Published online Jul 18, 2015. doi: 10.4254/wjh.v7.i14.1807
Peer-review started: January 22, 2015
First decision: April 10, 2015
Revised: May 7, 2015
Accepted: May 27, 2015
Article in press: May 28, 2015
Published online: July 18, 2015
Processing time: 183 Days and 9.7 Hours
The management of hepatic metastatic disease from solid tumors in adults has been extensively described and resection of metastatic liver lesions from colorectal adenocarcinoma, renal adenocarcinoma, breast cancer, testicular cancer, and neuroendocrine tumors (NET) have demonstrated therapeutic benefits in select patients. However, there are few reports in the literature on the management of hepatic metastatic disease in the pediatric and adolescent populations and the effectiveness of hepatic metastasectomy. This may be due to the much lower incidence of pediatric malignancies and the higher chemosensitivity of childhood tumors which make hepatic metastasectomy less likely to be required. We review liver involvement with metastatic disease from the main pediatric solid tumors, including neuroblastoma and Wilms tumor focusing on the management and treatment options. We also review other solid malignant tumors which may have liver metastases including germ cell tumors, gastrointestinal stromal tumors, osteosarcoma, desmoplastic small round cell tumors and NET. However, these histological subtypes are so rare in the pediatric and adolescent populations that the exact incidence and best management of hepatic metastatic disease are unknown and can only be extrapolated from adult series.
Core tip: Management of hepatic metastatic disease in pediatric and adolescent cancer patients is not as well delineated as for adults due to the lower incidence of pediatric malignancies and the higher chemosensitivity of childhood tumors. We review liver involvement by metastatic disease from the main pediatric and adolescent solid tumors focusing on management and treatment options.
- Citation: Fernandez-Pineda I, Sandoval JA, Davidoff AM. Hepatic metastatic disease in pediatric and adolescent solid tumors. World J Hepatol 2015; 7(14): 1807-1817
- URL: https://www.wjgnet.com/1948-5182/full/v7/i14/1807.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i14.1807
Cancer is the most common cause of disease-related mortality for children and adolescents 1-19 years of age[1]. More than 12000 children and adolescents younger than 20 years of age are diagnosed with cancer every year in United States with approximately 2300 deaths in this age group[2,3]. Primary liver malignancies are uncommon in children (annual incidence rate of 1.5 per million) and account for only 0.5% to 2% of all pediatric neoplasms (100-150 new cases/year in United States). The two main histologies are hepatoblastoma and hepatocellular carcinoma[1,4]. Hepatoblastoma is the most common malignant tumor of the liver in children with a higher incidence during the first year of life. Hepatocellular carcinoma is the second most common hepatic malignancy and occurrs primarily in adolescents[5]. The most common site of origin of liver metastases in children with solid tumors is neuroblastoma (NB) followed by Wilms tumor (WT). Other solid malignant tumors which may give liver metastases are germ cell tumors (GCT), gastrointestinal stromal tumors (GIST), osteosarcoma (OS), desmoplastic small round cell tumors and neuroendocrine tumors (NET)[6,7]. Table 1 summarizes liver involvement from pediatric solid tumors. Some histological subtypes are so rare in the pediatric population that the exact incidence of hepatic metastatic disease is unknown and extrapolated from series of adult patients. Although hepatic metastatic disease in adults is often associated with abnormal liver function tests, including a decreased serum albumin and elevated serum levels of transaminases, bilirubin and alkaline phosphatase, these findings are rarely seen in pediatric patients with hepatic tumor involvement. While the exact mechanisms underlying hepatic metastasis in children remain unclear, we briefly summarize tumor biology concepts underlying liver metastatic disease.
| Primary malignancy | Metastatic disease at diagnosis | Hepatic metastatic disease | Treatment options |
| NB | 50%-60% | 20%-30% | Surgery, chemotherapy and radiation therapy |
| WT | 10%-20% | 10%-15% | Surgery, chemotherapy and radiation therapy |
| GCT | 20%-30% | 15%-20%1 | Surgery and chemotherapy |
| GIST | 30%-40%1 | 15%-20%1 | Surgery and imatinib |
| OS | 15%-20%1 | 1%-3%1 | Surgery |
| DSRCT | 30%-50%1 | 30%-40%1 | HIPEC and surgery |
| NET | 30%-45%1 | 30%-45%1 | Surgery, HAE, cryoablation, radiofrequency ablation, liver transplant and radionuclides therapy |
Different treatment modalities have been used in the management of liver metastases in childhood including systemic chemotherapy, radiation therapy (RT), surgical resection, ablation techniques and image-guided interventional procedures (Table 1). Surgical resection of liver metastases from colorectal adenocarcinoma, renal adenocarcinoma, breast cancer, testicular cancer, and NET is feasible and has demonstrated therapeutic benefits in select adult patients[8-13]. The role of surgery for hepatic metastatic disease in pediatric malignancies is not as well described as for adults. This may be due to the lower incidence of malignancies in children and the higher chemosensitivity of pediatric histological subtypes. The decision to perform resection of liver metastases in pediatric cancer patients should be highly individualized with a clear understanding of tumor biology and chemosensitivity.
Patients whose primary tumor is under control and have adequate hepatic reserve for resection may be good candidates for liver metastasectomies. Some other patients may not be good surgical candidates but they may benefit from surgical relief of tumor biliary obstruction to improve liver function tests and permit the continuation of chemotherapy. Herein, we review liver involvement by metastatic disease from the main pediatric and adolescent solid tumors focusing on management and treatment options.
As dissemination of systemic metastasis to the liver in advanced stage pediatric solid neoplasms is limited, the liver remains a select host to pediatric solid cancers, particularly NB and WT. While the exact mechanisms underlying hepatic metastases remain unclear in these particular tumors, the general understanding of the interactions between metastatic tumor cells and the liver microenvironment involves a reciprocal dynamic between primary tumor and the hepatic microenvironment[14]. Metastatic cells arriving at the liver via the bloodstream encounter the microenvironment of the hepatic sinusoid. The interactions of the tumor cells with hepatic sinusoidal and extrasinusoidal cells (endothelial, Kupffer, stellate, and inflammatory cells) determine their fate. The sinusoidal cells may play a dual role, sometimes killing the tumor cells but also facilitating their survival and growth. Adhesion molecules participate in these interactions and may affect their outcome. In NB and WT, for instance, the association of various growth factors, cell adhesion molecules, and extracellular matrix proteins have been described for these tumors and have been shown to be involved in metastases[15,16]. Lastly, bone marrow-derived cells and chemokines play a part in the early struggle for survival of the metastases. Once the tumor cells have arrested and survived the initial onslaught, tumors can grow within the liver in 3 distinct patterns, reflecting differing host responses, mechanisms of vascularization, and proteolytic activity. While much has been accomplished in the understanding of the complex biology of liver metastases, in the following sections, we emphasize recent progress in the clinical management and treatment of hepatic metastases in advanced childhood tumors. We refer the reader to the references[17-19] for reviews on the current understanding of the biology of liver metastases.
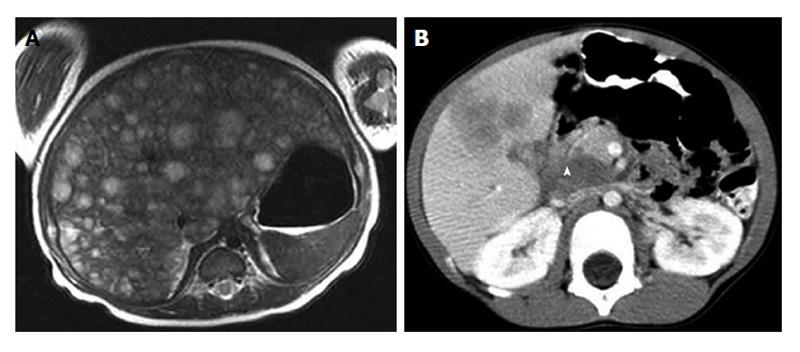
NB is the most common extracranial malignant solid tumor in the pediatric population, representing approximately 8%-10% of total cancer cases in children younger than 15 years of age[20]. More than 650 cases are diagnosed each year in North America (incidence of 10.54 cases per 1 million per year). The prognosis of NB is dependent on age at diagnosis, stage of disease, histology and molecular biologic characteristics of the tumor (e.g., amplification of MYCN-oncogene)[21-24]. Specifically, age less than 1 year is associated with a favourable prognosis, while MYCN-oncogene-amplification confers a poor prognosis[25-27]. Since NB is the most common pediatric extracranial solid tumor and the most frequent tumor which metastasizes to liver in children, knowledge of the management of hepatic metastatic disease from NB is particularly important. Approximately, 30% of NB patients with metastatic disease have liver involvement and two distinct clinical entities can be differentiated: stage 4S (or metastatic which metastases are confined to skin, liver and/or bone marrow in children younger than 18 mo, according to the International Neuroblastoma Risk Group Staging System) and stage 4 (or metastatic)[28]. Liver involvement is seen in approximately 80% of patients with stage 4S NB, whereas 10%-50% of stage 4 NB patients have liver metastasis[29]. Differences have been observed in the initial presentation of hepatic metastatic disease from NB in these 2 stages. Stage 4S NB is reported to usually present with multiple ill-defined nodules or diffuse liver involvement where stage 4 NB hepatic involvement presents more frequently with discrete liver nodules (Figure 1).
Stage 4S NB is defined by a localized primary tumor with dissemination limited to skin, liver, and/or bone marrow (involvement < 10%) in infants younger than 12 mo[30]. Bone marrow involvement > 10% or bone involvement is considered stage 4 disease. The first description of stage 4S NB by D’Angio et al[31] in 1971, reported frequent spontaneous tumor regression without adjuvant therapy. More recent reports have confirmed that stage 4S NB, with or without liver involvement, resolved in up to 50% of the cases without requiring therapy[32,33]. Overall survival is approximately 85%-92% in this group of patients and mortality is generally secondary to massive hepatic tumor infiltration causing respiratory compromise.
DuBois et al[34] reported the incidence of metastatic sites in stage 4 and 4S NB and the extent to which metastatic sites correlate with age, tumor biology, and survival. With regards to hepatic involvement, they showed that liver metastases were associated with a more favorable outcome overall, but in infants predicted a slightly greater event-free survival (EFS) and were associated with non-amplified MYCN and favorable histology tumors, while in children > 1 year at diagnosis, liver metastases were an unfavorable prognostic marker and associated with MYCN-amplified tumors. Although excellent outcome in stage 4S NB is common, there are subsets of infants with massive infiltration of the liver by tumor who experience significant morbidity and mortality secondary to respiratory compromise and symptoms of abdominal compartment syndrome with decreased venous return, renal impairment and coagulation disorders[35,36]. Nickerson et al[30] from the Children’s Cancer Study Group reported six deaths, five of which were in infants younger than 2 mo of age at diagnosis and were due to complications of extensive abdominal involvement with respiratory compromise or disseminated intravascular coagulation. Schleiermacher et al[37] reported that patients with stage 4S NB and progressive disease had a 20% mortality rate and suggested that the combination of etoposide and carboplatin may be more effective in these infants than radiation or vincristine and cyclophosphamide.
Multimodality therapy with surgery, radiation and chemotherapy has been used but the outcome of this approach has not yet been ascertained. In 2004, Weintraub et al[38] reported the first successful case of hepatic intra-arterial chemoembolization (HACE) in a neonate and 8 years later, they published a sequential treatment algorithm for infants with stage 4S NB and massive hepatomegaly based on initial observation without treatment, intravenous chemotherapy for those who have progressive disease and HACE for patients with progression despite chemotherapy[39]. Surgical management by partial hepatic resection or abdominal decompression with mesh placement in case of abdominal compartment syndrome has a high rate of associated complications and has rarely been shown to be effective[40].
Stage 4 NB patients under 1 year of age have an overall survival ranging from 70% to 93%, in contrast to overall survival between 35% and 60% for patients greater than 1 year. Patients with isolated liver metastases may benefit from resection of these lesions, resulting in prolonged survival and/or treatment reductions[41,42], but these clinical circumstances are rare and several factors including histological tumor characteristics and close evaluation of extrahepatic metastatic disease should be discussed before considering hepatic metastasectomies. There are some reports of stage 4S NB that recurs after initial regression or progresses to stage 4 with bone metastases. There are no guidelines for patients with responsive extrahepatic metastatic disease to therapy and persistent liver disease. The biology of the tumor may lead the therapeutic approach and tumors without MYC-N amplification may have a chance of survival and no indication for major liver resections[43-45].
French et al[46] investigated the long-term hepatic outcomes in infants with stage 4S and 4 NB, with a special focus on the impact of liver involvement and abdominal radiation. They reviewed 38 patients with available follow-up 5 years following diagnosis, assessing hepatic imaging and function (transaminases, bilirubin, alkaline phosphatase). For stage 4S, benign hepatic changes on imaging studies in patients treated with hepatic radiation as well as those who had hepatic involvement at diagnosis but did not receive radiation were observed. For infants with stage 4 and hepatic metastasis at diagnosis, none was found to have late hepatic imaging changes. Blood work was normal in both groups. They concluded that adverse hepatic outcomes after liver involvement or radiation in infants with stage 4S or 4 NB are rare and when they do occur, often resolve over time. Also, infants with NB and metastatic hepatic disease seem to be a specific risk-group for the development of focal nodular hyperplasia (FNH) of the liver, especially if they underwent chemotherapy and/or hepatic RT during treatment and it should be considered in patients with persistent late imaging changes[47]. Although FNH is a benign lesion that is typically managed conservatively in adults, most children with FNH undergo biopsy or resection because of increasing size, concerning symptoms or inability to rule out malignancy, especially in pediatric cancer survivors[48,49].
Long-term follow-up guidelines from the Children’s Oncology Group (COG) recommend yearly hepatic bloodwork screening (aspartate aminotransferase, alanine aminotransferase, and bilirubin) upon entry to the long-term follow-up clinic with repeat bloodwork only if clinically indicated in a patient that has received greater than 20-30 Gy to the liver. Bloodwork to check liver function is recommended by COG if there is an abnormality on screening bloodwork[50,51].
In summary, although stage 4 NB patients with isolated liver metastases may benefit from resection of these lesions, this is a rare clinical situation. Careful patient selection is indicated focusing on the histological tumor characteristics, evaluation of extrahepatic metastatic disease and tumor chemosensitivity. The role of surgery by partial liver resection or abdominal decompression with mesh or silo placement in stage 4S patients with massive hepatomegaly who are not responsive to chemotherapy/RT is also controversial and it has rarely been shown to be effective.
WT or nephroblastoma is the most common malignant renal tumor in children, representing approximately 6% of total cancer diagnoses among children younger than 15 years with 500 new cases in United States each year[1]. Overall survival for children with WT has been consistently above 90% since the 1980s. Prognosis depends on the stage of disease at diagnosis and histopathologic and molecular features of the tumor. According to the staging criteria, the primary renal tumor is assigned a local stage (1-3), which determines local therapy with or without RT[52-55]. Stage 4 WT is defined by hematogenous metastases or lymph node metastases outside the abdominopelvic region and it represents 10% of the patients[56]. The most common sites of metastatic spread of WT are the lungs, regional lymph nodes and liver. In the National Wilms Tumor Study Group (NWTSG), the lung was the only metastatic site in approximately 80% of patients presenting with stage 4 disease at diagnosis, whereas metastases were present in the liver with or without lung involvement in 15% of the patients[56-60].
Metastatic disease is recognized as a poor prognostic factor with a lower overall survival rate that ranges from 30%-50% for diffuse anaplastic WT to 85% for favorable histology WT[61]. Varan et al[62] reported results from 1971 to 2002 on 18 patients with liver metastases who were noted to have a lower overall survival than patients with pulmonary disease (16.6% vs 50.2%). These authors recommended a more intensive chemotherapy and more aggressive surgical treatment for patients with hepatic metastatic disease. Breslow et al[63] in the past have given a detailed analysis on the metastatic pattern of children with stage 4 WT from the NWTSG which showed no difference in survival according to metastatic site (liver and/or lung vs lung only). Szavay et al[64] observed a less favorable outcome in 29 patients with WT complicated by metastases of the liver primarily enrolled in the International Society of Pediatric Oncology (SIOP) and the German Pediatric Oncology Group studies, SIOP 93-01/GPOH study and the SIOP 2001/GPOH study. Two years later, Fuchs et al[65] published a series of a total of 45 patients enrolled in these two studies that corroborated the previous findings and suggested that successful complete surgical resection of the primary tumor and of liver metastases in children with WT improves survival.
Ehrlich et al[66] reported the largest series about the treatment and outcomes of patients with WT metastatic to the liver. They reviewed patients with favorable histology WT and hepatic metastasis at diagnosis treated on NWTS 4 and 5 to ascertain if they had a worse prognosis than other stage 4 disease. A total of 96 patients were identified. Twenty-two patients (22.9%) had a primary liver resection; 13 patients (13.5%) underwent liver resection after chemotherapy and/or RT. Seventy-one patients (67%) did not undergo surgery for their liver disease. In 14 patients, the liver disease disappeared with chemotherapy only. Eighty-two patients received abdominal RT. EFS for the patients with liver only metastatic favorable histology WT was 76% (95%CI: 58%, 87%) compared to 70% for patients with liver and lung involvement. EFS (95%CI) for the patients with primary resection of the liver metastases was 86% compared with 68% (P = 0.09) for the patients who did not have primary resection of liver metastases. This improved outcome may be the result of having limited hepatic disease and being a more appropriate surgical candidate. There was no significant difference in EFS for patients treated with chemotherapy compared with that of patients treated with chemotherapy and RT (P = 0.63). The EFS (95%CI) for patients who did not receive abdominal RT was 64% compared to 77% for patients who received abdominal RT without boost and 72% for patients who received abdominal RT with boost (P = 0.05). They concluded that liver metastases was not an independent adverse prognostic factor for children with stage 4 favorable histology WT. Although a more aggressive initial surgical approach for a child with WT and liver metastasis is not supported by this report, patients with residual liver disease after treatment with chemotherapy and/or RT that could be completely resected did well, suggesting there may be a role for complete surgical resection of residual metastases after adjuvant therapy. Furthermore, the impact of boost radiation to liver metastases on survival was not clear. The current approach for hepatic only metastatic disease WT patients depends on the tumor histology and type of protocol. Patients with favorable histology WT enrolled on the NWTSG protocol will undergo nephrectomy and lymph node sampling, followed by abdominal RT (planned according to local stage of renal tumor) and RT to sites of metastases and regimen DD-4A (vincristine, dactinomycin, doxorubicin × 24 wk)[61].
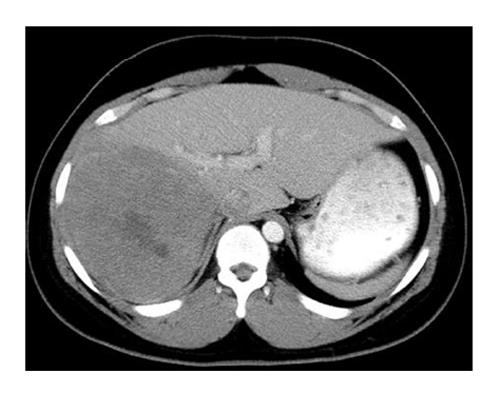
In conclusion, a role exists for complete surgical resection of residual metastases after adjuvant therapy in children with WT (Figure 2), a tumor that generally is very sensitive to chemotherapy.
GCT represent 7% of cancer diagnoses among children younger than 20% and 3.5% of cancer diagnoses for children younger than 15 with approximately 900 new cases under 20 years of age each year in United States[1]. After the introduction of cisplatin-based chemotherapy in the 1970s, the survival of children with GCTs substantially improved. For gonadal GCT, the 5-year survival rate has increased from 89% to 98% for children younger than 15 years and from 70% to 95% for adolescents aged 15 to 19 years. Extragonadal GCT 5-year survival rate has increased from 42% to 83% for children younger than 15 years[67-69]. The effectiveness of chemotherapy is monitored by decreases in serum tumor markers (alpha-fetoprotein and beta-HCG) which are produced by malignant GCT. Stage 4 disease includes distant metastases to liver, brain, bone, or lung. The presence of liver metastases represents an independent poor prognostic factor and one of the strongest indicators of a poor long-term outcome in adult patients with advanced GCTs. The literature suggests that liver resection in this age group age is feasible and safe[70]. Rivoire et al[71] reported 37 patients with a median age of 26 years (range, 14-47 years) who underwent liver resection for the treatment of metastatic GCT. Their results were favorable with a median survival of 54 mo and an overall 5-year survival rate of 62% which appeared to justify an aggressive surgical approach for treatment of patients with postchemotherapy residual hepatic metastatic disease. Interestingly, time to appearance of liver metastases, lesion distribution within the liver, timing of liver surgery, extent of resection, and size of resection margins were not of additional predictive value. They recommended close follow-up for patients with residual liver metastases measuring < 10 mm regardless of the primary tumor type and patient gender; close follow-up for male patients with residual metastases measuring > 30 mm regardless of the primary tumor type and delayed surgery for surviving patients with growing lesions even if they are teratomas; liver resection for male patients with metastases measuring 10-29 mm, particularly in the absence of embryonal carcinoma in the primary or mixed tumor; and liver resection for female patients with metastases measuring > 10 mm.
The differences between children and adults regarding the location of the primary GCT site, pattern of metastatic dissemination and the biology of childhood GCTs may limit the applicability of adult therapeutic approaches to children. A report from the Children’s Cancer Study Group showed that patients with malignant GCTs, (excluding dysgerminoma and tumors of the testis or brain) with more than one structure or organ involved at diagnosis increased the risk for adverse event[72,73]. In another study[74] from the Pediatric Oncology Group that aimed to investigate prognostic factors for pediatric extragonadal malignant GCT, patients older than 12 years of age with thoracic tumors had six times the risk of death compared with patients younger than 12 years of age with tumors at other sites. Metastatic disease at diagnosis was not a statistically significant prognostic factor for EFS. The role of postchemotherapy surgical exeresis of all residual hepatic metastatic disease may be justified for evaluation of the effectiveness of chemotherapy and resection of refractory disease, but this needs to be individualized.
GIST is a mesenchymal neoplasm of the gastrointestinal tract that originates from intestinal pacemaker cells, also known as interstitial cells of Cajal. It is typically seen in adults over the age of 40 and children are rarely affected. It has been estimated that there are 3300 to 6000 new GIST cases per year in the United States[75,76]. Of all GISTs, 1.4% to 2.7% occur in children and adolescents in large series[77]. A minority of GIST in pediatric patients (10%) can arise within the context of tumor predisposition syndromes such as Carney triad and Carney-Stratakis syndrome[78,79]. Pediatric GIST is commonly located in the stomach (gastric antrum) and usually occurs in adolescent females[80]. Histology in children is characterized by a predominance of epithelioid or epithelioid/spindle cell morphology and, unlike adult GIST, their mitotic rate does not appear to accurately predict clinical behavior[81]. Multifocal tumors and nodal metastases are common, which account for the high incidence of local recurrence seen in the pediatric population[82]. Pathogenesis in children and young adults may also differ from that of adult GIST, because activating mutations of KIT and platelet-derived growth factor receptor (PDGFR), which are seen in 90% of adult GIST, are present in only 11% of pediatric GIST. This fact is important in terms of therapeutic management. The administration of adjuvant imatinib mesylate, a selective tyrosine kinase inhibitor, has been shown to improve EFS in adult patients with GIST but this benefit is restricted to those with KIT and PDGFR mutations, and thus the use of this agent in pediatric GIST cannot be recommended if the mutation is not present[83-85]. Responses to imatinib in pediatric patients are uncommon and consist mainly of disease stabilization[86]. At presentation, approximately half of adult GISTs have already metastasized with the liver being the most frequent site of metastases. In this age group, gastric tumors of large size (> 5 cm) or arising from small intestine, colon, mesentery and omentum have a high frequency of recurrence and liver metastases. Few pediatric GISTs with hepatic metastatic disease have been reported[87].
The only definitive treatment for GIST is surgical resection, since it is highly resistant to conventional systemic chemotherapy and RT. The mainstay of surgical resection is to achieve a complete resection with negative margins in the primary and/or the metastatic disease[88]. Treatment varies based on whether a mutation is detected or not. For most pediatric patients with GIST and absence of KIT and PDGFR mutations, complete surgical resection of localized disease is recommended as long as it can be accomplished without significant morbidity. Since lymph node involvement is relatively common in younger patients, searching for overt or occult nodal involvement should be encouraged. Given the indolent course of the disease in pediatric patients, it is reasonable to withhold extensive and mutilative surgeries and to carefully observe children with locally recurrent or unresectable asymptomatic disease[89]. The few pediatric patients with KIT or PDGFR mutations should be managed according to adult guidelines and for those patients, resection of hepatic metastases following imatinib treatment may be curative when the primary disease has been eradicated and negative surgical resection margins are attained. Patients with solitary or limited hepatic metastases may be potential surgical candidates. However, a large tumor burden in the hepatic parenchyma may prohibit resection given the risk of insufficient remaining liver tissue and subsequent postoperative liver failure. Other treatment options may include thermal ablation (radiofrequency, laser, microwave, cryoablation), hepatic artery embolization and hepatic artery chemoembolization, but no experience has been reported in pediatric GIST patients[90,91].
In conclusion, the few pediatric patients with KIT or PDGFR mutations who present with solitary or limited hepatic metastases may be potential surgical candidates. Given the indolent course of GIST in pediatric patients with absence of KIT and PDGFR mutations, it is reasonable to withhold extensive hepatic resections, but further investigations are needed.
OS is the most common malignant bone tumor arising in children and adolescents. In the United States, 400 children and adolescents younger than 20 years of age are diagnosed with OS each year[1]. At diagnosis, 20% of patients will have radiographically detectable metastases, with the lung being the most common site. With improved survival of OS patients with pulmonary metastatic disease owing to a more aggressive treatment with surgery and intensified chemotherapy, the pattern of metastatic disease may be changing[92-95]. Moreover, new imaging modalities which are more sensitive at discovering new metastatic lesions are being incorporated in the tumor protocols.
Although extrapulmonary metastatic disease from OS is considered rare and generally occurs after the diagnosis of pulmonary metastases, a few studies also report some cases with presentation of isolated extrapulmonary metastases and no signs of lung invasion[96]. Hepatic metastatic disease from OS is extremely rare and few cases with or without simultaneous pulmonary metastases have been reported, although it is more commonly found at autopsy[97]. Daw et al[98] reported a case of ossified hepatic metastases detected at the time of diagnosis of a secondary OS. Complete resection of all disease is required for cure in patients with OS. Whereas pulmonary metastasectomy for OS has been shown to improve survival, surgical resection of hepatic metastases for this disease has been less well characterized[99,100]. Despite multimodal therapy including different chemotherapeutic agents, surgical resection and radiofrequency ablation, OS patients with hepatic metastatic disease have a poor prognosis and selection of surgical candidates must be individualized.
Desmoplastic small round cell tumor (DSRCT) is a rare malignant abdominal tumor with less than 300 cases reported in the literature. It typically arises in adolescents and young adult men and has a strong tendency to spread within the peritoneum but also to the liver and lungs[101]. It is classified as a small round cell tumor and it is characterized by a distinct immunohistochemical pattern and a recurrent, specific, chromosomal translocation [t (11; 22) (p13; p12)] which results in a chimeric EWS-WT1 fusion gene[102]. Most of the patients present with disseminated disease at diagnosis and the primary site of origin is frequently unknown. Because of this, it is associated with a very poor prognosis. Although surgery, chemotherapy, RT, radiofrequency ablation, hyperthermic intraperitoneal perfusion with chemotherapy (HIPEC) and combined therapy have been used in the treatment of DSRCT, no single therapy has been accepted as the standard strategy. Honoré et al[103] have recently published the largest series with a multimodal management of abdominal DSRCT. They reported on 38 patients with a median age of 27 years (range 13-57 years), but some adolescents were included. Nearly half of the patients at the time of diagnosis had extraperitoneal metastases with the liver involved in 78% of the cases. Different treatment modalities were used including systemic chemotherapy, surgery, HIPEC and RT. They concluded that the factors predictive of 3-year overall survival were the absence of extraperitoneal disease, complete surgical resection, postoperative whole abdominopelvic RT and postoperative chemotherapy. Patients with synchronous liver metastases treated with peritoneal cytoreductive surgery had an overall survival (14.8 mo) similar to patients treated with systemic chemotherapy alone. Therefore, no benefit of surgery was demonstrated in this group of patients. HIPEC had no impact on overall survival. In contrast, Hayes-Jordan et al[104,105] published the first report on the use of HIPEC in young children and showed that patients with disease limited to the abdominal cavity, including those with resectable liver metastases, were good candidates for HIPEC with good outcomes.
Therefore, conclusions about the best management of hepatic metastatic disease in DSRCT are difficult to draw. More studies in children and adolescents are necessary to elucidate if a different clinical behavior is documented in this age group.
NET are rarely seen in the pediatric population with an incidence rate of 2.8 per million[106]. Overall, appendiceal NET (carcinoids) are the most common subtype and they are usually found incidentally upon final histopathologic analysis in cases of a suspected appendicitis[107]. This tumor location is rarely associated with metastatic disease in children. Extra-appendiceal carcinoid tumors and neuroendocrine carcinomas are more poorly characterized and have a greater chance for metastatic spread compared with carcinoids arising in the appendix[108]. Broaddus et al[109] published 5 of 13 cases that were initially diagnosed in the liver, with no other primary sites identified. They concluded that it is not known if these tumors represent true primary hepatic neoplasms or metastases from asymptomatic, occult gastrointestinal, pancreatic, or pulmonary primary tumors. Although the definitive role of surgery in children with metastatic disease from NET has not been established, the management of hepatic metastases may include surgical resection[110]. In adults, cytoreductive surgery for hepatic metastases from gastrointestinal NETs has resulted in prolonged survival rates[111]. Other treatment options may include hepatic artery embolization, cryoablation, radiofrequency ablation, orthotopic liver transplantation and radionuclides therapy such as 131I-MIBG and 177Lu-octreotate[112-114].
In summary, there may be a potential role for surgical resection of liver metastases in pediatric patients with NET, but more experience is needed.
Hepatic metastatic disease and the benefit of hepatic metastasectomies in pediatric cancer patients are not as well delineated as for adults due to the lower incidence of pediatric malignancies and the higher chemosensitivity of childhood tumors. Patients with residual localized hepatic disease after neoadjuvant therapy and non-chemosensitive tumors may benefit from surgical resection, but careful patient selection remains critical.
P- Reviewer: Bouzianas DG, Bubnov RV, Gunay Y S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Ries LG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute 1999; . |
| 2. | Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5-26. [PubMed] |
| 3. | Parkin DM, Stiller CA, Draper GJ, Bieber CA. The international incidence of childhood cancer. Int J Cancer. 1988;42:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 300] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Buckley JD, Sather H, Ruccione K, Rogers PC, Haas JE, Henderson BE, Hammond GD. A case-control study of risk factors for hepatoblastoma. A report from the Childrens Cancer Study Group. Cancer. 1989;64:1169-1176. [PubMed] |
| 5. | Ross JA, Gurney JG. Hepatoblastoma incidence in the United States from 1973 to 1992. Med Pediatr Oncol. 1998;30:141-142. [PubMed] |
| 6. | La Quaglia MP. The surgical management of metastases in pediatric cancer. Semin Pediatr Surg. 1993;2:75-82. [PubMed] |
| 7. | Su WT, Rutigliano DN, Gholizadeh M, Jarnagin WR, Blumgart LH, La Quaglia MP. Hepatic metastasectomy in children. Cancer. 2007;109:2089-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Martin LW, Warren RS. Current management of colorectal liver metastases. Surg Oncol Clin N Am. 2000;9:853-876; discussion 877-878. [PubMed] |
| 9. | Fowler WC, Hoffman JP, Eisenberg BL. Redo hepatic resection for metastatic colorectal carcinoma. World J Surg. 1993;17:658-661; discussion 661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Robinson BJ, Rice TW, Strong SA, Rybicki LA, Blackstone EH. Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer? J Thorac Cardiovasc Surg. 1999;117:66-75; discussion 75-76. [PubMed] |
| 11. | Santoro E, Vitucci C, Carlini M, Carboni F, Santoro E, Sacchi M, Calisti A, Lepiane P. [Liver metastasis of breast carcinoma. Results of surgical resection. Analysis of 15 operated cases]. Chir Ital. 2000;52:131-137. [PubMed] |
| 12. | Buell JF, Rosen S, Yoshida A, Labow D, Limsrichamrern S, Cronin DC, Bruce DS, Wen M, Michelassi F, Millis JM. Hepatic resection: effective treatment for primary and secondary tumors. Surgery. 2000;128:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Wyczólkowski M, Klima W, Bieda W, Walas K. Spontaneous regression of hepatic metastases after nephrectomy and metastasectomy of renal cell carcinoma. Urol Int. 2001;66:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, Eefsen RL, Reynolds AR, Brodt P. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013;73:2031-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Lee S, Qiao J, Paul P, O’Connor KL, Evers MB, Chung DH. FAK is a critical regulator of neuroblastoma liver metastasis. Oncotarget. 2012;3:1576-1587. [PubMed] |
| 16. | Ghanem MA, van Steenbrugge GJ, Nijman RJ, van der Kwast TH. Prognostic markers in nephroblastoma (Wilms’ tumor). Urology. 2005;65:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Vidal-Vanaclocha F. The tumor microenvironment at different stages of hepatic metastasis. Liver metastasis: biology and clinical management. 1st ed. Dordecht (Netherlands): Springer 2011; 43-87. |
| 18. | Spicer J, Brodt P, Ferri LE. Role of inflammation in the early stages of liver metastasis. Liver metastasis: biology and clinical management. 1st ed. Dordecht (Netherlands): Springer 2011; 155-185. |
| 19. | Sceneay J, Smyth MJ, Möller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Brodeur GM, Castleberry RP. Neuroblastoma. Principles and practice of pediatric oncology. 2nd ed. Philadelphia: J. B. Lippincott 1993; 739-767. |
| 21. | Young JL, Ries LG, Silverberg E, Horm JW, Miller RW. Cancer incidence, survival, and mortality for children younger than age 15 years. Cancer. 1986;58:598-602. [PubMed] |
| 22. | Brodeur GM, Nakagawara A. Molecular basis of clinical heterogeneity in neuroblastoma. Am J Pediatr Hematol Oncol. 1992;14:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Breslow N, McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res. 1971;31:2098-2103. [PubMed] |
| 24. | Evans AE, D’Angio GJ, Propert K, Anderson J, Hann HW. Prognostic factor in neuroblastoma. Cancer. 1987;59:1853-1859. [PubMed] |
| 25. | Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1629] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 26. | Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1440] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 27. | Shimada H, Umehara S, Monobe Y, Hachitanda Y, Nakagawa A, Goto S, Gerbing RB, Stram DO, Lukens JN, Matthay KK. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer. 2001;92:2451-2461. [PubMed] |
| 28. | Look AT, Hayes FA, Shuster JJ, Douglass EC, Castleberry RP, Bowman LC, Smith EI, Brodeur GM. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991;9:581-591. [PubMed] |
| 29. | Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK, Nuchtern JG. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 704] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 30. | Nickerson HJ, Matthay KK, Seeger RC, Brodeur GM, Shimada H, Perez C, Atkinson JB, Selch M, Gerbing RB, Stram DO. Favorable biology and outcome of stage IV-S neuroblastoma with supportive care or minimal therapy: a Children’s Cancer Group study. J Clin Oncol. 2000;18:477-486. [PubMed] |
| 31. | D’Angio GJ, Evans AE, Koop CE. Special pattern of widespread neuroblastoma with a favourable prognosis. Lancet. 1971;1:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 32. | Katzenstein HM, Bowman LC, Brodeur GM, Thorner PS, Joshi VV, Smith EI, Look AT, Rowe ST, Nash MB, Holbrook T. Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: the pediatric oncology group experience--a pediatric oncology group study. J Clin Oncol. 1998;16:2007-2017. [PubMed] |
| 33. | Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, Stram D, Castleberry RP, Shimada H, Sandler A. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, Haase GM, Black CT, Perez C, Shimada H. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181-189. [PubMed] |
| 35. | van Noesel MM, Hählen K, Hakvoort-Cammel FG, Egeler RM. Neuroblastoma 4S: a heterogeneous disease with variable risk factors and treatment strategies. Cancer. 1997;80:834-843. [PubMed] |
| 36. | Martinez DA, King DR, Ginn-Pease ME, Haase GM, Wiener ES. Resection of the primary tumor is appropriate for children with stage IV-S neuroblastoma: an analysis of 37 patients. J Pediatr Surg. 1992;27:1016-1020; discussion 1020-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Schleiermacher G, Rubie H, Hartmann O, Bergeron C, Chastagner P, Mechinaud F, Michon J. Treatment of stage 4s neuroblastoma--report of 10 years’ experience of the French Society of Paediatric Oncology (SFOP). Br J Cancer. 2003;89:470-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Weintraub M, Bloom AI, Gross E, Revel-Vilk S, Shahroor S, Koplewitz BZ, Freeman AI. Successful treatment of progressive stage 4s hepatic neuroblastoma in a neonate with intra-arterial chemoembolization. Pediatr Blood Cancer. 2004;43:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Weintraub M, Waldman E, Koplewitz B, Bloom AI, Gross E, Freeman AI, Revel-Vilk S. A sequential treatment algorithm for infants with stage 4s neuroblastoma and massive hepatomegaly. Pediatr Blood Cancer. 2012;59:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Roberts S, Creamer K, Shoupe B, Flores Y, Robie D. Unique management of stage 4S neuroblastoma complicated by massive hepatomegaly: case report and review of the literature. J Pediatr Hematol Oncol. 2002;24:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Kushner BH, Kramer K, LaQuaglia MP, Modak S, Cheung NK. Liver involvement in neuroblastoma: the Memorial Sloan-Kettering Experience supports treatment reduction in young patients. Pediatr Blood Cancer. 2006;46:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Matthay KK. Is liver metastasis in neuroblastoma an indication for treatment reduction? Pediatr Blood Cancer. 2006;46:269-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Hero B, Simon T, Horz S, Berthold F. Metastatic neuroblastoma in infancy: what does the pattern of metastases contribute to prognosis? Med Pediatr Oncol. 2000;35:683-687. [PubMed] |
| 44. | Kato K, Ishikawa K, Toyoda Y, Kigasawa H, Aida N, Nishi T, Kusafuka T, Hara J, Ijiri R, Tanaka Y. Late recurrence of neuroblastoma stage 4S with unusual clinicopathologic findings. J Pediatr Surg. 2001;36:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Heij HA, Verschuur AC, Kaspers GJ, van Rijn RR, Adam JA, Aronson DC. Is aggressive local treatment necessary for diffuse liver involvement in patients with progression of stage 4s neuroblastoma to stage 4? J Pediatr Surg. 2008;43:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | French AE, Irwin MS, Navarro OM, Greenberg M, Nathan PC. Long-term hepatic outcomes in survivors of stage 4S and 4 neuroblastoma in infancy. Pediatr Blood Cancer. 2012;58:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Sugito K, Uekusa S, Kawashima H, Furuya T, Ohashi K, Inoue M, Ikeda T, Koshinaga T, Tomita R, Mugishima H. The clinical course in pediatric solid tumor patients with focal nodular hyperplasia of the liver. Int J Clin Oncol. 2011;16:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Benz-Bohm G, Hero B, Gossmann A, Simon T, Körber F, Berthold F. Focal nodular hyperplasia of the liver in longterm survivors of neuroblastoma: how much diagnostic imaging is necessary? Eur J Radiol. 2010;74:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 49. | Fernandez-Pineda I, Cabello-Laureano R. Differential diagnosis and management of liver tumors in infants. World J Hepatol. 2014;6:486-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, Constine LS, Wallace WH, Caron HN, Armenian SH. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 51. | Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979-4990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 529] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 52. | Charles AK, Vujanić GM, Berry PJ. Renal tumours of childhood. Histopathology. 1998;32:293-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Geller E, Smergel EM, Lowry PA. Renal neoplasms of childhood. Radiol Clin North Am. 1997;35:1391-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Green D, Coppes M, Breslow N. Wilms’ tumor. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott-Raven Publishers 1997; 733-759. |
| 55. | Breslow N, Beckwith JB, Ciol M, Sharples K. Age distribution of Wilms’ tumor: report from the National Wilms’ Tumor Study. Cancer Res. 1988;48:1653-1657. [PubMed] |
| 56. | Hamilton TE, Shamberger RC. Wilms tumor: recent advances in clinical care and biology. Semin Pediatr Surg. 2012;21:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Ehrlich PF, Hamilton TE, Grundy P, Ritchey M, Haase G, Shamberger RC; National Wilms' Tumor Study Group (National Wilms' Tumor Study 5). The value of surgery in directing therapy for patients with Wilms' tumor with pulmonary disease. A report from the National Wilms' Tumor Study Group (National Wilms' Tumor Study 5). J Pediatr Surg. 2006;41:162-167; discussion 162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Warmann SW, Furtwängler R, Blumenstock G, Armeanu S, Nourkami N, Leuschner I, Schenk JP, Graf N, Fuchs J. Tumor biology influences the prognosis of nephroblastoma patients with primary pulmonary metastases: results from SIOP 93-01/GPOH and SIOP 2001/GPOH. Ann Surg. 2011;254:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | de Kraker J, Lemerle J, Voûte PA, Zucker JM, Tournade MF, Carli M. Wilm’s tumor with pulmonary metastases at diagnosis: the significance of primary chemotherapy. International Society of Pediatric Oncology Nephroblastoma Trial and Study Committee. J Clin Oncol. 1990;8:1187-1190. [PubMed] |
| 60. | Berger M, Fernandez-Pineda I, Cabello R, Ramírez-Villar GL, Márquez-Vega C, Nustede R, Linderkamp C, Schmid I, Neth O, Graf N. The relationship between the site of metastases and outcome in children with stage IV Wilms Tumor: data from 3 European Pediatric Cancer Institutions. J Pediatr Hematol Oncol. 2013;35:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Dome JS, Cotton CA, Perlman EJ, Breslow NE, Kalapurakal JA, Ritchey ML, Grundy PE, Malogolowkin M, Beckwith JB, Shamberger RC. Treatment of anaplastic histology Wilms’ tumor: results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24:2352-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 62. | Varan A, Büyükpamukçu N, Cağlar M, Köksal Y, Yalçn B, Akyüz C, Kutluk T, Büyükpamukçu M. Prognostic significance of metastatic site at diagnosis in Wilms’ tumor: results from a single center. J Pediatr Hematol Oncol. 2005;27:188-191. [PubMed] |
| 63. | Breslow NE, Churchill G, Nesmith B, Thomas PR, Beckwith JB, Othersen HB, D’Angio GJ. Clinicopathologic features and prognosis for Wilms’ tumor patients with metastases at diagnosis. Cancer. 1986;58:2501-2511. [PubMed] |
| 64. | Szavay P, Luithle T, Graf N, Furtwängler R, Fuchs J. Primary hepatic metastases in nephroblastoma--a report of the SIOP/GPOH Study. J Pediatr Surg. 2006;41:168-172; discussion 168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Fuchs J, Szavay P, Luithle T, Furtwängler R, Graf N. Surgical implications for liver metastases in nephroblastoma--data from the SIOP/GPOH study. Surg Oncol. 2008;17:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Ehrlich PF, Ferrer FA, Ritchey ML, Anderson JR, Green DM, Grundy PE, Dome JS, Kalapurakal JA, Perlman EJ, Shamberger RC. Hepatic metastasis at diagnosis in patients with Wilms tumor is not an independent adverse prognostic factor for stage IV Wilms tumor: a report from the Children’s Oncology Group/National Wilms Tumor Study Group. Ann Surg. 2009;250:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Castleberry RP, Cushing B, Perlman E, Hawkins EP. Germ cell tumors. Principles and practice of pediatric oncology. Philadelphia: Lippincott-Raven 1997; 921-945. |
| 68. | Pinkerton CR. Malignant germ cell tumours in childhood. Eur J Cancer. 1997;33:895-901; discussion 901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Rogers PC, Olson TA, Cullen JW, Billmire DF, Marina N, Rescorla F, Davis MM, London WB, Lauer SJ, Giller RH. Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study--Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol. 2004;22:3563-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Hartmann JT, Candelaria M, Kuczyk MA, Schmoll HJ, Bokemeyer C. Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer. 1997;33:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 71. | Rivoire M, Elias D, De Cian F, Kaemmerlen P, Théodore C, Droz JP. Multimodality treatment of patients with liver metastases from germ cell tumors: the role of surgery. Cancer. 2001;92:578-587. [PubMed] |
| 72. | Ablin AR, Krailo MD, Ramsay NK, Malogolowkin MH, Isaacs H, Raney RB, Adkins J, Hays DM, Benjamin DR, Grosfeld JL. Results of treatment of malignant germ cell tumors in 93 children: a report from the Childrens Cancer Study Group. J Clin Oncol. 1991;9:1782-1792. [PubMed] |
| 73. | Cushing B, Giller R, Cullen JW, Marina NM, Lauer SJ, Olson TA, Rogers PC, Colombani P, Rescorla F, Billmire DF. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: a pediatric intergroup study--Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol. 2004;22:2691-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Marina N, London WB, Frazier AL, Lauer S, Rescorla F, Cushing B, Malogolowkin MH, Castleberry RP, Womer RB, Olson T. Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study. J Clin Oncol. 2006;24:2544-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49-vii55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Casali PG, Blay JY. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 78. | Otto C, Agaimy A, Braun A, Rädecke J, Hoeppner J, Illerhaus G, Werner M, Kontny U, Haller F. Multifocal gastric gastrointestinal stromal tumors (GISTs) with lymph node metastases in children and young adults: a comparative clinical and histomorphological study of three cases including a new case of Carney triad. Diagn Pathol. 2011;6:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296:1517-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 259] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Cypriano MS, Jenkins JJ, Pappo AS, Rao BN, Daw NC. Pediatric gastrointestinal stromal tumors and leiomyosarcoma. Cancer. 2004;101:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Haider N, Kader M, Mc Dermott M, Devaney D, Corbally MT, Fitzgerald RJ. Gastric stromal tumors in children. Pediatr Blood Cancer. 2004;42:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Durham MM, Gow KW, Shehata BM, Katzenstein HM, Lorenzo RL, Ricketts RR. Gastrointestinal stromal tumors arising from the stomach: a report of three children. J Pediatr Surg. 2004;39:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1647] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 84. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3111] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 85. | Pappo AS, Janeway K, Laquaglia M, Kim SY. Special considerations in pediatric gastrointestinal tumors. J Surg Oncol. 2011;104:928-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 86. | Janeway KA, Albritton KH, Van Den Abbeele AD, D’Amato GZ, Pedrazzoli P, Siena S, Picus J, Butrynski JE, Schlemmer M, Heinrich MC. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 87. | Janeway KA, Weldon CB. Pediatric gastrointestinal stromal tumor. Semin Pediatr Surg. 2012;21:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Prakash S, Sarran L, Socci N, DeMatteo RP, Eisenstat J, Greco AM, Maki RG, Wexler LH, LaQuaglia MP, Besmer P. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 89. | Ladd AP, Grosfeld JL. Gastrointestinal tumors in children and adolescents. Semin Pediatr Surg. 2006;15:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Takaki H, Litchman T, Covey A, Cornelis F, Maybody M, Getrajdman GI, Sofocleous CT, Brown KT, Solomon SB, Alago W. Hepatic artery embolization for liver metastasis of gastrointestinal stromal tumor following imatinib and sunitinib therapy. J Gastrointest Cancer. 2014;45:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 91. | Yamanaka T, Takaki H, Nakatsuka A, Uraki J, Fujimori M, Hasegawa T, Sakuma H, Yamakado K. Radiofrequency ablation for liver metastasis from gastrointestinal stromal tumor. J Vasc Interv Radiol. 2013;24:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Putnam JB, Roth JA, Wesley MN, Johnston MR, Rosenberg SA. Survival following aggressive resection of pulmonary metastases from osteogenic sarcoma: analysis of prognostic factors. Ann Thorac Surg. 1983;36:516-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 114] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | Goorin AM, Delorey MJ, Lack EE, Gelber RD, Price K, Cassady JR, Levey R, Tapper D, Jaffe N, Link M. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: analysis of 32 patients. J Clin Oncol. 1984;2:425-431. [PubMed] |
| 94. | Beron G, Euler A, Winkler K. Pulmonary metastases from osteogenic sarcoma: Complete resection and effective chemotherapy contributing to improved prognosis. Eur Pediatr Haematol Oncol. 1985;2:77-85. |
| 95. | Meyer WH, Schell MJ, Kumar AP, Rao BN, Green AA, Champion J, Pratt CB. Thoracotomy for pulmonary metastatic osteosarcoma. An analysis of prognostic indicators of survival. Cancer. 1987;59:374-379. [PubMed] |
| 96. | Shapiro RS, Mendelson DS, Norton KI, Janus C, Gendal ES, Hermann G. Case report: calcified liver metastases from osteosarcoma. J Comput Tomogr. 1988;12:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | O’Mara RE, Brettner A, Danigelis JA, Gould LV. 18 F uptake within metastatic osteosarcoma of the liver. A case report. Radiology. 1971;100:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Daw NC, Kaste SC, Hill DA, Kun LE, Pratt CB. Metastatic osteosarcoma to the liver after treatment for synovial sarcoma: a case report. Pediatr Hematol Oncol. 2001;18:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Skinner KA, Eilber FR, Holmes EC, Eckardt J, Rosen G. Surgical treatment and chemotherapy for pulmonary metastases from osteosarcoma. Arch Surg. 1992;127:1065-1070; discussion 1070-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Briccoli A, Rocca M, Salone M, Bacci G, Ferrari S, Balladelli A, Mercuri M. Resection of recurrent pulmonary metastases in patients with osteosarcoma. Cancer. 2005;104:1721-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Msika S, Gruden E, Sarnacki S, Orbach D, Philippe-Chomette P, Castel B, Sabaté JM, Flamant Y, Kianmanesh R. Cytoreductive surgery associated to hyperthermic intraperitoneal chemoperfusion for desmoplastic round small cell tumor with peritoneal carcinomatosis in young patients. J Pediatr Surg. 2010;45:1617-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Philippe-Chomette P, Kabbara N, Andre N, Pierron G, Coulomb A, Laurence V, Blay JY, Delattre O, Schleiermacher G, Orbach D. Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediatr Blood Cancer. 2012;58:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 103. | Honoré C, Amroun K, Vilcot L, Mir O, Domont J, Terrier P, Le Cesne A, Le Péchoux C, Bonvalot S. Abdominal desmoplastic small round cell tumor: multimodal treatment combining chemotherapy, surgery, and radiotherapy is the best option. Ann Surg Oncol. 2015;22:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Hayes-Jordan A, Green HL, Lin H, Owusu-Agyemang P, Fitzgerald N, Arunkumar R, Mejia R, Okhuysen-Cawley R, Mauricio R, Fournier K. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol. 2014;21:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 105. | Hayes-Jordan A, Green H, Lin H, Owusu-Agyemang P, Mejia R, Okhuysen-Cawley R, Cortes J, Fitzgerald NE, McAleer MF, Herzog C. Cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Surg Oncol. 2015;22:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Navalkele P, O’Dorisio MS, O’Dorisio TM, Zamba GK, Lynch CF. Incidence, survival, and prevalence of neuroendocrine tumors versus neuroblastoma in children and young adults: nine standard SEER registries, 1975-2006. Pediatr Blood Cancer. 2011;56:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 107. | Moertel CL, Weiland LH, Telander RL. Carcinoid tumor of the appendix in the first two decades of life. J Pediatr Surg. 1990;25:1073-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Soga J. Statistical evaluation of 2001 carcinoid cases with metastases, collected from literature: a comparative study between ordinary carcinoids and atypical varieties. J Exp Clin Cancer Res. 1998;17:3-12. [PubMed] |
| 109. | Broaddus RR, Herzog CE, Hicks MJ. Neuroendocrine tumors (carcinoid and neuroendocrine carcinoma) presenting at extra-appendiceal sites in childhood and adolescence. Arch Pathol Lab Med. 2003;127:1200-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 110. | Harring TR, Nguyen NT, Goss JA, O’Mahony CA. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol. 2011;2011:154541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Glazer ES, Tseng JF, Al-Refaie W, Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN, Curley SA. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford). 2010;12:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 112. | Kolbeck KJ, Farsad K. Catheter-based treatments for hepatic metastases from neuroendocrine tumors. AJR Am J Roentgenol. 2014;203:717-724. [PubMed] |
| 113. | Máthé Z, Tagkalos E, Paul A, Molmenti EP, Kóbori L, Fouzas I, Beckebaum S, Sotiropoulos GC. Liver transplantation for hepatic metastases of neuroendocrine pancreatic tumors: a survival-based analysis. Transplantation. 2011;91:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | Grąt M, Remiszewski P, Smoter P, Wronka KM, Grąt K, Lewandowski Z, Koperski L, Górnicka B, Pacho R, Zborowska H. Outcomes following liver transplantation for metastatic neuroendocrine tumors. Transplant Proc. 2014;46:2766-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |