Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.901
Revised: October 14, 2014
Accepted: November 7, 2014
Published online: December 27, 2014
Processing time: 106 Days and 13 Hours
Vitamin D is an important secosteroid hormone with known effect on calcium homeostasis, but recently there is increasing recognition that vitamin D also is involved in cell proliferation and differentiation, has immunomodulatory and anti-inflammatory properties. Vitamin D deficiency has been frequently reported in many causes of chronic liver disease and has been associated with the development and evolution of non-alcoholic fatty liver disease (NAFLD) and chronic hepatitis C (CHC) virus infection. The role of vitamin D in the pathogenesis of NAFLD and CHC is not completely known, but it seems that the involvement of vitamin D in the activation and regulation of both innate and adaptive immune systems and its antiproliferative effect may explain its importance in these liver diseases. Published studies provide evidence for routine screening for hypovitaminosis D in patients with liver disease. Further prospectives studies demonstrating the impact of vitamin D replacement in NAFLD and CHC are required.
Core tip: (Vitamin D and liver disease) vitamin D deficiency has been frequently reported in many causes of chronic liver disease and has been associated with the development and evolution of non-alcoholic fatty liver disease (NAFLD) and chronic hepatitis C (CHC) virus infection. The role of vitamin D in the pathogenesis of NAFLD and CHC is not completely known, but it seems that the involvement of vitamin D in the activation and regulation of both innate and adaptive immune systems and its antiproliferative effect may explain its importance in these liver diseases.
- Citation: Iruzubieta P, Terán &, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol 2014; 6(12): 901-915
- URL: https://www.wjgnet.com/1948-5182/full/v6/i12/901.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i12.901
Vitamin D insufficiency and deficiency are prevalent in almost half the healthy population of developed countries[1]. Most experts define vitamin D insufficiency as a 25(OH)D level below 75 nmol/L (30 ng/mL) and deficiency as levels below 50 nmol/L (20 ng/mL). It is estimated that one billion people suffer from deficiency or insufficiency of vitamin D[2]. In the United States, between 25% and 50% of the adult population has vitamin D deficiency[3]. In patients with chronic liver diseases, the prevalence of vitamin D deficits is much higher and practically universal[4]. Up to 93% of patients with chronic liver disease have insufficient vitamin D levels, and almost one-third of these show severe deficiency[5].
The outcome of vitamin D deficiency in terms of osteoporosis, osteomalacia and increased fracture risk is well known[6,7]. Furthermore, the association between vitamin D deficiency and the development of infections, cardiovascular, autoimmune and degenerative diseases and several types of cancer (colon, prostate and breast cancer) has also been reported[8]. Vitamin D is important in calcium homeostasis and has also been implicated in the mechanisms of cellular proliferation, differentiation and immunomodulation[9]. These effects are noted in the pathogenesis and treatment of many chronic liver diseases. In this review, we will focus on vitamin D functions involved in the development of chronic liver disease and on the relationship between vitamin D deficiency and the two main causes of chronic liver disease: chronic hepatitis C (CHC) virus infection and non-alcoholic fatty liver disease (NAFLD).
An evidence-based approach was used for this review. MEDLINE search was performed to September 2014 using the following MeSH terms: liver diseases, vitamin D, cholecalciferol, hepatitis C, Chronic, nonalcoholic fatty liver disease. Searches were limited to English language articles. References of suitable articles were searched for other appropriate articles.
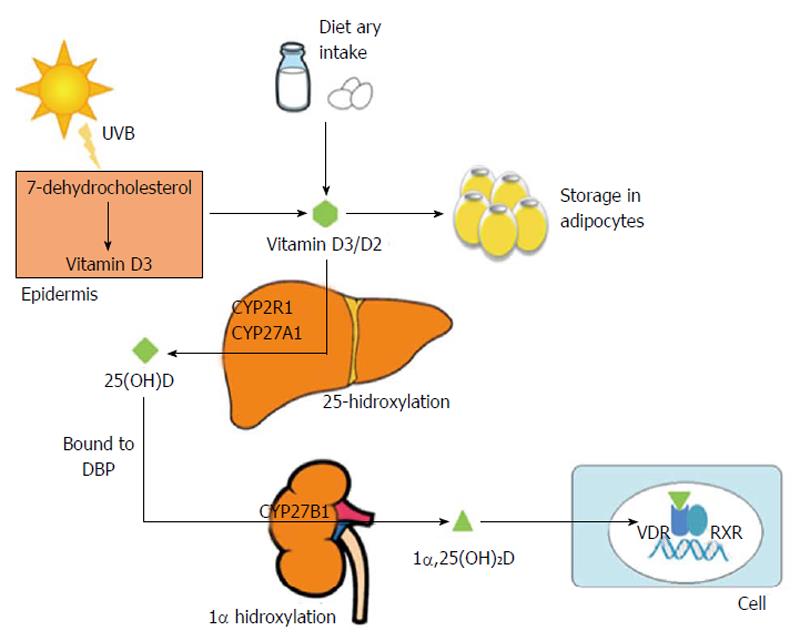
Under normal conditions, biogenesis from epidermal cells is the main source of vitamin D. In the skin, ultraviolet radiation from sun exposure transforms 7-dehydrocholesterol, a metabolite of cholesterol, into pre-vitamin D3, which is transformed into vitamin D3 (cholecalciferol). A small portion of vitamin D comes from dietary sources, such as milk and eggs, in the form of vitamin D2 (ergocalciferol) and D3 that is absorbed in the intestine by biliary acids[1,10]. Vitamin D synthesized from skin and from dietary sources may be stored in the adipocytes, or it may undergo hepatic 25-hydroxylation. This latter process is mediated by isoforms of the P450 cytochrome (CYP2R1, CYP27A1), the 25-hydroxylases, which produce 25-hydroxyvitamin D [25(OH)D] or calcidiol. The metabolite 25(OH)D, most abundant in blood, is an inactive form of vitamin D. It has a half-life of 2-3 wk and is a useful measure of vitamin D levels because it reflects the total amount of vitamin D from dietary sources, sun exposure and conversion from fatty deposits of the liver, and its concentration in plasma is the most reliable indicator of vitamin D status[11]. This vitamin D metabolite, like others, is a low-solubility lipophilic molecule that moves through the bloodstream attached to plasmatic proteins, the most prevalent of which is vitamin D-binding protein (DBP), also known as Gc. Up to 88% of serum 25(OH)D is attached to a DBP, a protein synthesized mainly in the liver that has anti-inflammatory and immunomodulating functions independent of its role as a vitamin D transporter[12,13]. 25(OH)D is hydroxylated in the proximal tubules of the kidney by 1α-hydroxylase (CYP27B1) that form 1α,25(OH)2D or calcitriol, the most biologically active and powerful metabolite of vitamin D[1]. CYP27B1 activity has been observed in the kidney and other tissues, including the liver, fat tissue and the cells of the innate immune system[14]. Finally, 24-hydroxylase, which is most abundant in the intestine and the kidney, catabolizes the calcitriol into an inactive metabolite that is excreted in bile[15] (Figure 1).
1α,25(OH)2D has a half-life of 4 h. It is transported via attachment to plasmatic proteins such as DBP and, as mentioned previously, conducts most of the biological effects of vitamin D by directly and indirectly controlling the expression of over 200 genes linked to angiogenesis, apoptosis, proliferation, differentiation and immunomodulation[1,16,17]. The biological effects of vitamin D are mediated by binding to the vitamin D receptor (VDR), belongs to the superfamily of nuclear steroid hormone receptors, which is expressed in more than 30 tissues, including the liver, the pancreatic islet cells, the epithelial cells of the gastrointestinal tract and the immune system cells[18]. Hence, vitamin D deficiency may be involved in several processes, such as cancer, diabetes mellitus (DM) and cardiovascular and autoimmune diseases[19-26]. Furthermore, the immune system cells, including macrophages, dendritic cells, and T and B lymphocytes, express CYP27A1 or CYP27B1 enzymes and thus can metabolize 25(OH)D to calcitriol. Calcitriol will then have an autocrine or paracrine function[19,20]. Vitamin D favors the innate response of the immune system and has a “self-regulatory” effect by limiting the adaptive response. On one hand, it stimulates the synthesis of antimicrobial peptides (cathelicidin and beta-defensin) and the chemotaxis and phagocytosis of the macrophages. On the other hand, it decreases the expression of class II complex molecules, co-stimulating molecules and the synthesis of Th1, Th2 and Th17 cytokines[19,20]. Finally, in addition to acting as a transcription factor, VDR seems to induce fast non-genomic responses by activating cellular signaling pathways. In this sense, has been shown presence of VDR in plasma membranes of intestinal, lung, kidney, muscle cells and osteoblasts, where it efficiently binds 1α,25(OH)2D[16,27,28].
The synthesis process of vitamin D includes regulatory mechanisms in each step, as follows: (1) in the skin, excess of vitamin D3 is destroyed by sunlight, thus preventing vitamin D3 intoxication from excessive sun exposure[29]; (2) the 25-hydroxylation of vitamin D is under-regulated. The levels of 25(OH)D increase according to the intake of vitamin D; thus, plasmatic levels of 25(OH)D are used to regulate vitamin D status; (3) in contrast, 1α-hydroxylase is highly regulated. Different factors are involved in its activity and expression, including serum calcium and phosphate, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). An elevated calcium serum concentration suppresses 1α-hydroxylase directly and indirectly by decreasing the PTH levels[30]; elevated plasmatic phosphate also decreases the expression and activity of 1α-hydroxylase through a mechanism that is not yet understood. This increase in serum phosphate seems to trigger an increase of FGF23 that inhibits 1α,25(OH)2D synthesis[31]. Furthermore, the synthesis and degradation of 1α,25(OH)2D is also controlled by local factors such as cytokines and growth factors, although this local production has no effect on the blood levels[32,33]. In the case of the macrophages, the expression of CYP27B1 and synthesis of 1α,25(OH)2D are induced by inflammatory cytokines, such as interferon (IFN)γ, and by toll-like receptor (TLRs) ligands, such as the lipopolysaccharide (LPS); (4) the 25-hydroxyvitamin D-24-hydroxylase (CYP24A1) catabolizes 1α,25(OH)2D to calcitroic acid, a biologically inactive bile-excreted metabolite[15]. The activity and expression of this enzyme, which is most abundant in intestine and kidney, is controlled by the levels of 1α,25(OH)2D, phosphate and PTH[34,35]; (5) the DBP protein may buffer the levels of free vitamin D which is correlated with the levels of active vitamin D, this prevents intoxication[36]. Additionally, DBP prevents catabolism and excretion of the hormone. The DBP levels decrease in liver disease, nephrotic syndrome and malnutrition; despite this modification, the concentration of 1α,25(OH)2D remains constant; and (6) 1α,25(OH)2D controls its own synthesis not only through the increase of CYP24A1 expression, as mentioned above, but also by directly or indirectly inhibiting CYP27B1 expression and providing a negative feedback pathway.
Therefore, we can conclude that multiple factors regulate vitamin D metabolism. The intake of vitamin D through diet or sun exposure is only one of many variables that determine its activity, another of these variables are DBP levels, the local synthesis of 1α,25(OH)2D (the autocrine or paracrine effect) and VDR expression.
As discussed previously, vitamin D plays an important role in reducing the risk of chronic diseases, including DM type 2, several types of cancer, and cardiovascular, autoimmune and infectious diseases. This role most likely results from the local production of 1α,25(OH)2D and its autocrine and paracrine actions in cellular proliferation and differentiation, apoptosis, insulin and renin secretion and interleukin (IL) and bactericidal protein production[1,16,17,19-23]. These effects may also be relevant in the pathogenesis of chronic liver diseases.
Vitamin D deficiency is extremely common in chronic liver disease patients. Up to 93% of these patients have some degree of vitamin insufficiency[4,5]. Even patients with mild liver disease are affected, although liver cirrhosis patients more commonly suffer from severe deficiency.
Several studies in general populations have shown that low levels of 25(OH)D significantly increase the risk of mortality from all causes, including cardiovascular diseases[37,38]. Regarding patients with chronic liver disease of varying etiologies, vitamin D deficiency has been associated with increased mortality[39,40], bacterial infections[41], portal hypertension complications[42] and fibrosis severity[43,44]. However, because the liver plays an important role in the metabolism and pleiotropic functions of vitamin D, the question is whether vitamin D deficiency is a consequence of liver disease or a contributor to the liver dysfunction.
Severe liver disease decreases vitamin D hydroxylation and albumin and DBP production, all of which are linked to low levels of 25(OH)D. Nevertheless, the vitamin D deficiency in chronic liver disease is only partly the result of a synthesis dysfunction of the liver, as evidenced by the fact that vitamin D deficiency is highly prevalent in non-cirrhotic patients[4]. The levels of 25(OH)D in cirrhotic patients normalize after vitamin D treatment, which indicates that the 25-hydroxylation is preserved[45,46]; and although DBP is moderately decreased in cirrhosis[47], vitamin D metabolites require only 5% of the DBP binding sites[48], indicating that liver dysfunction must be severe to decrease the DBP levels and contribute to vitamin D deficiency. Therefore, vitamin D deficiency in chronic liver disease requires several causes in addition to those mentioned above, including inadequate sun exposure, insufficient food intake, steroid use, jaundice-related deterioration of vitamin synthesis on the skin and decreased vitamin D absorption caused by intestinal edema secondary to portal hypertension or to cholestasis-induced bile salt disruption.
The observed association between vitamin D and liver disease is insufficient to establish a causal effect between vitamin D deficiency and the severity of chronic liver disease. Recent systematic and umbrella reviews has cast doubt on any causal link between vitamin D deficiency and non-skeletal health outcomes, suggesting that vitamin D deficiency is a marker of ill-health, rather than an important factor implicated in the pathogenesis of disease[49]. However, there is growing evidence that vitamin D is involved in the decrease of inflammation and fibrosis[43,50,51]. Proinflammatory signals in monocytes and macrophages may regulate the local metabolism of vitamin D, auto-inducing the expression of CYP27B1 and the local production of 1α,25(OH)2D, and thus controlling the excessive inflammatory response[33,52]. Almost 90% of the tissue macrophages are in the liver[53], which suggests that the liver production of active vitamin D is affected during inflammatory diseases of the liver. Furthermore, VDR is expressed in both macrophages and other non-parenchymal cells and biliary epithelial cells[54]. After activation, these cells increases the expression of cathelicidin, an antimicrobial peptide with anti-endotoxin activity[55], and inhibits the synthesis of biliary acids, thus protecting the hepatocytes from these acids[56,57]. Therefore, the relationship between vitamin D and hepatic physiopathology may result from signaling disruptions in non-parenchymal liver cells or extrahepatic cells[58].
It is important to mention that, together with diet intake and sun exposure, genetic factors substantially contribute to variations in 25(OH)D levels[59,60]. Several simple nucleotide polymorphisms of genes involved in the metabolism of VDR and vitamin D, such as DHCR7 (encode the 7-dehydrocholesterol reductase enzyme), CYP2R1, CYP24A1 and GC (encode DBP), have been strongly linked with the serum levels of 25(OH)D and its efficacy[59-62]. A recent study community-dwelling black Americans, as compared with whites, had low levels of total 25(OH)D and DBP, resulting in similar concentrations of estimated bioavailable 25(OH)D. Racial differences in the prevalence of common genetic polymorphisms provide a likely explanation for this observation[63]. Therefore, such genetic variations may be associated with the severity of chronic liver disease, and several polymorphisms of the VDR gene associated with primary biliary cirrhosis, autoimmune hepatitis, CHC and hepatocellular carcinoma have been identified[64-69].
The available data suggest that vitamin D supplements could be beneficial in terms of morbimortality[70,71]. Most experts consider of at least 75 nmol/L (30 ng/mL) as the most advantageous 25(OH)D level for reducing the risk of fractures, prevention of cancer and the risk of hypertension, and between 90-120 nmol/L (36-48 ng/mL) as the most optimal level[71]. In fact, a recent meta-analysis that included 73 cohort studies (849412 participants) and 22 controlled and randomized studies with over 30716 participants showed that vitamin D3 supplements significantly reduced mortality from any cause among older adults[72]. Few published prospective studies have examined the effects of supplements in chronic liver disease, and the results to date are contradictory, most likely because of issues with study designs, the quantity of vitamin D administered, the pre- or post-treatment measurements used and the presence of genetic polymorphisms that influence the biological activity of vitamin D. Nonetheless, vitamin D supplements are currently recommended to decrease the skeletal effects of vitamin D deficiency. In fact, the latest recommendation suggest that a 25(OH)D level over 20 ng/mL is sufficient to meet the vitamin D requirement[73]. However, the Endocrine Society Clinical Practice Guideline (ESCPG) suggested that vitamin D requirements may be greater for sick patients than for healthy individuals and blood level above 30 ng/mL may have additional health benefits in reducing the risk of various disease conditions[74]. In addition, the ESCPG suggest that 25(OH)D should be measured in chronic liver disease patients to identify those with levels under 20 ng/mL who would benefit from vitamin D supplements to reduce the risk of bone fracture[74]. Similarly, the guidelines of the European Association for the Study of the Liver recommend calcium (1000-1200 mg/d) and vitamin D (400-800 UI/d) supplements for cholestatic liver disease patients, although supplement use is supported by limited clinical data[75]. In fact, despite the frequency of vitamin D deficiency in liver disease patients, their calcium and PTH serum concentration levels are normal, which contradicts the possibility that regulatory mechanism of calcium metabolism is affected[76,77]. Our group has confirmed these results in cirrhotic patients of different etiologies; these patients showed vitamin D deficiencies[78] but had free vitamin D levels similar to those of healthy subjects (unpublished data). Consequently, the unaffected free vitamin D may be involved in the lack of correlation between the levels of 25(OH)D and calcium and PTH and may maintain calcium homeostasis without causing secondary hyperparathyroidism[79]. For this reason, several authors indicate that the levels of total and free 25(OH)D should be measured to identify the vitamin D status in chronic liver disease patients[76]. Nonetheless, these patients have a high prevalence of bone mass loss that can be explained by the previous data of vitamin D deficiency and by other interfering factors, such as the increase in pro-inflammatory cytokines[80-82], hypogonadism[83], elevated bilirubin levels[84] and steroid treatment[85].
Vitamin D maintains the normal skeletal architecture and plays roles in the cardiovascular[86,87] and nervous systems[88,89] and cellular proliferation and differentiation[90,91]. Furthermore, vitamin D may be relevant in the physiopathology of chronic liver diseases because of its effect on the immune system and its anti-fibrotic effect[51,92,93].
Several research lines suggest that vitamin D has beneficial effects in liver diseases by activating and regulating innate and adaptive immunity.Vitamin D increases innate immunity[23], stimulating the mechanisms associated with the elimination of pathogen agents through the secretion of antibacterial proteins, such as cathelicidin and beta-defensin, and favoring chemotaxis and macrophage phagocytosis[19,20,94,95]. An excessive immune response can cause tissue damage; in this sense, vitamin D promotes an adequate innate immune response by regulating the expression of several TLRs and by decreasing the production of proinflammatory cytokines[52]. An inverse relationship between vitamin D levels and the expression of TLR2, TLR4 and TLR9 in monocytes has been observed, as has a decrease in the expression of these innate immunity receptors after the administration of 1α,25(OH)2D[52,96,97]. These three TLRs are primarily related to the inflammation and fibrosis of the liver. A high-fat diet, alcohol consumption and structural changes in the intestinal mucosa resulting from chronic liver diseases (e.g., the loss of epithelial attachment, vascular congestion, defects of the mucosal immune system) alter the permeability of the mucosa, promoting an increase in intestinal bacteria translocation[98-100] and bacterial products, such as LPS, through the bloodstream; there, these bacteria bond to the TLRs, mainly TLR4, that are present such immune cells as hepatocytes, biliary epithelial cells, dendritic cells and hepatic stellate cells, triggering the synthesis of proinflammatory cytokines and fibrogenesis that ultimately result in liver damage[98,101]. However, vitamin D is involved not only in the regulation of TLR expression but also in intestinal permeability; it plays a role in intestine epithelial cell differentiation and in improving cell bonding[102,103], thus decreasing the bacterial products in the liver.
Regarding adaptive immunity, vitamin D seems to control an excessive immune response by decreasing the expression of class II HLA complex molecules and co-stimulator molecules and by modulating the T cell response[19,20,104]. The activation of naïve T cells has been shown to be vitamin D-dependent[105]; furthermore, it inhibits the development of Th1 (IL-2 and interferon-gamma proinflammatory cytokine producers) and Th9 and increases the number of Th2 cells (IL-4, 5 and 10 anti-inflammatory cytokine producers), thus affecting the polarization of T helper cells[106-108]. Additionally, 1α,25(OH)2D prevents the development of Th17 cells by inhibiting IL-6 and IL-23 production from the dendritic cells, and it induces the differentiation and expansion of regulatory T cells that secrete the anti-inflammatory cytokines IL-10 and transforming growth factor beta (TGF-β)[107,109,110]. This ability to modulate the adaptive immune system may explain the association between vitamin D deficiency and the risk of autoimmune diseases and liver damage.
Moreover, in vitro and in vivo studies of mouse models with liver fibrosis have reported that vitamin D has an anti-fibrotic effect due to ability to affect the pathological process of liver fibrosis at several stages, such as: inhibition of injury trigger, suppression of hepatic stellate cells activation and proliferation, reduction in accumulation of extracellular matrix and even degradation of collagen metalloproteinases activation and tissue inhibitor matrix metalloproteinases (TIMPs) inhibition[92,93]. Moreover, Ding et al[111] revealed an intersecting VDR/SMAD genomic circuit that regulates hepatic fibrogenesis and define a role for VDR as an endocrine checkpoint to modulate the wound-healing response in liver and VDR ligands as potential therapy for liver fibrosis[111]. In this regard, a recent study in mice showed that the active metabolite of vitamin D-1α,25(OH)2D may prevent liver fibrosis in the in-vivo model. However, it cannot ameloriate establish cirrhosis in an animal model[112].
Epidemiological studies show that vitamin D deficiency may increase the risk of acquiring viral infections such as influenza, human immunodeficiency virus and respiratory infections[113]. Chronic hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease; it is estimated to affect 130 to 150 million people worldwide, a significant number of whom also develop cirrhosis and hepatic cancer[114]. A high percentage of these patients (46% to 92%) have low vitamin D levels[50,115-117], and more than 25% suffer from severe deficiency[50,115,117]. It has been hypothesized that the high incidence of vitamin D deficiency in these patients may be caused by HCV’s effect on direct or indirect 25-hydroxylation through cytokine induction or oxidative stress[118,119] and that the virus may suppress 25(OH)D levels due to a disruption in lipid metabolism; as shown a recent study where HCV decreases the production of 7-dehydrocholesterol, the endogenous precursor of vitamin D[120].
As discussed previously, vitamin D inhibits fibrosis and modulates the innate and adaptive immune response, increases the production of antimicrobial peptides and inhibits proinflammatory cytokines. The anti-inflammatory action of vitamin D[19,20,50,94,95,104,106-110] can explain the improved therapeutic results of IFN and ribavirin (RBV) after the administration of vitamin D supplements[121-123], as some data indicate that proinflammatory cytokines and chemokines promote the persistence of HCV[124]. In this respect, a low Th1/Th2 ratio is an independent sustained viral response (SVR) factor in the treatment of the HCV genotype 1[125], and 1α,25(OH)2D favors Th2 in this balance, as mentioned previously[108]. Furthermore, several in-vitro studies have considered vitamin D a direct HCV antiviral agent[126-128]. Gal-Tanamy et al[127] showed that vitamin D increases VDR expression and inhibits HCV replication in human hepatocytes by inducing the expression of IFN beta and the IFN-stimulated gene (MxA) with different antiviral properties, thus producing a synergic effect with antiviral treatment[127]. In the same study, vitamin D or calcitriol added to the antiviral treatment had a synergic effect in the inhibition of HCV. In addition, in recent clinical studies have described an association between VDR polymorphisms on the response to IFN/RBV therapy in CHC[129,130].
The relevance of vitamin D in CHC has been reported in numerous studies that associated vitamin D deficiency with a greater degree of necrosis and fibrosis[40,50,68,131,132] and with a lower likelihood of a SVR to IFN-based therapies[50,115,121,123,133-135]. In fact, all of the patients who showed severe vitamin D deficiency had hardly any SVR, while 50% of those with normal levels or almost normal levels had SVR[50,121,123,136]. However other studies failed to find ant relationship between baseline vitamin D level and SVR and fibrosis[116,137-140] (Table 1). In addition, conflicting conclusions have been reached in two recent meta-analysis[130,141]. This may be due to limitations of the studies included: (1) the small number of patient; (2) majority had a cross-sectional studies that are subject to bias due to the possibility of reverse causation; (3) lack of vitamin D level assessment during therapy; and (4) characteristic of vitamin D assessment (seasonality, cut off values, methodology of vitamin D determination, ethnicity). In contrast, vitamin D has been shown to increase the probability of SVR when it is added to the antiviral treatment[121-123,142] (Table 1). Thus, futher clinical investigation on the effect of vitamin D supplementation in treating CHC are needed to confirm this item.
| Ref. | Year | Design | n | HCV genotype | Vitamin D deficiency | Outcome | P |
| Petta et al[50] | 2010 | Cohorts | 197 | 1 | 73% | Vitamin D levels (ng/mL): SVR: 26.6 No SVR: 23.7 | 0.05 |
| Bitetto et al[121] | 2011 | Cohorts | 42 | 1 and no 1 | Not stated | SVR according to the vitamin D levels (ng/mL): ≤ 10 ng/mL: 10% > 10 and ≤ 20 ng/mL: 30% > 20 ng/mL: 50% | < 0.05 |
| Bitetto et al[136] | 2011 | Cohorts | 211 | 1-5 | 46.4% | SVR according to the vitamin D levels (ng/mL): ≤ 10 ng/mL: 50% > 10 and ≤ 20 ng/mL: 60.9% > 20 ng/mL: 69% | 0.038 |
| Lange et al[115] | 2011 | Cohorts | 468 | 1-3 | 66% | SVR (genotype 2/3): Vitamin D deficit (< 10 ng/mL): 50% Without deficiency: 81% | < 0.0001 |
| SVR (genotype 1) Vitamin D deficit: 60% Without deficiency: 54% | 0.45 | ||||||
| Nseir et al[133] | 2011 | Cohorts | 80 | 1 | Not stated | Vitamin D levels (ng/mL): SVR: 42.1 No SVR: 27.3 | < 0.001 |
| Jazwinski et al[134] | 2011 | Cohorts | 82 | 1 | Not stated | Vitamin D levels (ng/mL): SVR: 23.3 No SVR: 19.3 | 0.82 |
| Abu-Mouch et al[123] | 2011 | Randomized prospective | 72 | 1 | 59% (with vitamin D supplementation) 60% (control group) | SVR: With vitamin D: 86% Control group: 42% | < 0.001 |
| Nimer et al[122] | 2012 | Randomized prospective | 50 | 2-3 | 60% (with vitamin D) 50% (control group) | SVR: With vitamin D: 95% Control group: 77% | < 0.001 |
| Lange et al[116] | 2012 | Cohorts | 269 | 1-4 | 74% | No significant association between SVR and 25(OH)D serum levels | 0.13 |
| Kitson et al[137] | 2013 | Cohorts | 274 | 1 | 48% | Vitamin D levels (ng/mL): SVR: 76.6 No SVR: 84.7 | 0.03 |
| Esmat et al[140] | 2014 | Randomized prospective | 101 | 4 | 95% | SVR: With vitamin D: 44% Control group: 68.6% | 0.22 |
| Yokoyama et al[142] | 2014 | Randomized prospective | 84 | 1b | Not stated | SVR: With vitamin D: 64.3% Control group: 50% | 0.19 |
| Grammatikos et al[138] | 2014 | Cohorts | 398 | 1 | Not stated | Vitamin D levels (ng/mL) SVR: 15.8 No SVR: 17.6 | 0.09 |
Furthermore, Bitteto et al[136] provided additional information in their study of the rs12979860 C/T polymorphism of IL28B. In their study, vitamin D levels were complementary to the rs12979860 C/T polymorphism of the IL28B for predicting SVR in CHC patients infected with difficult-to-treat genotypes (1, 4, 5). Another polymorphism, the CYP27B1-1260 polymorphism is also known to decrease the intracellular concentration of calcitriol in mononuclear cells and T lymphocytes[134] and is a known cofactor in immune response disruption in these cells. In fact, the study by Lange and colleagues confirms the lack of SVR in patients infected with the HCV 1, 2 and 3 genotypes who have this polymorphism[115]. This study also hypothesized that genotype 3 patients had low 25(OH)D levels, in contrast with previously published data[50,136]. We should, however, note that the definition of vitamin D deficiency differed among the three studies, a factor that should be considered when interpreting these results.
Vitamin D also favors the HCV response by improving the sensitivity to insulin[143-145]. Insulin resistance (IR) is considered one of the most important factors in predicting HCV patients’ response to IFN and RBV[146], and vitamin D is known to prevent DM type 2[144]. As β-pancreatic cells contain VDR, vitamin D deficiency may alter the balance between the intra- and extracellular calcium and interfere with insulin release[147].
Therefore, in theory, vitamin D deficiency may be linked to a lack of response to anti-viral treatment, while vitamin D supplementation may potentiate SVR.
NAFLD is a pathological clinical entity that includes a broad spectrum of liver conditions from steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis[148] and NAFLD is one of the main causes of chronic liver disease in developed countries, affecting 20% to 30% of the population[149,150]. Some NAFLD patients develop NASH and cirrhosis, while most others do not experience disease progression; however, the reason for these differences in progression are not known. NAFLD is generally related to at least one metabolic syndrome characteristic; in fact, liver conditions are considered part of the syndrome, and although their pathogenesis is not yet known, IR is a key factor in its development[151,152]. Several studies show a negative correlation between vitamin D levels and obesity, glucose intolerance, IR, metabolic syndrome and body mass index (BMI)[24-26,153-155]. Furthermore, vitamin D deficiency stimulates PTH, which has been linked to IR and an increase in the acute-phase reactant[156]. In support of this hypothesis, some studies show that vitamin D administration improves insulin secretion[145,157-160] and that its use decreases IR in patients with end-stage renal disease[161]. Moreover, VDR polymorphisms have been associated with IR and have an effect on insulin secretion and on the fasting glucose concentration[162]. Additionally, previous studies have shown that VDR knock-out mice developed hepatic steatosis[163]. Finally, studies have shown that vitamin D administration in mice activates the fibroblastic intestinal growth factor 15 (FGF15) (human ortholog FGF19). This intestinal hormone prevents IR and high-fat diet-induced obesity by inhibiting CYP7A1, an essential enzyme in the physiopathology of liver dyslipidemia[164]. This evidence suggests that vitamin D is linked to the development of NAFLD via its role in glucose metabolism by accelerating the conversion of proinsulin to insulin, while vitamin D deficiency has been associated with pancreatic β cell dysfunction and a greater prevalence of type 2 DM[153,164-167].
As in the case of CHC, vitamin D levels are lower in patients with NAFLD compared with healthy controls[43,167-174]. In addition, vitamin D deficiency in obese patients has been attributed to the accumulation of the vitamin D in adipose tissue[175-177]. Furthemore, vitamin D levels are inversely correlated with the severity of steatosis, necroinflammation and fibrosis independent of age gender, BMI, Homeostatic Model Assesment of IR score and presence of metabolic syndrome[43,168,178]. In a recent clinical study of adults with NAFLD, Targher et al[43] showed that the vitamin D levels had an effect on the development of hepatic steatosis and in the severity of the histological lesion. In fact, their hypothesis stated that patients with greater inflammation and fibrosis had lower vitamin D levels independent of other components of the metabolic syndrome. This observation was later confirmed in pediatric patients[179,180] (Table 2). Still, an association between vitamin D and NAFLD has been demonstrated that is independent of BMI or IR and metabolic syndrome[43,157,162]. Although causal conclusions are difficult to obtain from these studies, their results suggest that vitamin D deficiency plays a role in the development and progression of fatty liver, especially in terms of its anti-inflammatory potential. In fact, vitamin D reduces the risk for NAFLD in healthy men[181] and attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism[182].
| Ref. | Year | Design | n | NAFLD diagnosis | Vitamin D levels (ng/mL) | P |
| Targher et al[43] | 2007 | Cohorts prospective | 120 | Liver biopsy | Controls (60): 29.8 ± 6 Steatosis (10): 23.72 ± 8 NASH (50): 14.8 ± 9.2 | 0.001 |
| Manco et al[179] | 2010 | Cohorts prospective | 64 | Liver US | Without necroinflamation: 26.1 ± 10 With necroinflamation: 19.9 ± 9.8 Without fibrosis: 27.7 ± 10.3 With fibrosis: 17.1 ± 7.4 | 0.16 < 0.001 |
| Barchetra et al[168] | 2011 | Cohorts prospective | 262 | Liver US | Without NAFLD (100): 20.5 ± 9.7 NAFLD (162): 14.8 ± 9.2 | < 0.001 |
| Jablonski et al[169] | 2013 | Cohorts retrospective | 1214 | Liver US | Controls (607): 34 ± 8 NAFLD (607): 30 ± 7 | < 0.001 |
| Kasapoglu et al[171] | 2013 | Cohorts prospective | 613 | Liver US | Controls (275): 26,4 ± 9.8 NAFLD stage 1 (133): 20 ± 9.2 NAFLD stage 2 (106): 13.3 ± 6.7 NAFLD stage 3 (99): 8.8 ± 7.4 | < 0.05 |
| Black et al[170] | 2014 | Cohorts prospective | 994 | Liver US | Without NAFLD (838): 30.8 ± 9.6 NAFLD (156): 26.8 ± 8.8 | < 0.001 |
| Yildiz et al[174] | 2014 | Cohorts prospective | 101 | Liver US | Without NAFLD (43): 16.4 (IQR 12.4-24.8) NAFLD grade 1 (41): 14.2 (IQR 9.5-21.2) NAFLD grade 2 (17): 11.5 (IQR 7.5-16.7) | 0.005 |
| Dasarathy et al[178] | 2014 | Cohorts prospective | 148 | Liver biopsy | Controls (39): 35.7 ± 6 Steatosis (67): 25 ± 11.3 NASH (81): 18.1 ± 8.4 | < 0.01 |
| Nobili et al[180] | 2014 | Cohorts prospective | 73 | Liver biopsy | NASH (49) was associated with lower VD levels, i.e., -9.0 pg/mL when compared with that in children without NASH (24) | < 0.001 |
| Küçükazman et al[173] | 2014 | Cohorts prospective | 211 | Liver US | Without NAFLD (57): 20 ± 13.6 NAFLD (154): 12.3 ± 8.9 | < 0.001 |
Vitamin D deficiency has been linked to a systemic increase in inflammation markers[183,184], and systemic inflammation may play a central role in the pathogenesis and progression of NAFLD[185,186]. Increases in visceral adiposity promote the release of fatty acids and proinflammatory cytokines and activate inflammation pathways in the liver, prompting proinflammatory cytokine secretion that leads to liver damage[187]. Moreover, the obesity promotes the onset of NAFLD due to increased hepatic lipid synthesis secondary to excess free fatty acids; subsequent association with oxidative stress on mitochondrial and with the increase of proinflammatory cytokines can definitely trigger a progression of steatosis to NASH and cirrhosis[188]. Studies in vivo and in vitro have clearly documented that steatosis reduces oxidative activity controlled by cytochrome P450[189]. These inflammatory processes may be blocked by increasing the levels of 25(OH)D, and the development and progression of NAFLD may stop. In fact, vitamin D supplements have been shown to decrease inflammation markers[190-193] and increase anti-inflammatory cytokines[190]. It is known that vitamin D’s effects in the liver are not only exerted on the hepatocytes, given that these cells express very little VDR mRNA. In contrast, sinusoidal cells, Kupffer cells, hepatic stellate cells and immune system cells express VDR mRNA that is functionally active. Therefore, vitamin D deficiency may affect the activity/expression of macrophages, dendritic cells and T and B lymphocytes by favoring oxidative stress and the production of proinflammatory cytokines that lead to subclinical inflammation[18,19]. Furthermore, fibrosis is induced by TGF-β secretion that results from the increased secretion of the matrix metalloproteinase 9 inhibitor (TIMP-1)[194]. In fact, cell cultures show that vitamin D has an anti-inflammatory and an antifibrinolytic effect on hepatic stellate cells. Finally, animal models show that more severe histological lesions of NAFLD are associated with higher levels of mRNA of TLR2, 4 and 9, proinflammatory cytokines and oxidative stress markers in rats with a high-fat diet and deficient in vitamin D[195]. A recent study of experimentally NAFLD-induced rats showed that ultraviolet light exposure decreased hepatic stellate cell activity and TGF-β synthesis and stimulated the production of apolipoprotein E and adiponectin. Together, these findings translate into a beneficial effect on NAFLD, and a decrease in IR, steatosis, apoptosis, inflammation and intrahepatic fibrosis was hypothesized[196]. Thus, given the above-mentioned findings, we can conclude that extrahepatic signaling affects fibrosis and inflammation[187] and that the vitamin D-VDR axis may play a role in the initiation and progression of NAFLD.
Therefore, although the mechanisms of vitamin D’s control over hepatic lipid homeostasis and its link with inflammation are not fully known, recent research lines provide a more comprehensive understanding of its immune modulation capacity and of new therapeutic interventions for NAFLD.
The pleiotropic effects of vitamin D indicate a relationship between its deficiency and numerous chronic diseases, such as DM, cardiovascular, autoimmune and infectious diseases, several types of cancer and chronic liver diseases. In the case of chronic liver diseases, vitamin D seems to modulate the innate and adaptive immune system, which explains the association. Specifically, vitamin D deficiency has been associated with a greater risk of portal hypertension complications, mortality and increased histological severity in NAFLD and CHC, and a lower probability of viral response to HCV treatment with IFN based therapies. In fact, clinical studies suggest that these parameters may improve with vitamin D supplementation; however, prospective, randomized and placebo-controlled studies are required to establish firm conclusions.
P- Reviewer: Frick KK, Marzullo P, Mao S, Shaffer JA S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. [PubMed] |
| 2. | Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4-18. [PubMed] |
| 3. | Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771-777. [PubMed] |
| 4. | Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513-520. [PubMed] |
| 5. | Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 6. | Pérez-López FR. Vitamin D and its implications for musculoskeletal health in women: an update. Maturitas. 2007;58:117-137. [PubMed] |
| 7. | Looker AC, Mussolino ME. Serum 25-hydroxyvitamin D and hip fracture risk in older U.S. white adults. J Bone Miner Res. 2008;23:143-150. [PubMed] |
| 8. | Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290-304. [PubMed] |
| 9. | Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662-687. [PubMed] |
| 10. | DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S-1696S. [PubMed] |
| 11. | Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13-19. [PubMed] |
| 12. | Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539-8543. [PubMed] |
| 13. | Metcalf JP, Thompson AB, Gossman GL, Nelson KJ, Koyama S, Rennard SI, Robbins RA. Gcglobulin functions as a cochemotaxin in the lower respiratory tract. A potential mechanism for lung neutrophil recruitment in cigarette smokers. Am Rev Respir Dis. 1991;143:844-849. [PubMed] |
| 14. | Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1alpha-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103-109. [PubMed] |
| 15. | Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology. 1997;138:2233-2240. [PubMed] |
| 16. | Messa P, Alfieri C, Rastaldi MP. Recent insights into vitamin D and its receptor. J Nephrol. 2011;24 Suppl 18:S30-S37. [PubMed] |
| 17. | Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 18. | Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789-799. [PubMed] |
| 19. | Van Belle TL, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab. 2011;25:617-632. [PubMed] |
| 20. | Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685-698. [PubMed] |
| 21. | Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252-261. [PubMed] |
| 22. | Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol. 2012;109:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261-266. [PubMed] |
| 24. | Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 368] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 25. | Bellan M, Guzzaloni G, Rinaldi M, Merlotti E, Ferrari C, Tagliaferri A, Pirisi M, Aimaretti G, Scacchi M, Marzullo P. Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc Diabetol. 2014;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Vitamin D concentration, obesity, and risk of diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 27. | Boland RL. VDR activation of intracellular signaling pathways in skeletal muscle. Mol Cell Endocrinol. 2011;347:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 28. | Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660-2671. [PubMed] |
| 29. | Holick MF, Garabedian M. Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. Primer on the metabolic bone diseases and disorders of mineral metabolism. Washington, DC: American Society for Bone and Mineral Research 2006; 129-137. |
| 30. | Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. Constitutive expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology. 1999;140:2027-2034. [PubMed] |
| 31. | Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843-9849. [PubMed] |
| 32. | Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37-47. [PubMed] |
| 33. | Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770-1773. [PubMed] |
| 34. | Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1-9. [PubMed] |
| 35. | Wu S, Finch J, Zhong M, Slatopolsky E, Grieff M, Brown AJ. Expression of the renal 25-hydroxyvitamin D-24-hydroxylase gene: regulation by dietary phosphate. Am J Physiol. 1996;271:F203-F208. [PubMed] |
| 36. | Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67:589-596. [PubMed] |
| 37. | Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 38. | Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf). 2011;75:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 39. | Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, Gustot T, Degré D, Vercruysse V, Deltenre P. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 40. | Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, Obermayer-Pietsch B, Stauber RE. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Malham M, Jørgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, Dahlerup JF. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Anty R, Tonohouan M, Ferrari-Panaia P, Piche T, Pariente A, Anstee QM, Gual P, Tran A. Low Levels of 25-Hydroxy Vitamin D are Independently Associated with the Risk of Bacterial Infection in Cirrhotic Patients. Clin Transl Gastroenterol. 2014;5:e56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517-524. [PubMed] |
| 44. | Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, Silecchia G, Leonetti F, Fraioli A, Picardi A. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Skinner RK, Sherlock S, Long RG, Wilis MR. 25-Hydroxylation of vitamin D in primary biliary cirrhosis. Lancet. 1977;1:720-721. [PubMed] |
| 46. | Compston JE. Hepatic osteodystrophy: vitamin D metabolism in patients with liver disease. Gut. 1986;27:1073-1090. [PubMed] |
| 47. | Masuda S, Okano T, Osawa K, Shinjo M, Suematsu T, Kobayashi T. Concentrations of vitamin D-binding protein and vitamin D metabolites in plasma of patients with liver cirrhosis. J Nutr Sci Vitaminol (Tokyo). 1989;35:225-234. [PubMed] |
| 48. | White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab. 2000;11:320-327. [PubMed] |
| 49. | Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 775] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 50. | Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 325] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 51. | Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 565] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 52. | Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zügel U, Steinmeyer A, Pollak A, Roth E. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361-370. [PubMed] |
| 53. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [PubMed] |
| 54. | Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034-1042. [PubMed] |
| 55. | D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, Wendum D, Firrincieli D, Coilly A, Fouassier L, Corpechot C, Poupon R, Housset C. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 56. | Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486-14494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 58. | Khan AA, Chow EC, van Loenen-Weemaes AM, Porte RJ, Pang KS, Groothuis GM. Comparison of effects of VDR versus PXR, FXR and GR ligands on the regulation of CYP3A isozymes in rat and human intestine and liver. Eur J Pharm Sci. 2009;37:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D’Agostino RB, Ordovas JM, O’Donnell CJ, Dawson-Hughes B, Vasan RS. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458-464. [PubMed] |
| 60. | Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16:371-378. [PubMed] |
| 61. | Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1202] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 62. | Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 597] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 63. | Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 64. | Tanaka A, Nezu S, Uegaki S, Kikuchi K, Shibuya A, Miyakawa H, Takahashi S, Bianchi I, Zermiani P, Podda M. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol. 2009;50:1202-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Fan L, Tu X, Zhu Y, Zhou L, Pfeiffer T, Feltens R, Stoecker W, Zhong R. Genetic association of vitamin D receptor polymorphisms with autoimmune hepatitis and primary biliary cirrhosis in the Chinese. J Gastroenterol Hepatol. 2005;20:249-255. [PubMed] |
| 66. | Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35:126-131. [PubMed] |
| 67. | Halmos B, Szalay F, Cserniczky T, Nemesanszky E, Lakatos P, Barlage S, Schmitz G, Romics L, Csaszar A. Association of primary biliary cirrhosis with vitamin D receptor BsmI genotype polymorphism in a Hungarian population. Dig Dis Sci. 2000;45:1091-1095. [PubMed] |
| 68. | Baur K, Mertens JC, Schmitt J, Iwata R, Stieger B, Eloranta JJ, Frei P, Stickel F, Dill MT, Seifert B. Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients. Liver Int. 2012;32:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, Fumolo E, Bignulin S, Cmet S, Minisini R. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16:3016-3024. [PubMed] |
| 70. | Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. [PubMed] |
| 71. | Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2014;810:500-525. [PubMed] |
| 72. | Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 73. | The National Academies Collection: Reports funded by National Institutes of Health; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. The National Academies Collection: Reports funded by National Institutes of Health. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US) 2011; . [PubMed] |
| 74. | Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. [PubMed] |
| 75. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1200] [Article Influence: 75.0] [Reference Citation Analysis (1)] |
| 76. | Corey RL, Whitaker MD, Crowell MD, Keddis MT, Aqel B, Balan V, Byrne T, Carey E, Douglas DD, Harrison ME. Vitamin D deficiency, parathyroid hormone levels, and bone disease among patients with end-stage liver disease and normal serum creatinine awaiting liver transplantation. Clin Transplant. 2014;28:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Chen CC, Wang SS, Jeng FS, Lee SD. Metabolic bone disease of liver cirrhosis: is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol. 1996;11:417-421. [PubMed] |
| 78. | Terán A, Fábrega E, Moraleja I, Iruzubieta P, García-Inzueta MT, Crespo J, Amado JA, Pons-Romero F. Eje calcio-vitamina D-PTH en la cirrosis hepática. Existe un hipoparatiroidismo relativo en el paciente cirrótico? Gatroenterol Hepatol. 2013;36:83-84. |
| 79. | Bikle DD, Halloran BP, Gee E, Ryzen E, Haddad JG. Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Invest. 1986;78:748-752. [PubMed] |
| 80. | Nakchbandi IA, van der Merwe SW. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 81. | Axmann R, Böhm C, Krönke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60:2747-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 82. | Fábrega E, Orive A, García-Suarez C, García-Unzueta M, Antonio Amado J, Pons-Romero F. Osteoprotegerin and RANKL in alcoholic liver cirrhosis. Liver Int. 2005;25:305-310. [PubMed] |
| 83. | Karan MA, Erten N, Tascioglu C, Karan A, Sindel D, Dilsen G. Osteodystrophy in posthepatitic cirrhosis. Yonsei Med J. 2001;42:547-552. [PubMed] |
| 84. | Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581-2586. [PubMed] |
| 85. | Mitra R. Adverse effects of corticosteroids on bone metabolism: a review. PM R. 2011;3:466-471; quiz 471. [PubMed] |
| 86. | Liu L, Chen M, Hankins SR, Nùñez AE, Watson RA, Weinstock PJ, Newschaffer CJ, Eisen HJ. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol. 2012;110:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387-392. [PubMed] |
| 88. | Cai Q, Tapper DN, Gilmour RF, deTalamoni N, Aloia RC, Wasserman RH. Modulation of the excitability of avian peripheral nerves by vitamin D: relation to calbindin-D28k, calcium status and lipid composition. Cell Calcium. 1994;15:401-410. [PubMed] |
| 89. | Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861-7864. [PubMed] |
| 90. | Elias J, Marian B, Edling C, Lachmann B, Noe CR, Rolf SH, Schuster I. Induction of apoptosis by vitamin D metabolites and analogs in a glioma cell line. Recent Results Cancer Res. 2003;164:319-332. [PubMed] |
| 91. | Valrance ME, Welsh J. Breast cancer cell regulation by high-dose Vitamin D compounds in the absence of nuclear vitamin D receptor. J Steroid Biochem Mol Biol. 2004;89-90:221-225. [PubMed] |
| 92. | Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 93. | Neeman R, Abramovitch S, Sharvit E, Elad-Sfadia G, Haklai R, Kloog Y, Reif S. Vitamin D and S-farnesylthiosalicylic acid have a synergistic effect on hepatic stellate cells proliferation. Dig Dis Sci. 2014;59:2462-2469. [PubMed] |
| 94. | Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482-496. [PubMed] |
| 95. | Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 96. | Murillo G, Nagpal V, Tiwari N, Benya RV, Mehta RG. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol. 2010;121:403-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford). 2010;49:1466-1471. [PubMed] |
| 98. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [PubMed] |
| 99. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] |
| 100. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. [PubMed] |
| 101. | Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010;3:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 102. | Holt PR, Arber N, Halmos B, Forde K, Kissileff H, McGlynn KA, Moss SF, Kurihara N, Fan K, Yang K. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113-119. [PubMed] |
| 103. | Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208-G216. [PubMed] |
| 104. | Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 105. | von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 386] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 106. | Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S-1708S. [PubMed] |
| 107. | Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. [PubMed] |
| 108. | Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974-4980. [PubMed] |
| 109. | Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23-33. [PubMed] |
| 110. | Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490-3497. [PubMed] |
| 111. | Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 517] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 112. | Abramovitch S, Sharvit E, Weisman Y, Bentov A, Brazowski E, Cohen G, Volovelsky O, Reif S. Vitamin D inhibits development of liver fibrosis in animal model but cannot ameliorate established cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;Epub ahead of print. [PubMed] |
| 113. | Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 268] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 114. | World Health Organization. Fact sheet Nº164. [updated 2014 April]. Available from: http: //www.who.int/mediacentre/factsheets/fs164/en/. |
| 115. | Lange CM, Bojunga J, Ramos-Lopez E, von Wagner M, Hassler A, Vermehren J, Herrmann E, Badenhoop K, Zeuzem S, Sarrazin C. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 116. | Lange CM, Bibert S, Kutalik Z, Burgisser P, Cerny A, Dufour JF, Geier A, Gerlach TJ, Heim MH, Malinverni R. A genetic validation study reveals a role of vitamin D metabolism in the response to interferon-alfa-based therapy of chronic hepatitis C. PLoS One. 2012;7:e40159. [PubMed] |
| 117. | Choudhary NS, Tomar M, Chawla YK, Bhadada SK, Khandelwal N, Dhiman RK, Duseja A, Bhansali A. Hepatic osteodystrophy is common in patients with noncholestatic liver disease. Dig Dis Sci. 2011;56:3323-3327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Ramos-Lopez E, Kahles H, Weber S, Kukic A, Penna-Martinez M, Badenhoop K, Louwen F. Gestational diabetes mellitus and vitamin D deficiency: genetic contribution of CYP27B1 and CYP2R1 polymorphisms. Diabetes Obes Metab. 2008;10:683-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Bellecave P, Sarasin-Filipowicz M, Donzé O, Kennel A, Gouttenoire J, Meylan E, Terracciano L, Tschopp J, Sarrazin C, Berg T. Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system. Hepatology. 2010;51:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 120. | Clark PJ, Thompson AJ, Vock DM, Kratz LE, Tolun AA, Muir AJ, McHutchison JG, Subramanian M, Millington DM, Kelley RI. Hepatitis C virus selectively perturbs the distal cholesterol synthesis pathway in a genotype-specific manner. Hepatology. 2012;56:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 121. | Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, Bignulin S, Cmet S, Fontanini E, Falleti E. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int. 2011;24:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 122. | Nimer A, Mouch A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J Gastroenterol. 2012;18:800-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 123. | Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184-5190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 124. | Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P, Mondal R, Pithawalla R, He W, Dekhtyar T. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45:1413-1421. [PubMed] |
| 125. | Shirakawa H, Matsumoto A, Joshita S, Komatsu M, Tanaka N, Umemura T, Ichijo T, Yoshizawa K, Kiyosawa K, Tanaka E. Pretreatment prediction of virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients using viral and host factors. Hepatology. 2008;48:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 126. | Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, Kato N. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother. 2007;51:2016-2027. [PubMed] |
| 127. | Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, Zemel R. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology. 2011;54:1570-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 128. | Matsumura T, Kato T, Sugiyama N, Tasaka-Fujita M, Murayama A, Masaki T, Wakita T, Imawari M. 25-Hydroxyvitamin D3 suppresses hepatitis C virus production. Hepatology. 2012;56:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 129. | Falleti E, Cmet S, Fabris C, Fattovich G, Cussigh A, Bitetto D, Ceriani E, Lenisa I, Dissegna D, Ieluzzi D. Genetic polymorphisms of vitamin D pathway predict antiviral treatment outcome in slow responder naïve patients with chronic hepatitis C. PLoS One. 2013;8:e80764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 130. | García-Álvarez M, Pineda-Tenor D, Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, Resino S. Relationship of vitamin D status with advanced liver fibrosis and response to hepatitis C virus therapy: A meta-analysis. Hepatology. 2014;60:1541-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 131. | Terrier B, Carrat F, Geri G, Pol S, Piroth L, Halfon P, Poynard T, Souberbielle JC, Cacoub P. Low 25-OH vitamin D serum levels correlate with severe fibrosis in HIV-HCV co-infected patients with chronic hepatitis. J Hepatol. 2011;55:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 132. | Petta S, Grimaudo S, Marco VD, Scazzone C, Macaluso FS, Cammà C, Cabibi D, Pipitone R, Craxì A. Association of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patients. J Viral Hepat. 2013;20:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Nseir W, Gali M, Mouch SA, Djibre A, Nassar F, Assy N. Baseline serum HDL and vitamin D levels are strongly associated with SVR in chronic hepatitis C naïve genotype 1 patients. J Hepatol. 2011;54 Suppl 1:S450. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 134. | Jazwinski A, Clark PJ, Tillmann HL, Muir AJ. Vitamin D and treatment response in African American patients with HCV genotype 1. Hepatology. 2011;54 Suppl S1:853. |
| 135. | Weintraub SJ, Fleckenstein JF, Marion TN, Madey MA, Mahmoudi TM, Schechtman KB. Vitamin D and the racial difference in the genotype 1 chronic hepatitis C treatment response. Am J Clin Nutr. 2012;96:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 136. | Bitetto D, Fattovich G, Fabris C, Ceriani E, Falleti E, Fornasiere E, Pasino M, Ieluzzi D, Cussigh A, Cmet S. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology. 2011;53:1118-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 137. | Kitson MT, Dore GJ, George J, Button P, McCaughan GW, Crawford DH, Sievert W, Weltman MD, Cheng WS, Roberts SK. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 138. | Grammatikos G, Lange C, Susser S, Schwendy S, Dikopoulos N, Buggisch P, Encke J, Teuber G, Goeser T, Thimme R. Vitamin D levels vary during antiviral treatment but are unable to predict treatment outcome in HCV genotype 1 infected patients. PLoS One. 2014;9:e87974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Corey KE, Zheng H, Mendez-Navarro J, Delgado-Borrego A, Dienstag JL, Chung RT. Serum vitamin D levels are not predictive of the progression of chronic liver disease in hepatitis C patients with advanced fibrosis. PLoS One. 2012;7:e27144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Esmat G, El Raziky M, Elsharkawy A, Sabry D, Hassany M, Ahmed A, Assem N, El Kassas M, Doss W. Impact of Vitamin D Supplementation on Sustained Virological Response in Chronic Hepatitis C Genotype 4 Patients Treated by Pegylated Interferon/Ribavirin. J Interferon Cytokine Res. 2014;Epub ahead of print. [PubMed] |
| 141. | Kitson MT, Sarrazin C, Toniutto P, Eslick GD, Roberts SK. Vitamin D level and sustained virologic response to interferon-based antiviral therapy in chronic hepatitis C: A systematic review and meta-analysis. J Hepatol. 2014;61:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 142. | Yokoyama S, Takahashi S, Kawakami Y, Hayes CN, Kohno H, Kohno H, Tsuji K, Aisaka Y, Kira S, Yamashina K. Effect of vitamin D supplementation on pegylated interferon/ribavirin therapy for chronic hepatitis C genotype 1b: a randomized controlled trial. J Viral Hepat. 2014;21:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 143. | Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hyppönen E, Dunger DB, Ramos-Lopez E, Badenhoop K. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56:2616-2621. [PubMed] |
| 144. | Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 145. | Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003;57:258-261. [PubMed] |
| 146. | Grasso A, Malfatti F, De Leo P, Martines H, Fabris P, Toscanini F, Anselmo M, Menardo G. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 147. | Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14:505-520. [PubMed] |
| 148. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [PubMed] |
| 149. | Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16, vii. [PubMed] |
| 150. | Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 151. | Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 555] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 152. | Kraegen EW, Cooney GJ. Free fatty acids and skeletal muscle insulin resistance. Curr Opin Lipidol. 2008;19:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 153. | Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228-1230. [PubMed] |
| 154. | Stein EM, Strain G, Sinha N, Ortiz D, Pomp A, Dakin G, McMahon DJ, Bockman R, Silverberg SJ. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf). 2009;71:176-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 155. | Liu E, Meigs JB, Pittas AG, McKeown NM, Economos CD, Booth SL, Jacques PF. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr. 2009;139:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 156. | McCarty MF. Secondary hyperparathyroidism promotes the acute phase response -- a rationale for supplemental vitamin D in prevention of vascular events in the elderly. Med Hypotheses. 2005;64:1022-1026. [PubMed] |
| 157. | Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 158. | von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 441] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 159. | Inomata S, Kadowaki S, Yamatani T, Fukase M, Fujita T. Effect of 1 alpha (OH)-vitamin D3 on insulin secretion in diabetes mellitus. Bone Miner. 1986;1:187-192. [PubMed] |
| 160. | Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 161. | Mak RH, Wong JH. The vitamin D/parathyroid hormone axis in the pathogenesis of hypertension and insulin resistance in uremia. Miner Electrolyte Metab. 1992;18:156-159. [PubMed] |
| 162. | Hitman GA, Mannan N, McDermott MF, Aganna E, Ogunkolade BW, Hales CN, Boucher BJ. Vitamin D receptor gene polymorphisms influence insulin secretion in Bangladeshi Asians. Diabetes. 1998;47:688-690. [PubMed] |
| 163. | Zuñiga S, Firrincieli D, Lasnier E, Miquel JF, Housset C, Chignard N. Invalidation of the vitamin D nuclear receptor promotes liver steatosis. Hepatology. 2010;52 Suppl 1:1058A. |
| 164. | Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 165. | Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820-825. [PubMed] |
| 166. | Bourlon PM, Faure-Dussert A, Billaudel B. The de novo synthesis of numerous proteins is decreased during vitamin D3 deficiency and is gradually restored by 1, 25-dihydroxyvitamin D3 repletion in the islets of langerhans of rats. J Endocrinol. 1999;162:101-109. [PubMed] |
| 167. | Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017-2029. [PubMed] |
| 168. | Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 169. | Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, Chonchol M. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 170. | Black LJ, Jacoby P, She Ping-Delfos WC, Mori TA, Beilin LJ, Olynyk JK, Ayonrinde OT, Huang RC, Holt PG, Hart PH. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol. 2014;29:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 171. | Kasapoglu B, Turkay C, Yalcin KS, Carlioglu A, Sozen M, Koktener A. Low vitamin D levels are associated with increased risk for fatty liver disease among non-obese adults. Clin Med. 2013;13:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Seo JA, Eun CR, Cho H, Lee SK, Yoo HJ, Kim SG, Choi KM, Baik SH, Choi DS, Yim HJ. Low vitamin D status is associated with nonalcoholic Fatty liver disease independent of visceral obesity in Korean adults. PLoS One. 2013;8:e75197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 173. | Küçükazman M, Ata N, Dal KA, Yeniova AÖ, Kefeli AE, Basyigit S, Aktas B, Akin KO, A Ladio Lu K, Ure OS. The association of vitamin D deficiency with non-alcoholic fatty liver disease. Clinics (Sao Paulo). 2014;69:542-546. [PubMed] |
| 174. | Yildiz I, Erol OB, Toprak S, Cantez MS, Omer B, Kilic A, Oguz F, Uysalol M, Yekeler E, Unuvar E. Role of vitamin D in children with hepatosteatosis. J Pediatr Gastroenterol Nutr. 2014;59:106-111. [PubMed] |
| 175. | Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690-693. [PubMed] |
| 176. | Yanoff LB, Parikh SJ, Spitalnik A, Denkinger B, Sebring NG, Slaughter P, McHugh T, Remaley AT, Yanovski JA. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf). 2006;64:523-529. [PubMed] |
| 177. | Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 178. | Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, Khiyami A, McCullough AJ, Dasarathy S. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:e118-e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 179. | Manco M, Ciampalini P, Nobili V. Low levels of 25-hydroxyvitamin D(3) in children with biopsy-proven nonalcoholic fatty liver disease. Hepatology. 2010;51:2229; author reply 2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 180. | Nobili V, Giorgio V, Liccardo D, Bedogni G, Morino G, Alisi A, Cianfarani S. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol. 2014;170:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 181. | Rhee EJ, Kim MK, Park SE, Park CY, Baek KH, Lee WY, Kang MI, Park SW, Kim SW, Oh KW. High serum vitamin D levels reduce the risk for nonalcoholic fatty liver disease in healthy men independent of metabolic syndrome. Endocr J. 2013;60:743-752. [PubMed] |
| 182. | Yin Y, Yu Z, Xia M, Luo X, Lu X, Ling W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest. 2012;42:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 183. | Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does vitamin D modulate asymmetric dimethylarginine and C-reactive protein concentrations? Am J Med. 2010;123:335-341. [PubMed] |
| 184. | Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340-1349. [PubMed] [DOI] [Full Text] |
| 185. | Day CP. Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med. 2006;6:19-25. [PubMed] |
| 186. | Targher G. Non-alcoholic fatty liver disease and cardiovascular disease risk. Curr Cardiovasc Risk Rep. 2010;4:32-39. |
| 187. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1815] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 188. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [PubMed] |
| 189. | Donato MT, Lahoz A, Jiménez N, Pérez G, Serralta A, Mir J, Castell JV, Gómez-Lechón MJ. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab Dispos. 2006;34:1556-1562. [PubMed] |
| 190. | Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754-759. [PubMed] |
| 191. | Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008;52:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 192. | Bucharles S, Barberato SH, Stinghen AE, Gruber B, Piekala L, Dambiski AC, Custodio MR, Pecoits-Filho R. Impact of cholecalciferol treatment on biomarkers of inflammation and myocardial structure in hemodialysis patients without hyperparathyroidism. J Ren Nutr. 2012;22:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 193. | Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70-80. [PubMed] |
| 194. | Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787-796. [PubMed] |
| 195. | Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 196. | Nakano T, Cheng YF, Lai CY, Hsu LW, Chang YC, Deng JY, Huang YZ, Honda H, Chen KD, Wang CC. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. J Hepatol. 2011;55:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (1)] |