations of blood coagulation genes were detected: homozygous 4G/4G polymorphism in plasminogene activator inhibitor 1 (PAI-1) gene, 677TT polymorphism in methylene tetrahydrofolate reductase gene, heterozygous G20210A polymorphism in prothrombin gene. His family history was unremarkable for coronary artery disease and for venous thromboembolic events. The level of homocysteine was normal.
| Mutation | Result |
| FV Leiden | -/- |
| G20210A in prothrombin gene | +/- |
| C10034T in γ-phibrinogen gene | -/- |
| Methylene tetrahydrofolate reductase 677TT polymorphism | +/- |
| 4G/5G plasminogen activator inhibitor 1 | +/+ |
| G29926C in THBS gene (thrombospondine-4 gene) | -/- |
| G10976A in VII factor gene | -/- |
| С807Т in GpIa gene | -/- |
| Т1565С in GpIIIa gene | -/- |
| CYP2C9*2 ( cytochrome Р450 gene) | -/- |
| CYP2C9*3 (cytochrome Р450 gene) | -/- |
| G1639A in VKORC 1 gene (vitamin K hypoxide reductase gene) | -/- |
| I/D-polymorphism in ACE gene (anghiotensin-converting ferment gene) | -/- |
The patient continues to take dabigatran etexilate up to the present. No relapses of thrombosis occurred during the medical supervision period. Laboratory test values dynamics are shown in Figure 1.
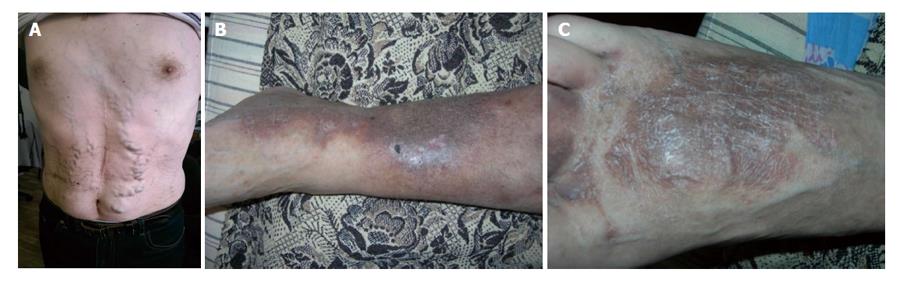
At the moment of presentation to our clinic the patient’s state by the physical examination revealed as satisfactory. He was of normosthenic type, body temperature was 36.7 °C in axillary. Lipodermatosclerosis of skin legs, multiple small superficial ulcers on skin legs in stage of cicatrization were revealed. Varicose veins on the anterior surface of abdominal wall, delicate wire mesh livedo reticularis on skin of shoulders and thighs (Figure 3) were noted. Respiration rate was 16/min, heart rate - 82 beats in min, systolic/diastolic blood pressure was 140/80 mmHG. There were no consciousness disturbances. The patient oriented to time and place and was cooperative. Any focal, meningeal symptoms were not identified. Our patient did not fulfill the American College of Rheumatology[18] classification criteria for systemic lupus erythematosus or other autoimmune disease. Autoimmune and viral hepatitis, myeloproliferative disease and paroxysmal nocturnal hemoglobinuria were excluded. The echo-KG revealed dilatation of the left atrium, functional extension of the trunk of the pulmonary artery, thickening of the mitral valve with mitral regurgitation +2.
Dynamic Doppler examination of his lower limbs was performed. On the right leg revealed postthrombotic syndrome: thrombosis of posterior tibial vein, of peroneal vein, popliteal, sural, superficial, deep, common femoral veins, external and common iliac veins in weak recanalization stage (except iliac veins that have no recanalization), and on the left leg thrombosis of popliteal, sural, superficial, deep, common femoral vein, external and common iliac veins and great saphenous vein on the shank in partial recanalization stage of various intensity levels (except iliac veins that have no recanalization).
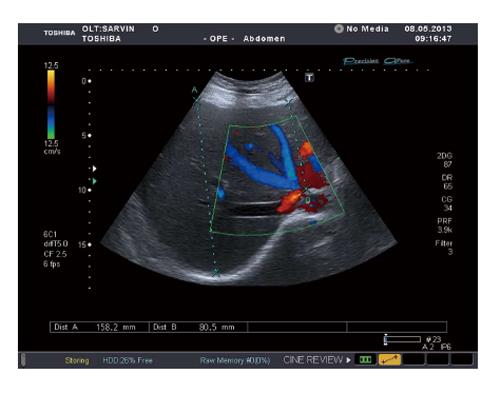
The patient was eventually discharged home and followed up satisfactorily as an outpatient. He showed dramatic improvement and remained asymptomatic with no further recurrence of his ascites. His liver function test showed marked improvement. Repeated color Doppler ultrasound and CT showed better hepatic perfusion and hepatic venous flow with better recanalization of the inferior vein cava (IVC) and hepatic veins (Figure 4). His condition remained under satisfactory control while on prolonged coagulation.
BCS is a rare disease with a potentially dismal outcome if not treated optimally. The classical BCS is a clinical and pathological entity, characterized by structural and functional abnormalities of the liver resulting from obstruction of the outflow of hepatic venous blood[3]. Ascites, hepatomegaly and abdominal pain constitute the classic triad of BCS of hepatic vein but also an extensive thrombosis of the IVC.
Several myeloproliferative disorders and hypercoagulable states have been implicated as possible causes of BCS. These include polycythaemia vera, essential thrombocythaemia, paroxysmal nocturnal haemoglobinuria, antithrombin, protein C and protein S deficiency, resistance to activated protein C, factor V Leiden (FVL), G20210A factor II gene mutations, use of oral contraceptives, pregnancy and postpartum state[8,9,12,18-20]. The factor V G1691A mutation and the prothrombin G20210A mutation are the 2 most common causes of hereditary thrombophilia. Numerous studies have shown that these 2 gene mutations alone or in combination with other risk factors can increase the occurrence and recurrence of venous thromboembolism[19-22]. In one systematic review based on the meta-analysis, it was shown the FVL mutation was associated with an increased risk of BCS, portal vein thrombosis (PVT) without cirrhosis, and PVT in cirrhosis, however, the prothrombin G20210A mutation was associated with PVT, but not BCS[23].
The relationship between LA and BCS was first described in 1984 by Pomeroy et al[21]. Several other cases were reported afterwards[12,18-22]. The association of BCS with APS seems to be rare. For this reason, in one published series of 177 patients with BCS, no such association was reported[24].
We described a case of a patient whose disease began at the age of 15 with ileofemoral thrombosis of the left leg. Subsequently, secondary to irregular taking of anticoagulants, the development of ileofemoral thrombosis on the right side was noted, accompanied by development of postthrombotic syndrome in both legs, as well as by IVC thrombosis - a stenosis of its infrarenal part and non-occlusive thrombosis of retrohepatic segment. In January 2011 an ascites gradually appeared and then because of the accumulation of large quantity of liquid, a laparocentesis was performed resulting in evacuation of 11 liters of liquid; gradual rising of liver failure was noted. An additional thrombosis risk factor was heterozygous prothrombin gene (G20210A) mutation. Besides that, the patient showed polymorphism in PAI-1 gene (4G/4G genotypes of PAI-1). The role of this gene’s polymorphism in thrombosis development is still a matter of discussion. In a study of 357 patients with different types of thrombosis and 281 unrelated healthy controls by Balta et al[25], it was found that the 4G/4G genotype of PAI-1 was associated with a higher risk of thrombosis (OR = 1.7; 95%CI: 1.1-2.5). Stronger association was observed in a subgroup of 33 patients with PVT wherein 4G/4G and 4G/5G genotypes showed 10- and 6-fold increases respectively in the risk of developing portal vein thrombosis. No statistically significant association was found between 4G/4G genotype and other thrombosis groups in this study. Most probably, the presence of inherent thrombophilias (prothrombin gene mutation, 4G/4G PAI-1 genotype) was a background for the development of thrombosis. The combination of these inherent thrombophilias with aPL without long-lasting anticoagulation therapy after the first episode of thrombosis lead to relapses and the thrombosis of the IVC with development of BCS and further progression of liver damage.
Management of BCS, from simple medical treatment to liver transplantation, depends on the acute and chronic evolution of the disease and on the degree of hepatic insufficiency. The management of BCS includes anticoagulation and thrombolysis, percutaneous transhepatic stent angioplasty, and transjugular intrahepatic portosystemic shunt, but the effect of these approaches varies greatly. BCS in patients progressing to cirrhosis is an indication for liver transplantation[26,27]. Anticoagulation therapy is the first line treatment of BCS secondary to obstruction IVC and PVT. Long-term anticoagulation with oral vitamin K antagonists such as warfarin is the cornerstone treatment in APS also[28-30]. These drugs have a delayed onset of action, food and drug interactions, and variable pharmacokinetics/pharmacodynamics so regular laboratory monitoring and dose adjustments are required to maintain the international normalized ratio in the therapeutic range. New oral anticoagulants that selectively inhibit either thrombin (dabigatran etexilate) or factor Xa (rivaroxaban, apixaban) have now gained approval in many countries for several clinical indications. Unlike other than warfarin, these drugs have a rapid onset of action and a relatively wide therapeutic range such that coagulation monitoring is not required. In the described case the drug of choice of anticoagulant therapy was dabigatran etexilate, which the patient continued to take after surgery. Dabigatran etexilate is a new oral direct thrombin inhibitor that was approved in the United States and in Canada for the prevention of thromboembolic events in patients with atrial fibrillation, as well as in Europe and Canada for the prevention of venous thromboembolism[31]. We have not found the use of this drug in patients with APS and BCS for treatment and prevent venous thromboembolic events.
In conclusion, it is described a case report of BCS development in young man aged 22, with definite APS and inherent thrombophilia (heterozygous prothrombin gene mutation and homozygous 4G/4G polymorphism in PAI-1 gene). The disease began at the age of 15 with ileofemoral thrombosis of the left leg, with further development of ileofemoral thrombosis on the right side, secondary to irregular taking of anticoagulants, with relapses of the disease. An ascites and an evident hepatic insufficiency were noted after 5 years from the onset. Ultrasound Doppler examination showed non-occlusive thrombus in retro hepatic segment of the IVC. BCS led to the development of liver cirrhosis with its evident functional deficiency and the development of multiple organ failure. The patient underwent orthotopic liver transplantation. At present time his state is good, blood flow in the liver is restored and its function is not impaired. We report about the first the successful use of dabigatran etexilate for prolonged anticoagulation therapy in APS patient with BCS after orthotopic liver transplantation.
The author expresses thanks to Dr. Semenova MN for receiving and discussion of liver image by Ultrasound Doppler examination and Professor Alexandrova EN for receiving and discussion of laboratory data. The author expresses his gratitude to Professor Patrushev LI for obtaining data on immunogenetic polymorphisms of the coagulation system and assistance in preparing this article.
Presents a clinical case of a 22-year-old male with antiphospholipid syndrome and developed a severe form of Budd-Chiari syndrome (BCS) required orthotopic liver transplantation.
Primary antiphospholipid syndrome (bilateral ileofemoral thrombosis, trophic ulcers on the skin of the left shank, livedo reticularis, positive tests for antiphospholipid antibodies), BCS (refractory ascites, hepatosplenomegaly, stenosis of lower infrarenal part of inferior vena cava and non-occlusive thrombosis of retrohepatic segment of inferior vena cava), condition after orthotopic liver transplantation.
Inherent thrombophilia, systemic lupus erythematosus, autoimmune and viral hepatitis, myeloproliferative disease and paroxysmal nocturnal hemoglobinuria.
Patient had high levels of liver enzyme level (alanine aminotransferase, aspartate aminitransferase, bilirubine), creatinine, total levels antiphospholipid antibodies > 100 (normal range: 0-20), positive test for lupus anticoagulant, high level of IgG-anti-β2GPI and the next mutations of blood coagulation genes: homozygous 4G/4G polymorphism in plasminogene activator inhibitor 1 gene, 677TT polymorphism in methylene tetrahydrofolate reductase gene, heterozygous G20210A mutation in prothrombin gene.
Contrast-enhanced computed tomography of the abdomen showed no visualization of the hepatic veins, the presence of venous collaterals, enlarged liver, ascites and stenosis of infrarenal part of the inferior vena cava and non-occlusive thrombosis of retrohepatic segment of the inferior vena cava.
The liver biopsy was not done due to prolonged prothrombin time, histologic examination did not perform.
The patient underwent orthotopic liver transplantation and subsequently continues to take immunosuppressive drugs (Mycophenolate mofetil, Tarcrolimus, Methylprednisolone) in combination with anticoagulants (Nadroparinum calcium which was replaced for dabigatran etexilate for prolonged anticoagulation).
The antiphospholipid syndrome is an acquired thrombophilic disorder in which autoantibodies are produced to a variety of phospholipids determinants of cell membranes or phospholipid binding proteins.
Clinical manifestations of antiphospholipid syndrome depend on the localization of thrombosis, which can lead to serious consequences such as BCS requiring liver transplantation, and Dabigatran etexilate is the drug of choice for long-term anticoagulant therapy in the prevention of recurrence of thrombosis.
The authors present a rare and complicated case with underline antiphospholipid (aPL) syndrome subsequently suffering BCS, and S/P liver transplantation. After that, the patient’s aPL syndrome is well controlled by dabigatran etexilate. The case report is impressive.
P- Reviewer: Dolapcioglu C, Ohkohchi N, Tzeng JE S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Nasonov EL, Rheshetnyak TM. Antiphospholipid syndrome: prevention, treatment and prognosis. Antiphospholipid syndrome. Moscow: Litterra, (in Russ) 2004; 337-378. |
| 2. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [PubMed] |
| 3. | Erkan D, Pierangeli SS. Antiphospholipid syndrome: Insights and highlights from the 13th International Congress on Antiphospholipid Antibodies. New York: Springer Science Business Media 2012; 23-113. [DOI] [Full Text] |
| 4. | Asherson RA, Cervera R, Piette J-Ch, Shoenfeld Y. The antiphospholipid syndrome II: Autoimmune thrombosis. New York: Elsevier 2002; 3-445. |
| 5. | Reshetnyak TM. Antiphospholipid syndrome: diagnosis and clinical manifestations (a lecture). 2014;56-71 Available from: URL: http://www.rheumatolog.ru/sites/default/files/Pdf/npr/npr_1_2014.pdf. |
| 6. | Saigal R, Kansal A, Mittal M, Singh Y, Ram H. Antiphospholipid antibody syndrome. J Assoc Physicians India. 2010;58:176-184. [PubMed] |
| 7. | Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1425] [Cited by in RCA: 1350] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 8. | Castro I, Ríos JJ, Iniesta N, Perez I, Robles A, Gil A. Acute and fulminant Budd-Chiari syndrome in a well-anticoagulated patient with primary antiphospholipid syndrome. Lupus. 2005;14:979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Yagi K, Kawano M, Haraki T, Higashikata T, Ueda K, Okada T, Koni I, Mabuchi H. Long-term efficacy of immunoadsorbent plasmapheresis in a patient with Budd-Chiari syndrome due to antiphospholipid syndrome: case report with nine-year follow-up. Lupus. 2004;13:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Tektonidou MG. Renal involvement in the antiphospholipid syndrome (APS)-APS nephropathy. Clin Rev Allergy Immunol. 2009;36:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Ramon I, Mathian A, Bachelot A, Hervier B, Haroche J, Boutin-Le Thi Huong D, Costedoat-Chalumeau N, Wechsler B, Karmali R, Velkeniers B. Primary adrenal insufficiency due to bilateral adrenal hemorrhage-adrenal infarction in the antiphospholipid syndrome: long-term outcome of 16 patients. J Clin Endocrinol Metab. 2013;98:3179-3189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Uthman I, Khamashta M. The abdominal manifestations of the antiphospholipid syndrome. Rheumatology (Oxford). 2007;46:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Cervera R, Espinosa G, Cordero A, Oltra MR, Unzurrunzaga A, Rossiñol T, Plaza J, Bucciarelli S, Ramos-Casals M, Ingelmo M, Asherson RA, Font J; Catastrophic Antiphospholipid Syndrome (CAPS) Registry Project Group. Intestinal involvement secondary to the antiphospholipid syndrome (APS): clinical and immunologic characteristics of 97 patients: comparison of classic and catastrophic APS. Semin Arthritis Rheum. 2007;36:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Veraldi GF, Zecchinelli MP, Furlan F, Genco B, Minicozzi AM, Segattini C, Pacca R. Mesenteric revascularisation in a young patient with antiphospholipid syndrome and fibromuscular dysplasia: report of a case and review of the literature. Chir Ital. 2009;61:659-665. [PubMed] |
| 15. | Kalman DR, Khan A, Romain PL, Nompleggi DJ. Giant gastric ulceration associated with antiphospholipid antibody syndrome. Am J Gastroenterol. 1996;91:1244-1247. [PubMed] |
| 16. | Johnsen SJ, Valborgland T, Gudlaugsson E, Bostad L, Omdal R. Thrombotic microangiopathy and the antiphospholipid syndrome. Lupus. 2010;19:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Ilkgül O, Içöz G, Dayangaç M, Tokat Y, Ozütemiz O. A case of antiphospholipid antibody syndrome with Budd-Chiari and colonic ulcers complicated with gastrointestinal hemorrhage. Turk J Gastroenterol. 2004;15:115-116. [PubMed] |
| 18. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3499] [Article Influence: 269.2] [Reference Citation Analysis (0)] |
| 19. | Sciascia S, Mario F, Bertero MT. Chronic Budd-Chiari syndrome, abdominal varices, and caput medusae in 2 patients with antiphospholipid syndrome. J Clin Rheumatol. 2010;16:302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Diz-Kucukkaya R, Demir K, Yenerel MN, Nalcaci M, Kaymakoglu S, Inanc M. Coexistence of homozygous factor V Leiden mutation and antiphospholipid antibodies in two patients presented with Budd-Chiari syndrome. Haematologia (Budap). 2002;32:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Pomeroy C, Knodell RG, Swaim WR, Arneson P, Mahowald ML. Budd-Chiari syndrome in a patient with the lupus anticoagulant. Gastroenterology. 1984;86:158-161. [PubMed] |
| 22. | Espinosa G, Font J, García-Pagan JC, Tàssies D, Reverter JC, Gaig C, Cervantes F, Cervera R, Bosch J, Ingelmo M. Budd-Chiari syndrome secondary to antiphospholipid syndrome: clinical and immunologic characteristics of 43 patients. Medicine (Baltimore). 2001;80:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Qi X, Ren W, De Stefano V, Fan D. Associations of coagulation factor V Leiden and prothrombin G20210A mutations with Budd-Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1801-12.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, Sood GK, Mitra SK, Khanna SK, Walia BS. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 214] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Balta G, Altay C, Gurgey A. PAI-1 gene 4G/5G genotype: A risk factor for thrombosis in vessels of internal organs. Am J Hematol. 2002;71:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Mancuso A. Budd-Chiari syndrome management: Lights and shadows. World J Hepatol. 2011;3:262-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Levit DA, Chvanov EA, Petrishchev YuI, Levit AL Maintenance of Minute Circulation Volume during Orthotopic Liver Transplantation. General Reanimatology (in Russ). 2011;7:23-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48-49:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, Brey R, Crowther M, Derksen R, Erkan D, Krilis S, Machin S, Pengo V. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 2011;20:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 30. | Ruiz-Irastorza G, Khamashta MA. The treatment of antiphospholipid syndrome: a harmonic contrast. Best Pract Res Clin Rheumatol. 2007;21:1079-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Cheng JW, Vu H. Dabigatran etexilate: an oral direct thrombin inhibitor for the management of thromboembolic disorders. Clin Ther. 2012;34:766-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Reshetnyak TM, Radenska-Lopovok SG, Aleksandrova YeN, Kondratyeva LV, Shtivelband IB, Novikov AA, Mach ES, Nasonov YeL. Lung Pathology in Antiphospholipid Syndrome. General Reanimatology (in Russ). 2005;1:34-43. [DOI] [Full Text] |