Published online Mar 27, 2014. doi: 10.4254/wjh.v6.i3.155
Revised: December 16, 2013
Accepted: January 17, 2014
Published online: March 27, 2014
Processing time: 167 Days and 22.3 Hours
Nested stromal-epithelial tumours (NSETs) of the liver have been reported to be extremely unusual primary hepatic neoplasms. To date, few cases have been described in the literature. NSETs have been defined as non-hepatocytic and non-biliary tumours of the liver consisting of nests of epithelial and spindled cells, myofibroblastic stroma and variable intralesional calcification and ossification. Here, we report a case of a young female who underwent liver resection for a large hepatic lesion that proved to be a calcifying NSET on pathological examination. Details about the clinical and histopathological features of the tumour are reported.
Core tip: Rare cases of hepatic nested stromal-epithelial tumours (NSETs), consisting of non-hepatocytic mixed stromal and epithelial neoplasms with associated calcification and ossification, have been previously described. To date, NSETs’ behaviour and prognosis are completely unclear. We report the case of a 23-year-old female who underwent liver resection for a large hepatic, calcifying NSET. Details about preoperative imaging and the clinical and histopathological features of this very rare hepatic tumour are reported.
- Citation: Procopio F, Tommaso LD, Armenia S, Quagliuolo V, Roncalli M, Torzilli G. Nested stromal-epithelial tumour of the liver: An unusual liver entity. World J Hepatol 2014; 6(3): 155-159
- URL: https://www.wjgnet.com/1948-5182/full/v6/i3/155.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i3.155
Nested stromal-epithelial tumours (NSETs) of the liver are very rare primary tumours characterised by unexampled clinicopathological features. To date, only isolated cases[1] and small series have been reported in the literature[2-4]. NSETs have been defined as a non-hepatocytic and non-biliary tumour of the liver consisting of nests of epithelial and spindled cells with associated myofibroblastic stroma and variable intralesional calcification and ossification[2]. This rare liver malignancy appears solid on imaging and has been reported to be isolated or occasionally associated with hormone cortisol-related syndrome. Herein, we describe a patient who underwent radical hepatectomy for a large calcifying NSET of the liver.
In January 2012, a 23-year-old female was referred to our unit for recurrent dull abdominal pain associated with abdominal distension and dyspepsia. The patient had a past history of being negative for hepatitis but positive for the consumption of oral contraceptives during the previous 5 years.
On physical examination, a palpable mass in the upper abdomen was revealed. No Cushingoid or other clinical features were evident.
Laboratory tests revealed an altered serum level of aspartate aminotransferase (36 IU/L; normal range: 5-30), alanine aminotransferase (297 IU/L; normal range: 5-35), alkaline phosphatase (62 IU/L; normal range: 4-150) and gamma-glutamyltransferase (285 IU/L; normal range: 6-32). Virological markers for hepatitis B and C yielded negative results. Tumour markers, including carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP), were negative, whereas the level of carbohydrate antigen 19-9 (CA19-9) was elevated (98 IU/mL; normal value: < 40).
Abdominal computed tomography (CT) revealed a well-circumscribed liver lesion of 16 cm in size involving the left hemiliver, part of the right anterior section (S5-8)[5] and the paracaval portion of the caudate lobe (S1pc). Extensive vascular invasion including the left hepatic vein (LHV) and middle hepatic vein (MHV) at the hepatocaval confluence and the left portal pedicle (LPP) was evident. The lesion appeared solid and heterogeneous, with a rim-like enhancement on the arterial phase and a gradual centripetal enhancement on delayed phases. Multiple intralesional calcifications were also evident.
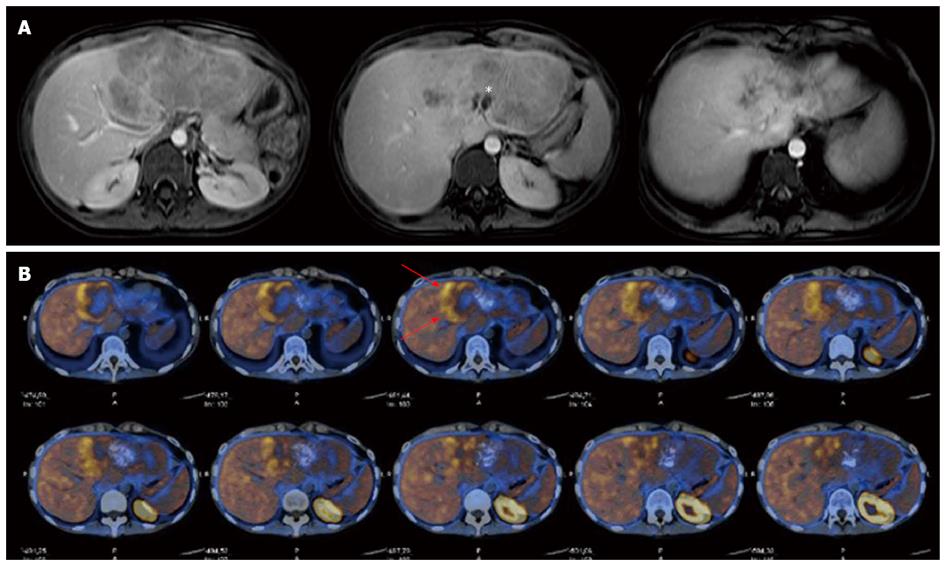
On magnetic resonance imaging (MRI), the tumour showed hypointensity on T1-weighted images and hyperintensity on both T2- and diffusion-weighted images (DWI), with small central necrotic collections. An inhomogeneous pattern with subcentimetric calcifications showing mostly hypointensity on both T1- and T2-weighted images was depicted. On gadolinium-enhanced images, the lesion showed a heterogeneous enhancement pattern on the arterial phase and washout in the portal and parenchymal phases (Figure 1A). The hepatocyte-specific delayed phase (Primovist, Bayer-Schering, Berlin, Germany) showed a hypointense lesion on T1-weighted images, with well-defined margins and a hyperintense capsule.
The work-up was completed with total-body 11C-choline positron emission tomography (PET), which showed only slight pathological uptake of the tracer into the liver (Figure 1B). Based on these preoperative findings, a percutaneous lesion biopsy was not considered, and the patient was candidated to liver resection with a presumptive diagnosis of fibrolamellar hepatocellular carcinoma (HCC) or hepatocholangiocarcinoma.
At laparotomy, peritoneal carcinomatosis was excluded. During liver exploration, a huge, hard liver tumour entirely occupying the left hemiliver, part of S5-8 and the S1pc was confirmed. On intraoperative ultrasonography (IOUS), no additional lesions were detected, and the tumour showed well-defined margins and a heterogeneous echogenicity, with several intralesional hyperechoic spots due to multiple calcifications. Extensive involvement of the LHV, MHV and LPP was confirmed. Once the intraoperative staging was completed, the patient underwent an extended left hepatectomy with left and middle hepatic vein resection for radical removal of the mass. The specimen weighed 2000 g. Intraoperative blood loss was 100 mL, and the patient did not receive a blood transfusion. The postoperative course was uneventful, and the patient was discharge on the 10th postoperative day. Currently, the patient is alive and disease free 21 mo after surgery.
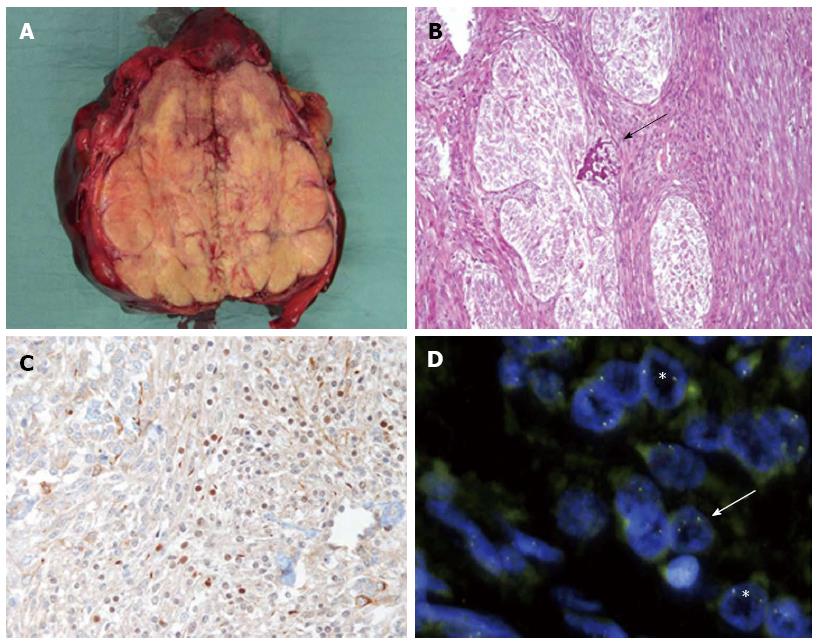
Grossly, the tumour had well-defined margins, was arranged in yellow lobules with several granular and rasping foci and was 16 cm in size (Figure 2A). On histology, the tumour was composed of well-defined nests of epithelial cells separated by strands of stromal cells and focally admixed with calcifying material (Figure 2B). The epithelial component was represented by polygonal to spindle-shaped cells, devoid of overt cytological atypia; stained positive for CKpool, CK19 and CD56; and stained negative for Hep Par-1 (hepatocyte paraffin 1) and AFP. Scattered nuclei were also immunoreactive for WT1 (Figure 2C). The stromal component stained positive for vimentin and smooth muscle actin. The mitotic index was < 5 mitoses/10 HPFs, and 10%-15% of cells stained positive for Ki67.
Several neoplastic nuclei were positive for the EWS-WT1 fusion transcript (Figure 2D) but negative for the SYT-SSX fusion transcript.
Stromal-epithelial tumours are extremely rare conditions of the liver, and very few cases have been previously described[1-4]. The unusual entity defined as an NSET typically displays an arrangement of cellular nests composed of spindled or epithelioid cells surrounded by desmoplastic stroma and associated with variable calcifications or ossifications. To our knowledge, few cases of NSETs have been reported[1-4,6]: of these, only one has been described in Asian descendants[6].
This primary liver lesion represents an unexampled clinicopathological entity with an unclear pathogenesis. However, based on immunohistochemical studies showing an intimate correlation of tumoural cell nests with bile ducts and the expression of specific antigens, as well as the shared expression of CD56 by both components, the origin of this tumour in a hepatic mesenchymal precursor cell with primitive differentiation along the bile duct lineage is strongly suspected[3,4]. The expression of WT1 is also in favour of this hypothesis, as this multifunctional protein is a requisite component of the mesenchymal-to-epithelial transformation during certain processes in organogenesis[7].
NSETs predominantly occur in young females and are more frequently located in the right hemiliver. Conversely, in the case described here, the tumour occupied the left hemiliver.
The development of this tumour may occur as an isolated condition or in association with a hormone-related syndrome, such as Cushing syndrome. Heerema-McKenney et al[3] reported two cases of NSETs occurring in paediatric patients that were associated with Cushing syndrome due to an elevated adrenocorticotropic hormone (ACTH) level[4]. Additionally, Rod et al[8] described a 17-year-old female patient affected by a large hepatic NSET causing mild Cushingoid syndrome secondary to moderate-to-high ACTH secretion. In our experience, Cushing-like symptoms were not evident.
From a clinical standpoint, one of the main differential diagnoses is a mixed epithelial and mesenchymal hepatoblastoma, although very few cases have been reported in adults[9]. However, this tumour shows components of foetal and/or embryonal hepatocyte differentiation and lacks the typical stromal architecture of NSETs. Synovial sarcomas and desmoplastic small round cell tumours (DSRCTs) are other diagnostic possibilities to consider that can be distinguished based on specific histologic features. Indeed, in the current study, the absence of a demonstrable carcinoma component and the SYT-SSX fusion transcript helped to exclude the diagnosis of synovial sarcoma. However, cases of synovial sarcoma with extensive calcification and osteoid formation have been reported[10,11]. In our case, the histologic features of the NSET were slightly reminiscent of those of DSRCTs[12,13], as both tumours exhibited nests of WT1-positive cells and were positive for the EWS-WT1 fusion transcript. The NSET, however, can be distinguished from DSRCTs by the NSET’s typical arrangement of myofibroblastic collars and fibrovascular supporting stroma, which differed from the fibrous stroma of DSRCTs. However, the NSET was not immunoreactive for desmin, whereas the opposite is observed in DSRCTs. In the case reported here, based on preoperative imaging features, a diagnosis of fibrolamellar HCC or eventually mixed hepatocholangiocarcinoma was considered.
Interestingly, the patient had taken oral contraceptive pills (OCPs) during the previous 5 years. As reported for hepatic adenoma[14], a possible role for OCPs in the occurrence of the NSET could be considered. However, no staining for hepatocyte antigens was demonstrated in any tumour cells, which led us to exclude a likely correlation with hepatic adenoma. Furthermore, a lack of progesterone and oestrogen receptors in tumoural cells contributed to doubt about the hypothetical correlation between NSET occurrence and OCP consumption. However, more studies on a larger number of cases are likely needed before a possible correlation can be determined.
NSET prognosis remains an unclear matter, but based on current information, this tumour seems to have low proliferation activity and an indolent course, behaving as a low-grade malignancy featuring unusual extrahepatic spread and a possible presentation since childhood. However, specific tumoural features, a large size and vascular invasion seem to be associated with a higher probability of recurrence. Brodsky et al[15] reported experiences of recurrent NSETs of the liver with lymph node metastasis after partial hepatectomy. Hommann et al[16] reported a case of lung metastasis after liver transplantation for an unresectable NSET. Based on these experiences, although the risk of relapse seems negligible, a careful postoperative follow-up is recommended.
To date, the benefit of systemic chemotherapy and the most appropriate regimen to adopt remain poorly defined. In paediatric experience, preliminary results showing a minimal response for an unresectable NSET when a hepatoblastoma and sarcoma protocol regimen was adopted have been reported[3]. However, this topic remains completely unexplored.
Surgery seems to be the pivotal therapeutic approach, remaining the best strategy to guarantee longer survival and a better prognosis[1,3]. Our experience attempts to convey more information about the reliability of the surgical approach in the case of a resectable NSET. In this sense, our clinical experience confirms that liver resection allows the safe attainment of complete tumour clearance, even in advanced disease. Conversely, considering the low tendency of NSETs to relapse and previous unsuccessful experiences, at least for oncological control, liver transplantation generally should not be recommended, at least as a first choice[16]. Liver transplantation would be potentially useful for those patients with unresectable but not extrahepatic disease.
In conclusion, this report aimed to clarify the clinical history, therapy, imaging pattern and histopathological features of a very rare primary liver tumour that is still poorly characterised. Awareness of hepatic NSET occurrence may help to identify additional cases, enlarging knowledge about NSETs’ clinical behaviour and prognostic features and limiting the possibility that these tumours could be misdiagnosed and confused with other aggressive liver malignancies.
Symptoms were featured by dull abdominal pain associated with abdominal distension and dyspepsia.
Palpable mass in the upper abdomen. Cushingoid clinical features can be sometimes detected.
Mixed epithelial and mesenchymal hepatoblastoma, synovial sarcoma and desmoplastic small round cell tumour are the main differential diagnosis. Histologic and immunohistochemical analysis help to distinguish them.
Laboratory tests revealed altered serum level of AST, ALT, alkaline phosphatase and gamma glutamyltransferase. Virological markers for hepatitis B and C and tumour markers including carcinoembryonic antigen, alpha-fetoprotein were negative, while, the CA19-9 was elevated.
Preoperative imaging (Computed tomography, Magnetic resonance imaging scan) revealed a well-circumscribed liver lesion involving the left hemiliver. The lesion appeared solid and heterogeneous with a rim-like enhancement at contrast phase with multiple intra-lesional calcifications. An extensive vascular invasion was evident.
Nested stromal-epithelial tumours (NSETs) are a non-hepatocytic and non-biliary tumor of the liver consisting in nests of epithelial and spindled cells with associated myofibroblastic stroma and variable intralesional calcification and ossification.
Surgery seems the pivotal therapeutic approach remaining the best strategy to guarantee longer survival and a better prognosis.
Heerema-McKenney et al, Rod et al reported cases of NSETs occurring in pedriatic patients and associated with a Cushing syndrome. Brodsky et al reported experiences of recurrent NSET of the liver with lymph-node metastasis after partial hepatectomy. Hommann et al reported a case of lung metastasis after liver transplantation for unresectable NSET.
WT1 is a mutifunctional zinc-finger protein involved in mesenchyme-to-epithelium transformation which suggests an origin of NSET in a hepatic mesenchymal precursor cell with primitive differentiation along the bile duct lineage. EWS-WT1 and SYT-SSX fusion transcript are genes can help to distinguish NSETs from other liver malignancy.
NSETs occur predominantly in young female and their development may occur as an isolated condition, otherwise associated with hormone-related syndrome, such as Cushing syndrome. From a clinical standpoint, histologic and immunohistochemical studies are essential to distinguish NSETs from other malignancy because of lack of a typical imaging pattern.
In the present study, the authors report a case with a nested stromal-epithelial tumor in the liver, which has been rarely reported worldwide. They fully examined the pathological features of the tumor, by using immunohistochemical analysis. This report has originality, figures are clear, and the discussion is well written.
P- Reviewers: Homayounfar K, Ji G, Ivanovski PI, Matsuda Y, Nanashima A S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Grazi GL, Vetrone G, d’Errico A, Caprara G, Ercolani G, Cescon M, Ravaioli M, Del Gaudio M, Vivarelli M, Zanello M. Nested stromal-epithelial tumor (NSET) of the liver: a case report of an extremely rare tumor. Pathol Res Pract. 2010;206:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Heywood G, Burgart LJ, Nagorney DM. Ossifying malignant mixed epithelial and stromal tumor of the liver: a case report of a previously undescribed tumor. Cancer. 2002;94:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Heerema-McKenney A, Leuschner I, Smith N, Sennesh J, Finegold MJ. Nested stromal epithelial tumor of the liver: six cases of a distinctive pediatric neoplasm with frequent calcifications and association with cushing syndrome. Am J Surg Pathol. 2005;29:10-20. [PubMed] |
| 4. | Makhlouf HR, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal-epithelial tumors of the liver: a clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol. 2009;33:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Terminology Committee of the International Hepato-Pancreato-Biliary Association. Terminology of liver anatomy and resections. HPB Surg. 2000;2:333-339. |
| 6. | Wang Y, Zhou J, Huang WB, Rao Q, Ma HH, Zhou XJ. Calcifying nested stroma-epithelial tumor of the liver: a case report and review of literature. Int J Surg Pathol. 2011;19:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Sainio K, Hellstedt P, Kreidberg JA, Saxén L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development. 1997;124:1293-1299. [PubMed] |
| 8. | Rod A, Voicu M, Chiche L, Bazille C, Mittre H, Louiset E, Reznik Y. Cushing’s syndrome associated with a nested stromal epithelial tumor of the liver: hormonal, immunohistochemical, and molecular studies. Eur J Endocrinol. 2009;161:805-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Remes-Troche JM, Montaño-Loza A, Meza-Junco J, García-Leiva J, Torre-Delgadillo A. Hepatoblastoma in adult age. A case report and literature review. Ann Hepatol. 2006;5:179-181. [PubMed] |
| 10. | Holla P, Hafez GR, Slukvin I, Kalayoglu M. Synovial sarcoma, a primary liver tumor--a case report. Pathol Res Pract. 2006;202:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Srivastava A, Nielsen PG, Dal Cin P, Rosenberg AE. Monophasic synovial sarcoma of the liver. Arch Pathol Lab Med. 2005;129:1047-1049. [PubMed] |
| 12. | Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 388] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Ordóñez NG. Desmoplastic small round cell tumor: I: a histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol. 1998;22:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Rosenberg L. The risk of liver neoplasia in relation to combined oral contraceptive use. Contraception. 1991;43:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Brodsky SV, Sandoval C, Sharma N, Yusuf Y, Facciuto ME, Humphrey M, Yeh YA, Braun A, Melamed M, Finegold MJ. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: case report and review of literature. Pediatr Dev Pathol. 2008;11:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hommann M, Kaemmerer D, Daffner W, Prasad V, Baum RP, Petrovitch A, Sauerbrey A, Katenkamp K, Kaufmann R, Settmacher U. Nested stromal epithelial tumor of the liver--liver transplantation and follow-up. J Gastrointest Cancer. 2011;42:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |