Published online Dec 27, 2014. doi: 10.4254/wjh.v6.i12.894
Revised: October 9, 2014
Accepted: October 28, 2014
Published online: December 27, 2014
Processing time: 106 Days and 6.7 Hours
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, and is the most common type of chronic liver diseases in the majority of developed countries. NAFLD shows a wide spectrum of disorders including simple steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis. While simple steatosis is recognized to be benign and stable, NASH is considered to be an aggressive form of the disease progressing to cirrhosis. Currently, differentiation between NASH and simple steatosis can be done only by liver biopsy. Despite many proposals and revisions, the histological criteria for the differentiation have not been perfected yet. In this review article, the changes in the histopathologic criteria of NAFLD during the last three decades are summarized, and perspectives of the future changes are demonstrated. The discussion focuses on how pathologists have been dealing with “hepatocellular ballooning”. Loose criteria, in which hepatocellular ballooning was not required for the diagnosis of NASH, were applied in many clinical studies published in around 2000’s, whereas a strict criterion based on the presence/absence of hepatocellular ballooning was approved recently. Hence, simple and reliable methods of identifying ballooned hepatocytes are being sought. Clinical and pathological predictors of NAFLD-related hepatocarcinogenesis will also be sought in the future.
Core tip: The differentiation between nonalcoholic steatohepatitis and simple steatosis can be done only by liver biopsy. Through many proposals and revisions, the histological criteria for the differentiation have been changed. The changes in the criteria during the last three decades are exhibited in this review article, with a special interest in “hepatocellular ballooning”.
- Citation: Ikura Y. Transitions of histopathologic criteria for diagnosis of nonalcoholic fatty liver disease during the last three decades. World J Hepatol 2014; 6(12): 894-900
- URL: https://www.wjgnet.com/1948-5182/full/v6/i12/894.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i12.894
In newly proposed disease entities, or even in already established ones, the definitions and diagnostic criteria may be revised repeatedly. The revisions are led by alterations in recognition of the disease, changes in morbidity and social healthcare strategy in each era, elucidation of the pathologic mechanisms, etc. Hence, these changes probably occur more frequently in a disease of unknown etiology. Nonalcoholic fatty liver disease (NAFLD) and its aggressive form, nonalcoholic steatohepatitis (NASH), are representative examples.
Although NASH/NAFLD have generally been accepted as independent diseases since Ludwig’s monumental publication in 1980[1], minor revisions regarding definition, criteria (mainly histopathologic features and a cutoff level of alcohol consumption) and diagnostic algorithm have continued to be made. A goal of the revisions is establishment of accurate selection criteria to extract NAFLD cases that are most likely to progress to cirrhosis or to hepatocellular carcinoma (HCC). The selected patients become subjects of follow-up and therapeutic interventions[2,3]. NAFLD is considered to be the most common chronic liver disease in the majority of developed countries, and clarification of the high-risk group of NAFLD patients is the most critical issue in current hepatology.
Noninvasive clinical methods, which can evaluate the degree of steatosis and can diagnose NAFLD in some cases, have been developed[4,5]. However, since they cannot evaluate inflammatory activity, the diagnosis of NASH still requires histological examination[4]. It is not possible to perform liver biopsy in every NAFLD patient, and thus, the detailed pathobiological and clinicopathologic characteristics of NASH/NAFLD have not yet been elucidated. Consequently, the histopathologic criteria for the diagnosis of NASH/NAFLD have changed repeatedly. The ambiguous and wandering criteria have confused general pathologists.
What are the reliable histopathologic markers of true NASH? No one can provide an appropriate answer to this substantial query. I review the 30-year history of the revision process that contained many trials and errors (Table 1). This review may not only introduce a clue to the answer, but also provide a direction for future studies on NASH/NAFLD.
| Ref. | Year | Steatosis | Inflammatory cell infiltration | Hepatocellular necrosis | Hepatocellular ballooning | Mallory-Denk body | Pericellular fibrosis |
| Ludwig et al[1] | 1980 | ≥ Moderate | + | + | |||
| Falchuk et al[23] | 1980 | ≥ Moderate | + | + | |||
| Diehl et al[26] | 1988 | ≥ Mild | + | +1 | +1 | ||
| Nagore et al[25] | 1988 | ≥ Mild | + | + | + | + | |
| Lee et al[35] | 1989 | ≥ Mild | + | + | |||
| Powell et al[28] | 1990 | ≥ Moderate | + | ||||
| Wanless et al[29] | 1990 | ≥ 5% | + | ||||
| Bacon et al[36] | 1994 | ≥ Minimal | + | ||||
| Laurin et al[37] | 1996 | ≥ Minimal | + | ||||
| George et al[38] | 1998 | ≥ Minimal | + | ||||
| Younossi et al[32] | 1998 | > 1/3 | + | + | |||
| Matteoni et al[33] | 1999 | > 1/3 | + | + | +2 | ||
| Brunt et al[41] | 1999 | > 0% | + | ||||
| Dixon et al[44] | 2001 | ≥ Mild | + | + | +1 | +1 | |
| Neuschwander-Tetri et al[2] | 2003 | ≥ 5% | + | + | |||
| Bedossa et al[51] | 2012 | > 5% | + | +3 |
The proposal of NASH as a new disease entity by Ludwig et al[1] in 1980 was truly the first milestone in NASH/NAFLD research. Historically, many pathologists prior to Ludwig focused on fatty livers and cirrhosis associated with morbid obesity or diabetes[6-13]. Histologic pictures shown in the earlier reports were of NASH/NAFLD according to the current diagnostic criteria. They had noticed even some morphological features of this type of fatty livers rather different from alcoholic fatty livers, such as low percentages of Mallory-Denk body and siderosis and frequent nuclear glycogen[11-13]. However, due to the facts that most fatty livers did not progress to fibrosis and cirrhosis[13,14], and that livers could physiologically store a certain amount of lipid, there had been for a long time controversy regarding the pathologic significance of lipid accumulation in livers. In other words, fatty change was considered as an innocent bystander, not harmful, and an accompanying phenomenon caused by hepatotoxic pathogens[15].
There was no obvious definition of the physiological level of hepatic fat. Galambos et al[16] studied hepatic histopathologic findings corresponding to abnormal laboratory test results in obese patients. In that study, the authors defined > 33% fatty change as an abnormal/pathologic condition. There was no explanation about how the authors determined 33% as the normal limit. This fact indicates that the value of 33% was acceptable without any explanations as the normal limit at that time.
Undiscovered hepatitis C virus (HCV)[17] might have disturbed to recognize NASH/NAFLD as independent hepatic disorders. Especially HCV genotype 3 is now known to be able to cause prominent hepatic steatosis as well as necroinflammation[18]. Pathologists might have misunderstood NASH as viral hepatitis, and simultaneously, HCV-related hepatitis as primary steatotic liver disease. The potential overlap of NASH/NAFLD and HCV-related hepatitis is still a focus of debate[19,20].
In that era, earlier than Ludwig’s, many reports concerning NASH/NAFLD were published from Japanese institutes[7,21,22]. Although the medical interest had not been directed to metabolic syndrome, Japanese pioneer researchers investigated fatty liver disorders with keen observations and deep insights. Surprisingly, they suggested that fatty change was a first step of NAFLD progression to cirrhosis and dysfunctions of hepatocellular organelles including endoplasmic reticulum were pivotal[7,22]. These are completely identical with the present recognitions of pathological mechanisms of NASH/NAFLD.
The current disease concept and terminology of NASH were established only by a single pathologic report entitled “Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease” written by Ludwig et al[1] in 1980. At present, it is well recognized that the contribution of this breakthrough article to hepatology is too large to be estimated. The report consisted of clinicopathologic reviews of twenty cases of NASH. Their inclusion criteria, namely diagnostic criteria of NASH, were extremely simple and clear: non-habitual drinkers with liver damage that was indistinguishable from alcoholic injury histologically. The authors proposed to categorize all types of liver damages fulfilling the criteria into one disease entity named NASH. A little confusion might have arisen because NASH included fatty liver disorders associated with nutritional disturbances and even drug-induced damage as well as those associated with morbid obesity and diabetes. In addition, the definition of “nonalcoholic” became a big issue; they excluded only obvious alcohol abusers.
In the same year (1980), Falchuk et al[23] published their article entitled “Pericentral Hepatic Fibrosis and Intracellular Hyalin in Diabetes Mellitus”, and suggested that an inflammatory hepatic disorder associated with diabetes mellitus was an intermediate illness between fatty liver and cirrhosis. The contents of the papers by Ludwig et al[1] and Falchuk et al[23] complemented each other, and emphasized the independence and importance of NASH among chronic liver diseases. However, the etiopathology of NASH was not elucidated, and the concept of NASH did not gain complete acceptance for about 20 years. As proof, some articles very similar to the earlier studies than 1980 were published, and different terms such as diabetic hepatitis and fatty liver hepatitis were used instead of NASH[24,25]. A study seeming to have repeated the Ludwig’s original work also appeared. It was not surprising that the results of those studies validated the presence of the new disease, NASH, and its progressive nature[25,26].
A growing interest produced one substantial question: what kind of fatty liver disorders is truly progressive? Two scientific streams, which still influence today’s research trends, sprang from the query. The diagnostic criteria in the streams have been gradually modified.
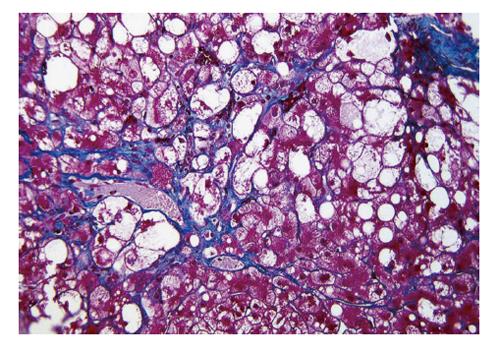
One of the scientific streams was to highlight specific findings of NASH/NAFLD. Clain et al[27] systematically reviewed previous papers on NAFLD, and concluded that the presence of perivenular/pericellular fibrosis (Figure 1) potentially indicated a progressive disease[9]. Powell et al[28] confirmed that NASH was actually a slowly progressing disease, and tried to classify NASH on the basis of steatosis, inflammation, Mallory-Denk bodies and fibrosis. However, they could not find a relationship with prognosis. Wanless et al[29] accentuated the importance of hepatocellular ballooning (Figure 1), and made a diagnosis of NASH according to the presence of steatosis and ballooning. In that article, histologically abnormal lipid accumulation was defined as fatty change that affected more than 5% of hepatocytes. There was no obvious evidence for the definition of “more than 5%”. A previous biochemical analysis revealed that normal livers (livers of healthy persons) could store lipids comprising less than 5% of liver weight[30]. Accordingly, “more than 5%” has been used as a standard value for defining pathologic hepatic lipid accumulation until now. Teli et al[31] defined NASH as hepatic steatosis with lobular inflammation, hepatocellular ballooning, Mallory-Denk bodies, and hepatocellular necrosis. They suggested that non-NASH NAFLD (namely, simple steatosis, and steatosis with portal inflammation) did not progress to NASH and cirrhosis. This stream led to the NAFLD classification of Younossi et al[32] and to Matteoni’s classification[33]. Hepatocellular ballooning was a key finding for their classification. Later they insisted on the necessity of hepatocellular ballooning in diagnosis of NASH (Table 1)[34]. In their original classification, however, they used a term “steatohepatitis” for type 2 NAFLD, which was steatosis with lobular inflammation but without ballooning.
The other scientific stream was to establish a score system based on semi-quantitative analyses of the histological severity of liver damage. The trial was initiated by Diehl et al[26], followed by Lee et al[35] They evaluated the degree of steatosis, inflammatory cell infiltration, hepatocellular damage, Mallory-Denk bodies, and fibrosis using four- or five-step scales. Unfortunately, they failed to find a relationship between the scores and prognoses. Bacon et al[36] also performed a semi-quantitative analysis using a system similar to Diehl’s system, but did not described its relationship with prognosis. Whilst the cutoff alcohol consumption ensuring “nonalcoholic” was strict (less than 20 g ethanol/d; it is almost same as the current standard, < 30 g for men and < 20 g for women[3]), they used a very loose histological criterion for diagnosing NASH. The minimal diagnostic requirements were only steatosis and inflammatory cell infiltration into lobular areas. Various clinical studies of NASH using loose diagnostic criteria similar to theirs were published thereafter[37,38]. Finally, even simple steatosis was recognized to be one aspect in the spectrum NASH and to be an ongoing change potentially progressing to more severe NASH or cirrhosis[39]. The well-known “two-hit theory” presented in the same year was generated through analogous hypothetical thinking[40]. Brunt’s system[41] was established and published in such research background. They evaluated the severity of NASH using a histological score composed of inflammation grade and fibrosis stage similar to the METAVIR score[42]. The NASH severity score was used in many clinical studies, and contributed to the subsequent flowering of research on NASH. Unexpectedly, however, fairly mild fatty liver disorders were implicated in NASH cohorts by being diagnosed as grade 1 NASH. The original purpose of Brunt’s system was to show a standard for evaluation of the severity of NASH. They did not seem to intend primarily to present a diagnostic criterion of NASH[43]. The expanded understanding (or misunderstanding) of NASH had rapidly spread, apart from the inventors’ idea.
About ten years after the flowering of NASH/NAFLD research in Western countries, Japanese hepatologists also began to study NASH/NAFLD aggressively, due to the wide prevalence of metabolic syndrome in the beginning of the 21st century. Many NASH/NAFLD researchers in Japan understood that the minimum requirements for diagnosis of NASH were Brunt’s grade 1 (without hepatocellular ballooning) and Matteoni’s type 2 NAFLD. Their recognitions had been conserved even after the decision of the AASLD Single Topic Conference in 2002[2], in which hepatocellular ballooning was officially recommended as a factor in the diagnosis of NASH. They did not notice the controversy about NASH diagnostic criteria[44] and the kaleidoscope changes of the criteria in that period.
In 2005, the NAFLD activity score (NAS), which was considered as a type of modified Brunt’s system, was developed and published by Kleiner et al[45] This also led to a simplistic recognition that a NAS of over 5 points is NASH[46], and led to further confusion in the laboratory and clinical settings.
On the other hand, Younossi et al[47] confirmed that hepatocellular ballooning was a predictor of liver-related death in their follow-up study, and insisted that Matteoni’s classification was superior to Brunt’s system or NAS. Brunt et al[48,49] also cautioned about the presence of cases of non-NASH NAFLD showing NAS ≥ 5 and cases of NASH showing NAS ≤ 4, to avoid misusage of NAS. Through their discussions and controversies[49], the diagnostic criteria of NASH/NAFLD were revised and standardized hastily. Hepatocellular ballooning finally became the most important finding in the diagnosis of NASH.
However, the difficulty in correctly identifying hepatocellular ballooning subsequently became a critical issue[50]. To overcome this problem, Bedossa et al[51] proposed a semi-quantitative method in which all hepatocytes with clear reticular cytoplasm were defined as ballooning and graded by the cellular size. The same research group has recently published a new diagnostic algorithm in which hepatocellular ballooning is a root node of the binary tree[52].
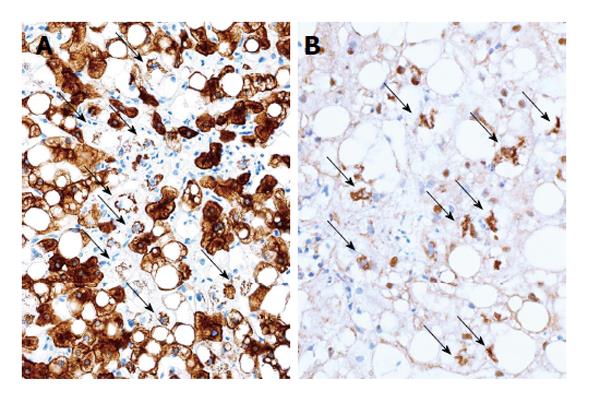
Examination of specific markers for ballooning has been recommended as a method to determine it objectively. Hepatocellular ballooning is a result of degeneration and fragmentation of cytoskeleton intermediate filaments, cytokeratin (CK) 8/18, and an aggregates of the degenerated CK 8/18 is a Mallory-Denk body[53]. Immunohistochemical staining for CK 18, ubiquitin and p62 can be applied to detect hepatocellular ballooning. A negative result for the presence of CK 18 in hepatocytes can be interpreted as degenerative disappearance of CK 18, and the presence of ubiquitin-, p62-, and CK 18-positive intracellular inclusions indicates aggregation of degenerated CK 18 (Figure 2). The usefulness of ubiquitin immunohistochemistry in the diagnosis of NASH was first suggested in 2000[44,54]. Recently, the significance of these special stainings has been reconfirmed[55,56].
Alternatively, controversy about such excessive weighting to hepatocellular ballooning in the diagnosis may arise. Perivenular/pericellular fibrosis (Figure 1) should be highlighted more because of its close association to hepatocellular ballooning and its potential linkage to cirrhosis[9]. The diagnostic criteria of NASH/NAFLD still remain to be improved.
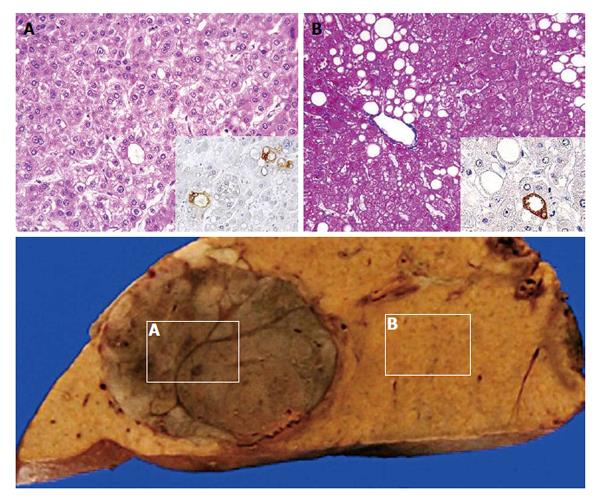
Although the histological criteria of NASH/NAFLD have been revised during the last three decades, there has been an absolute premise: cirrhosis is a final advanced form of NAFLD. However, recent reports of HCC associated with NAFLD may deny the central dogma of the NASH/NAFLD concept. Surprisingly, considerable numbers of such HCCs arose from non-cirrhotic steatotic livers or even from livers with simple steatosis (Figure 3)[57-59]. Accumulation of cellular damage without major morphological changes and acceleration of cellular senescence may lead to hepatocarcinogenesis[59,60]. The facts will possibly lead to a paradigm shift in medical strategies for NAFLD.
How do we select which NAFLD patients to follow up? What is a true prognostic factor of NAFLD? Is it a histological finding? These are the ultimate themes for NAFLD researchers.
While reviewing the 30-year history of changes in the histological criteria for the diagnosis of NASH/NAFLD, the importance of hepatocellular ballooning in the diagnosis of NASH, imperfectness of the present criteria, and necessity of exploring new predictors of hepatocarcinogenesis were elucidated. Further collection of evidence is necessary to solve these problems, and pathologists will play central roles in this process.
The author thanks Mr. Shogo Ikura, University of Tokyo, for helping preparation of the manuscript.
P- Reviewer: Carvalho-Filho RJ, Grassi A S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 2. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 582] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 4. | Grandison GA, Angulo P. Can NASH be diagnosed, graded, and staged noninvasively? Clin Liver Dis. 2012;16:567-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Zelman S. The liver in obesity. AMA Arch Intern Med. 1952;90:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Hano T. Pathohistological study on liver cirrhosis in diabetes mellitus. Kobe J Med Sci. 1968;14:87-106. [PubMed] |
| 8. | Kern WH, Heger AH, Payne JH, DeWind LT. Fatty metamorphosis of the liver in morbid obesity. Arch Pathol. 1973;96:342-346. [PubMed] |
| 9. | Marubbio AT, Buchwald H, Schwartz MZ, Varco R. Hepatic lesions of central pericellular fibrosis in morbid obesity, and after jejunoileal bypass. Am J Clin Pathol. 1976;66:684-691. [PubMed] |
| 10. | Adler M, Schaffner F. Fatty liver hepatitis and cirrhosis in obese patients. Am J Med. 1979;67:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 237] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Robbers H, Strohfeldt P, Krüger C. Differential diagnosis between diabetic and alcoholic fatty liver. A study of 171 diabetics and 100 chronic alcoholics. Ger Med Mon. 1968;13:124-125. [PubMed] |
| 12. | Christoffersen P, Petersen P. Morphological features in non-cirrhotic livers from patients with chronic alcoholism, diabetes mellitus or adipositas. A comparative study. Acta Pathol Microbiol Scand A. 1978;86A:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Massarrat S, Jordan G, Sahrhage G, Korb G, Bode JC, Dölle W. Five-year follow-up study of patients with nonalcoholic and nondiabetic fatty liver. Acta Hepatogastroenterol (Stuttg). 1974;21:176-186. [PubMed] |
| 14. | Hilden M, Juhl E, Thomsen AC, Christoffersen P. Fatty liver persisting for up to 33 years. A follow-up of the inversen-roholm liver biopsy material. Acta Med Scand. 1973;194:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Thaler H. Relation of steatosis to cirrhosis. Clin Gastroenterol. 1975;4:273-280. [PubMed] |
| 16. | Galambos JT, Wills CE. Relationship between 505 paired liver tests and biopsies in 242 obese patients. Gastroenterology. 1978;74:1191-1195. [PubMed] |
| 17. | Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2495] [Cited by in RCA: 2343] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 18. | Mihm S, Fayyazi A, Hartmann H, Ramadori G. Analysis of histopathological manifestations of chronic hepatitis C virus infection with respect to virus genotype. Hepatology. 1997;25:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 173] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Patel A, Harrison SA. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol Hepatol (N Y). 2012;8:305-312. [PubMed] |
| 20. | Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E, Nascimbeni F, Loria P. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World J Gastroenterol. 2014;20:7089-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Itoh S, Igarashi M, Tsukada Y, Ichinoe A. Nonalcoholic fatty liver with alcoholic hyalin after long-term glucocorticoid therapy. Acta Hepatogastroenterol (Stuttg). 1977;24:415-418. [PubMed] |
| 22. | Itoh S, Tsukada Y, Motomura Y, Ichinoe A. Five patients with nonalcoholic diabetic cirrhosis. Acta Hepatogastroenterol (Stuttg). 1979;26:90-97. [PubMed] |
| 23. | Falchuk KR, Fiske SC, Haggitt RC, Federman M, Trey C. Pericentral hepatic fibrosis and intracellular hyalin in diabetes mellitus. Gastroenterology. 1980;78:535-541. [PubMed] |
| 24. | Nasrallah SM, Wills CE, Galambos JT. Hepatic morphology in obesity. Dig Dis Sci. 1981;26:325-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Nagore N, Scheuer PJ. The pathology of diabetic hepatitis. J Pathol. 1988;156:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056-1062. [PubMed] |
| 27. | Clain DJ, Lefkowitch JH. Fatty liver disease in morbid obesity. Gastroenterol Clin North Am. 1987;16:239-252. [PubMed] |
| 28. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 991] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 29. | Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 763] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 30. | Kwiterovich PO, Sloan HR, Fredrickson DS. Glycolipids and other lipid constituents of normal human liver. J Lipid Res. 1970;11:322-330. [PubMed] |
| 31. | Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 564] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 32. | Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, Rybicki L, McCullough AJ. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11:560-565. [PubMed] |
| 33. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2345] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 34. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 35. | Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [PubMed] |
| 37. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 361] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 39. | James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 266] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3130] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 41. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2888] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 42. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3082] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 43. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 617] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 44. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 45. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8248] [Article Influence: 412.4] [Reference Citation Analysis (5)] |
| 46. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1756] [Cited by in RCA: 1822] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 47. | Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 477] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 48. | Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 954] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 49. | Brunt EM, Kleiner DE, Behling C, Contos MJ, Cummings OW, Ferrell LD, Torbenson MS, Yeh M. Misuse of scoring systems. Hepatology. 2011;54:369-370; author reply 370-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Lackner C. Hepatocellular ballooning in nonalcoholic steatohepatitis: the pathologist’s perspective. Expert Rev Gastroenterol Hepatol. 2011;5:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 649] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 52. | Bedossa P, FLIP Pathology Consortium. FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 460] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 53. | Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, Pramoonjago P, Simmons W, Scruggs H, Rosenbaum N. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Banner BF, Savas L, Zivny J, Tortorelli K, Bonkovsky HL. Ubiquitin as a marker of cell injury in nonalcoholic steatohepatitis. Am J Clin Pathol. 2000;114:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Guy CD, Suzuki A, Burchette JL, Brunt EM, Abdelmalek MF, Cardona D, McCall SJ, Ünalp A, Belt P, Ferrell LD, Diehl AM; Nonalcoholic Steatohepatitis Clinical Research Network. Costaining for keratins 8/18 plus ubiquitin improves detection of hepatocyte injury in nonalcoholic fatty liver disease. Hum Pathol. 2012;43:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | Ikura Y, Kim SR, Iwai Y, Muragaki Y. Diagnostic values of ubiquitin-positive hepatocellular inclusions in pathologic diagnosis of nonalcoholic steatohepatitis. J Hepatol. 2013;58:S539-S540. [DOI] [Full Text] |
| 57. | Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008;132:1761-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 58. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 59. | Ikura Y, Mita E, Nakamori S. Hepatocellular carcinomas can develop in simple fatty livers in the setting of oxidative stress. Pathology. 2011;43:167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |