Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.243
Revised: January 17, 2014
Accepted: February 16, 2014
Published online: April 27, 2014
Processing time: 180 Days and 14.6 Hours
AIM: To propose an alternative model of hepatic encephalopathy (HE) in mice, resembling the human features of the disease.
METHODS: Mice received two consecutive intraperitoneal injections of thioacetamide (TAA) at low dosage (300 mg/kg). Liver injury was assessed by serum transaminase levels (ALT) and liver histology (hematoxylin and eosin). Neutrophil infiltration was estimated by confocal liver intravital microscopy. Coagulopathy was evaluated using prolonged prothrombin and partial thromboplastin time. Hemodynamic parameters were measured through tail cuff. Ammonia levels were quantified in serum and brain samples. Electroencephalography (EEG) and psychomotor activity score were performed to show brain function. Brain edema was evaluated using magnetic resonance imaging.
RESULTS: Mice submitted to the TAA regime developed massive liver injury, as shown by elevation of serum ALT levels and a high degree of liver necrosis. An intense hepatic neutrophil accumulation occurred in response to TAA-induced liver injury. This led to mice mortality and weight loss, which was associated with severe coagulopathy. Furthermore, TAA-treated mice presented with increased serum and cerebral levels of ammonia, in parallel with alterations in EEG spectrum and discrete brain edema, as shown by magnetic resonance imaging. In agreement with this, neuropsychomotor abnormalities ensued 36 h after TAA, fulfilling several HE features observed in humans. In this context of liver injury and neurological dysfunction, we observed lung inflammation and alterations in blood pressure and heart rate that were indicative of multiple organ dysfunction syndrome.
CONCLUSION: In summary, we describe a new murine model of hepatic encephalopathy comprising multiple features of the disease in humans, which may provide new insights for treatment.
Core tip: The study of hepatic encephalopathy is crucial for development of new therapies but has been dampened by the absence of murine models resembling the disease in patients. We showed that sequential thioacetamide injections cause extensive liver injury in mice, leading to increased ammonia levels, electroencephalography alterations and brain edema. In line with this, mice presented with poor psychomotor activity and survival rate. Liver injury and brain function impairment by thioacetamide resulted in systemic alterations such as coagulopathy, hemodynamic instability and lung inflammation, consistent with multiple organ failure. Therefore, this alternative model may provide tools for new therapeutic insights for hepatic encephalopathy.
- Citation: Gomides LF, Marques PE, Faleiros BE, Pereira RV, Amaral SS, Lage TR, Resende GHS, Guidine PAM, Foureaux G, Ribeiro FM, Martins FP, Fontes MAP, Ferreira AJ, Russo RC, Teixeira MM, Moraes MF, Teixeira AL, Menezes GB. Murine model to study brain, behavior and immunity during hepatic encephalopathy. World J Hepatol 2014; 6(4): 243-250
- URL: https://www.wjgnet.com/1948-5182/full/v6/i4/243.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i4.243
Acute liver failure (ALF) is a rare but severe clinical syndrome. It is typically characterized by jaundice, coagulopathy and encephalopathy resulting from sudden hepatic dysfunction without preexisting liver disease[1]. Drug-induced liver injury (DILI) is the main cause of ALF in the United States and Europe[2], whereas in developing countries viral hepatitis is the most important etiology. Although liver injuries may result from a wide range of situations, drug overdose may become the most important etiology of ALF worldwide in a few years[2] as life expectancy increases and the medicalization of the older population tends to increase[3]. Moreover, the worldwide cause of ALF tends towards DILI due to increasing public health measures (e.g., vaccination)[1], making it an emergent widespread problem. ALF has a high mortality rate of approximately 30% due to multiple organ dysfunction, hepatic encephalopathy and sepsis. Unfortunately, therapeutic options are very limited since liver transplantation is the only definitive therapy available. Besides that, patients are kept under clinical management and supportive care until spontaneous liver recovery[1,4].
Hepatic encephalopathy (HE) is one of the major complications in ALF because of rapid brain edema, intracranial hypertension and cerebral herniation[5]. Clinically, HE is a neuropsychiatric syndrome that comprises a wide range of signs and symptoms from subtle altered mental status to stupor and coma[6]. The severity of HE has prognostic implications since patient outcome worsens as HE progresses. There is a chance of 65%-70% of spontaneous liver recovery in mild HE and less than 20% in severe HE[7]. Moreover, patients with ALF who manifest increased intracranial pressure during the course of illness are more likely to develop sepsis[8]. Hence, understanding HE pathophysiology is crucial for development of new and specific therapies that would slow down its progression and increase chances of better outcomes.
In this sense, a murine model that reproduces features of HE in humans would be key to improve our understanding in HE pathophysiology and may also be useful for drug testing and development. Here, we propose an alternative murine model of HE using sequential systemic thioacetamide (TAA) injections in a lower dosage instead of a single higher dose administration.
Female C57BL/6 mice and Lysm-eGFP (eGFP-expressing neutrophils) had free access to food and water. TAA-induced liver injury protocol was based on two consecutive days of treatment with thioacetamide (300 mg/kg) administered intraperitoneally. Treated groups received the first dose in time zero and an additional dose after 24 h. Controls received vehicle following the same chronogram. Mice were sacrificed after 24 or 48 h and liver, lung, blood and brain samples were collected for further analysis. All mice received glucose replacement (12/12 h; 5%; s.c.) and glycemia was monitored (12/12 h) with commercially available reactive strips and a glucometer (Accu-Chek, Performa). Body temperature was maintained at 37 °C by a thermal pad. Psychomotor activity was graded following an adapted clinical score, in which 0 = normal behavior; 1 = mild lethargy; 2 = decreased motor activity, poor gesture control, diminished pain perception; 3 = Severe ataxia, no spontaneous righting reflex; 4 = no righting reflex, no reaction to pain stimuli; and 5 = death[9]. All procedures were approved by Animal Care and Use Committee in UFMG (CEBIO n°051/2011). The investigation conformed to the standards of Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, 1996 revision). In a separate set of experiments, the liver was imaged using confocal intravital microscopy as described previously[10]. Lysm-eGFP mice received propidium iodide (200 μL of a 100 μmol/L stock solution; i.v) prior to the surgical procedure in order to visualize necrotic cells.
Immediately after decapitation, the brain was rapidly removed from the cranial cavity and fast-frozen in liquid nitrogen. Subsequently, brain samples were macerated using a pestle in perchloric acid (ice-cold; 1mol/L) and centrifuged at 10000 g for 10 min at 4 °C. The supernatant was collected for immediate dosage. Also, blood samples were centrifuged for 7 min (7000 g) and plasma was collected. Ammonia concentration was estimated using Ammonia Assay kit (Sigma, United States) following manufacturer instructions.
The alanine aminotransferase enzyme is present in the cytoplasm of hepatocytes and is highly specific for the liver. The measurement of serum alanine aminotransferase (ALT) is a gold-standard marker of liver damage. To determine the activity of ALT, blood samples were centrifuged and the serum was collected and dosed using a kinetic kit (Bioclin, Brazil).
Mean arterial pressure (MAP) and heart rate (HR) were evaluated by a volume pressure recording sensor and an occlusion tail-cuff, which measures mice blood pressure and HR noninvasively (Kent Scientific Corporation, Torrington, CT, United States)[11]. Mice were acclimated to the restraint and tail cuff inflation for two days before the beginning of the experiments. The restraint platform was maintained at 36-38 °C. In each session, mice were placed in an acrylic box restraint, and the tail was inserted into a compression cuff that measured the blood pressure 10 times. Following the measurement cycle, the average of these values was considered for each mouse. MAP and HR were evaluated at 0, 24 and 48 h after TAA administration.
Surgery procedures: Mice (n = 8) were anesthetized using a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Prophylactic treatment with antibiotics (enrofloxacin; 10 mg/kg; s.c.) was done in order to prevent post-surgical infections. The animals were submitted to surgery for electroencephalography (EEG) electrode implantation in the right and left parietal cortices. The electrodes for superficial EEG recordings were made with surgical screws (Fine Science Tools; model 19010-00; Foster City, CA, United States) previously soldered to Teflon-coated stainless-steel wires (A&M Systems; model 7916; Carlsborg, WA, United States) and introduced bilaterally in the parietal bones. A reference electrode, also made with surgical screws, was inserted in the nasal bone. All the electrodes were soldered to a common pin connector and anchored to the cranium with dental acrylic. Following surgery, animals were allowed to recover for at least 4 days before EEG recordings.
Experimental protocol: Mice were injected with thioacetamide in the same regime (n = 5) or saline (n = 3). Each mouse was recorded in three consecutive days, namely: day 1, before drug injection; days 2 and 3, after first and second drug injections, respectively. Mice were recorded for a period of 10 min each day. Bioelectrical activity was recorded with a video-EEG recording system (8:00-11:00 am). The EEG signal from both parietal cortices was amplified (1000 × gain) and filtered (1 Hz High pass, 500 Hz Low pass) by a signal conditioner (Aisha4 - Kananda® Ltda). Data were sampled at 1 kHz (12 bit DI-148U A/D converter - DATAQ® Instruments) and recorded in a computer hard disk for offline analyses. Spectral analysis and 3D rendering of EEG spectrum were done using MatLab® scripts.
MR image experiments were acquired using 4.7T NMR system (Oxford Systems) controlled by a UNITY Inova-200 imaging console (Varian). The imaging protocol consisted of coronal T2-weighted (TR = 3000 ms, TE = 50 ms) spin echo multislice scans, 16 contiguous 1 mm thick slices. Mice (n= 12) were anesthetized with halothane (4% induction, 1.5% maintenance) and oxygen (1.5 l/min) delivered by a facemask in a head holder to minimize artifact movements. Animals anesthetized for the duration of an imaging experiment (50 min) recovered with no apparent difficulty and could be used for subsequent imaging studies. Each mouse was imaged in three consecutive days, namely: day 1, before drug injection; days 2 and 3, after first and second drug injections, respectively. Brain masks, based on the anatomical scans, were done using a tablet driver (Bamboo Tablet Driver, V5.2.5 WIN, WACOM Technology Corporation, United States) and MeVisLab software (MeVis Medical Solutions AG, Fraunhofer). Additionally, densitometry analysis was done using MatLab® scripts and repeated measures. ANOVA was used to compare densitometry values on different days.
Statistical analyzes were performed using one-way ANOVA (Tukey’s post test) and Student’s t test. P values less than 0.05 were considered statistically significant. All data are presented as mean ± SE.
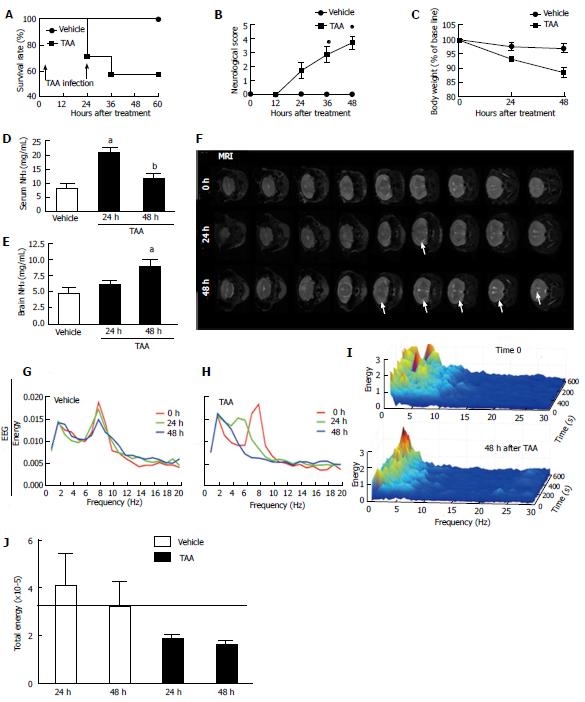
As shown in Figure 1A, around 30% of mice treated with a single dose of TAA died after 24 h and more than 40% succumbed due to a second TAA administration. Also, a progressive increase in neurological score (reflecting a decreased neuropsychomotor activity) was observed (Figure 1B) and, probably due to such reduced mobility, TAA-treated mice presented with a significant weight loss in comparison to controls throughout the experiment (Figure 1C). In addition, TAA treatment caused serum hyperammonemia after 24 h (Figure 1D), which was also detected in the brain in later timepoints (48 h; Figure 1E). Taking into account the edematogenic effects of cerebral ammonia accumulation, we imaged mice brains using magnetic resonance (MR) to investigate potential morphological alterations, including suggestive areas of fluid accumulation. MR revealed a discrete but diffuse brain edema in TAA-treated mice, which was not observed in controls (Figure 1F). Increased fluid accumulation was more evident following 48 h of TAA treatment; however, such a feature was not prominent. In fact, no significant differences were observed in brain densitometry during analysis of MRI images (data not shown), suggesting that a mild brain edema was occurring in this model. To further dissect the neurological effects of TAA poisoning, we submitted mice to daily EEG registration throughout the experimental protocol. Analysis of the EEG spectrum revealed that while untreated mice had coincident EEG records during all experimental protocol (Figure 1G), TAA administration caused a progressive decrease in higher frequencies waves (theta and alpha; 4-8 and 8-13 Hz, respectively) with a concomitant increase of lower frequency ones (mainly delta; up to 4 Hz; Figure 1H-I). Such an EEG profile, with concentration of energy mainly in delta waves region and significant reduction in total energy (Figure 1J), confirmed that repeated TAA administration led to a diffuse brain lesion and metabolic encephalopathy.
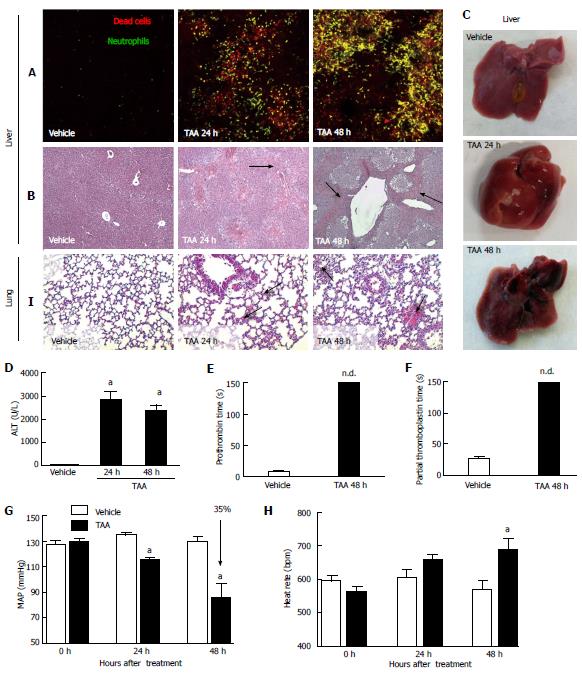
It is well established that impaired liver function caused by extensive hepatocyte death leads to a general deficiency in metabolism, including ammonia depuration and synthesis of coagulation cascade factors[3]. Liver intravital microscopy revealed a marked increase in necrotic cells (stained by propidium iodide; in red) following TAA exposure (Figure 2A), which was also confirmed by histology analysis (Figure 2B). Also, we observed an exuberant neutrophil accumulation in liver necrotic areas, which increased throughout TAA intoxication process (Figure 2A; eGFP-expressing cells). Macroscopically, livers from TAA treated mice displayed extensive areas of necrosis (Figure 2C), which became more obvious after repeated TAA administration (48 h). Significantly higher serum levels of alanine aminotransferase (ALT) also indicated massive liver injury, which was sustained during the whole experimental period (Figure 2D). In fact, a complete lack of hemostatic function also confirmed hepatotoxicity and organ failure, suggesting that synthesis of liver-derived coagulation factors was seriously impaired. While controls had normal hemostatic parameters (MAP and HR), TAA-treated mice had a prolonged (undetermined) prothrombin and partial thromboplastin time (Figure 2E, F). Moreover, while control mice had normal hemodynamic parameters, the TAA-treated group had a significant drop in MAP (approximately 35%) with concomitant tachycardia, suggesting that mice might be evolving to a hemodynamic shock (Figure 2G, H). In this direction, we hypothesized that in our model TAA might also cause multiple organ failure and remote injury.
We have previously shown that necrosis-derived products may reach systemic circulation and trigger remote inflammatory responses in organs including the lungs[12]. Here, we evaluated the potential of TAA in induction of lung inflammation. Histopathological analysis showed that TAA-induced liver injury also caused pulmonary injury. After 24 h, lungs from mice had increased cellularity in lung parenchyma, alveolar edema and hemorrhage in comparison to controls (Figure 1I; arrows). Those observations were found to be more pronounced after 48 h, showing increased tissue damage, alveolar hemorrhage and lung architecture disruption, suggesting that lethality induced by TAA can also be due to exacerbation of lung inflammatory response in this model.
The hepatotoxic ability of TAA is well described in the literature and this drug has been extensively used in rat models of ALF and HE in sequential repeated dose administration[9,13-16]. Once in circulation, TAA is absorbed and bioactivated by hepatocytes. This metabolite will finally modify aminolipids and proteins, causing cell damage and death[17]. In mice, acute TAA poisoning causes centrilobular necrosis and an increase in plasma transaminases and bilirubin. We have shown that following two daily TAA injections, mice developed a massive and progressive liver damage and failure, with hyperammonemia in serum and brain, with discrete signals of cerebral edema.
Hyperammonemia is a hallmark of HE pathophysiology. In patients with ALF, the arterial ammonia level is directly related to severity of HE and development of intracranial hypertension[18]. Also, persistent hyperammonemia seems to be more important than a transient increase in the ammonia level regarding the occurrence of intracranial hypertension[19]. Most studies in mice used a single dose of a hepatotoxic drug which caused a briefer period of illness[20-22]. To supersede this, we developed a novel protocol using a lower TAA dose (300 mg/kg) and repeated administration (2 daily doses), trying to mimic a more realistic scenario closer to severe HE. We found that under these conditions, mice progressively presented with neuropsychomotor deficiencies (as assessed by neurological score)[23], which was accompanied by persistent liver injury and failure. Interestingly, serum ammonia concentration peaked after the first TAA administration, decreasing at 48 h. However, cerebral ammonia gradually increased throughout the experimental protocol, reaching significantly higher levels 48 h after TAA, suggesting that probably excessive serum levels of ammonia might be transferred to the brain due to deficient liver ammonia clearance. The mechanisms responsible for ammonia-mediated encephalopathy and brain edema are still under debate; however, it is accepted that increased blood-derived ammonia intensifies glutamine synthesis via amidation of glutamate, generating a hyperosmotic environment within brain cells (mainly astrocytes)[24]. Consequently, cerebral liquid accumulation leads to intracranial hypertension, herniation and coma. In agreement with this, magnetic resonance imaging from mice treated with TAA revealed a discrete, but crescent-shaped and diffuse brain edema[25]. In addition, through analysis of the EEG spectrum we found diffuse a brain lesion compatible with metabolic encephalopathy[26], suggesting that our DILI model reproduces some of the main clinical manifestations of HE[27]. In conjunct, our data suggest that this model may be suitable for further studies involving ALF, hyperammonemia and brain edema.
We also investigated the mechanisms involved in liver damage triggered by TAA in mice. Intravital microscopy revealed that large areas of liver necrosis were infiltrated by neutrophils and such sterile inflammation has been described as a key factor for injury amplification[12]. Furthermore, we also observed repercussions in hemodynamic functions and inflammatory infiltration in the lungs. In this sense, the innate immune response triggered by necrosis-derived products may add to direct TAA-mediated hepatotoxicity to establish not only ALF, but also remote organ injury. Accordingly, TAA-treated mice displayed a severe impairment in hemostatic function compatible with an end-stage liver failure, with coagulopathy, hypovolemic shock and possible multiple organ failure. Thus, these factors should be also evaluated in future studies primarily dedicated to the liver or central nervous system.
In conclusion, we have shown that repeated lower doses of TAA might constitute as a novel murine model of HE and ALF, which reproduce several features of human disease. Also, we provided read-outs for disease grade and severity that comprise behavioral changes, brain edema and ammonemia, hemodynamic parameters and inflammatory response.
We would like to thank CNPq, Fapemig, PRONEX and CAPES for financial support.
Acute liver failure (ALF) is a rare but severe clinical syndrome. ALF is the result of massive liver injury, which can be caused mostly by drugs or viruses. During the disease, patients may suffer hepatic encephalopathy, multiple organ dysfunction and sepsis, leading to a high mortality rate. The incidence of ALF is increasing worldwide and liver transplantation and supportive medical care are the only therapies available for ALF.
Hepatic encephalopathy (HE) is one of the major complications in ALF because of rapidly progressing brain edema and hypertension. Hence, understanding HE pathophysiology is crucial for development of new and specific therapies that would slow down its progression and increase chances of better outcomes. In this sense, a murine model that reproduces most features of HE in humans would be key to improve our understanding of HE pathophysiology and may also be useful for drug testing and development.
This study provided a new model to study hepatic encephalopathy and acute liver failure in mice. In this model, mice are treated twice with a low dose thioacetamide, causing extensive liver injury and characteristic complications of human HE, including high ammonia levels, altered electroencephalography (EEG), brain edema and poor neuropsychomotor function. In addition, mice presented with a systemic response similar to multiple organ failure syndrome, where lung inflammation, coagulopathy and hemodynamic alterations were observed.
This is a new model to study ALF and HE in mice, the most used animal model. Also, this is the first animal model to present such similar symptoms to the human disease, making it a more useful research tool for this biomedical area.
The most important terms in this article are: ALF, HE and thioacetamide.
The authors describe a murine model of acute hepatic failure to study hepatic encephalopathy by sequential administration of thioacetamide intraperitoneally. The hypothesis is convenient, the methodology for acute hepatic failure was suitable, the evaluation of hepatic encephalopathy was convenient and sophisticated, the results were interesting and the discussion appropriate.
P- Reviewers: Galvao FHF, Naglaa A, Yamasaki T S- Editor: Song XX L- Editor: Roemmele A E -Editor: Wu HL
| 1. | Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ichai P, Samuel D. Epidemiology of liver failure. Clin Res Hepatol Gastroenterol. 2011;35:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O’Grady JG, Wendon J. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74-80. [PubMed] |
| 4. | Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Vaquero J, Chung C, Blei AT. Brain edema in acute liver failure. A window to the pathogenesis of hepatic encephalopathy. Ann Hepatol. 2003;2:12-22. [PubMed] |
| 6. | Shawcross DL, Wendon JA. The neurological manifestations of acute liver failure. Neurochem Int. 2012;60:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 8. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [PubMed] |
| 9. | Farjam M, Dehdab P, Abbassnia F, Mehrabani D, Tanideh N, Pakbaz S, Imanieh MH. Thioacetamide-induced acute hepatic encephalopathy in rat: behavioral, biochemical and histological changes. Iran Red Crescent Med J. 2012;14:164-170. [PubMed] |
| 10. | McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 923] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 11. | Hartman RE, Kamper JE, Goyal R, Stewart JM, Longo LD. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav. 2012;105:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaço JG. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Norton NS, McConnell JR, Rodriguez-Sierra JF. Behavioral and physiological sex differences observed in an animal model of fulminant hepatic encephalopathy in the rat. Physiol Behav. 1997;62:1113-1124. [PubMed] |
| 14. | Chen TM, Subeq YM, Lee RP, Chiou TW, Hsu BG. Single dose intravenous thioacetamide administration as a model of acute liver damage in rats. Int J Exp Pathol. 2008;89:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Ashworth CT, Werner DJ, Glass MD, Arnold NJ. Spectrum of fine structural changes in hepatocellular injury due to thioacetamide. Am J Pathol. 1965;47:917-951. [PubMed] |
| 16. | Mangipudy RS, Chanda S, Mehendale HM. Tissue repair response as a function of dose in thioacetamide hepatotoxicity. Environ Health Perspect. 1995;103:260-267. [PubMed] |
| 17. | Hajovsky H, Hu G, Koen Y, Sarma D, Cui W, Moore DS, Staudinger JL, Hanzlik RP. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem Res Toxicol. 2012;25:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55:98-104. [PubMed] |
| 19. | Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jørgensen L, Larsen FS. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2006;26:21-27. [PubMed] |
| 20. | Avraham Y, Grigoriadis N, Poutahidis T, Vorobiev L, Magen I, Ilan Y, Mechoulam R, Berry E. Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol. 2011;162:1650-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Bélanger M, Côté J, Butterworth RF. Neurobiological characterization of an azoxymethane mouse model of acute liver failure. Neurochem Int. 2006;48:434-440. [PubMed] |
| 22. | Miranda AS, Rodrigues DH, Vieira LB, Lima CX, Rachid MA, Vidigal PV, Gomez MV, Reis HJ, Guatimosim C, Teixeira AL. A thioacetamide-induced hepatic encephalopathy model in C57BL/6 mice: a behavioral and neurochemical study. Arq Neuropsiquiatr. 2010;68:597-602. [PubMed] |
| 23. | Norenberg MD. Astroglial dysfunction in hepatic encephalopathy. Metab Brain Dis. 1998;13:319-335. [PubMed] |
| 24. | Butterworth RF. Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis. 2002;17:221-227. [PubMed] |
| 25. | Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, Jones I, Towner RA, Castro-Faria-Neto HC. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307-318. [PubMed] |
| 27. | Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |